Artificial Intelligence-Driven Analysis of Antimicrobial-Resistant and Biofilm-Forming Pathogens on Biotic and Abiotic Surfaces
Abstract
:1. Introduction
2. Clinical Significance of Biofilm-Forming Microbial Infection
3. Limitations of Detection and Analysis of Biofilms Using Biochemical and Microscopic Techniques
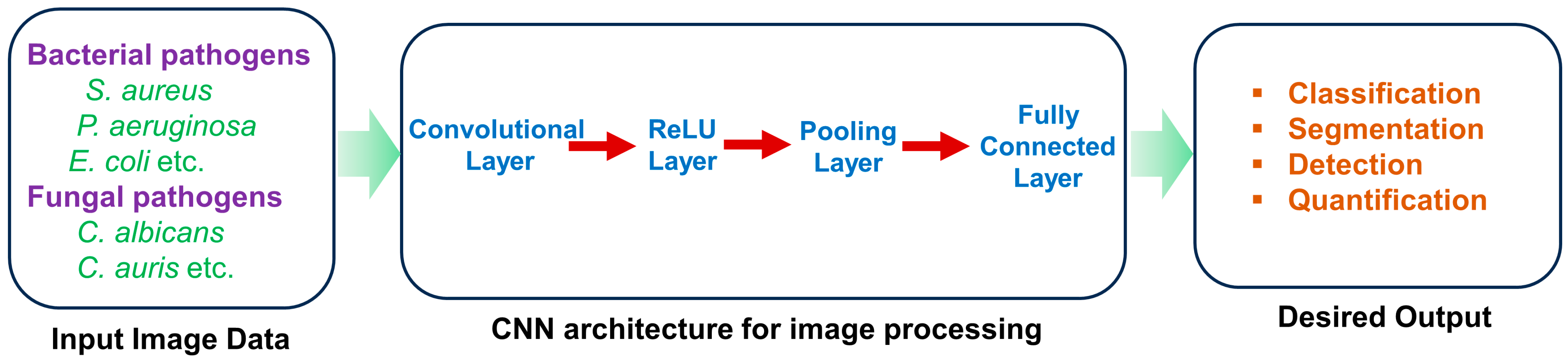
4. Application of Artificial Intelligence for the Analysis of Biofilm of Microbial Pathogens on the Biotic and Abiotic Surfaces
5. Application of Artificial Intelligence for Detection of the Effects of Various Environmental Factors on Biofilm Formation by Microbial Pathogens
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nabadda, S.; Kakooza, F.; Kiggundu, R.; Walwema, R.; Bazira, J.; Mayito, J.; Mugerwa, I.; Sekamatte, M.; Kambugu, A.; Lamorde, M. Implementation of the World Health Organization global antimicrobial resistance surveillance system in Uganda, 2015–2020: Mixed-methods study using national surveillance data. JMIR Public Health Surveill. 2021, 7, e29954. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2019; US Department of Health and Human Services, Centres for Disease Control and Prevention: Atlanta, GA, USA, 2019.
- ECDC; EMEA. The Bacterial Challenge–Time to React a Call to Narrow the Gap between Multidrug-Resistant Bacteria in the EU and Development of New Antibacterial Agents; ECDC & EMEA: Solna, Sweden, 2009. [Google Scholar]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef]
- Mah, T.-F.C.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881. [Google Scholar] [CrossRef]
- Khan, F.; Bamunuarachchi, N.I.; Pham, D.T.N.; Tabassum, N.; Khan, M.S.A.; Kim, Y.M. Mixed biofilms of pathogenic Candida-bacteria: Regulation mechanisms and treatment strategies. Crit. Rev. Microbiol. 2021, 47, 699–727. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Sutherland, I.W. The biofilm matrix—An immobilized but dynamic microbial environment. Trends Microbiol. 2001, 9, 222–227. [Google Scholar] [CrossRef]
- Ma, L.; Conover, M.; Lu, H.; Parsek, M.R.; Bayles, K.; Wozniak, D.J. Assembly and development of the Pseudomonas aeruginosa biofilm matrix. PLoS Pathog. 2009, 5, e1000354. [Google Scholar] [CrossRef]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef]
- Parsek, M.R.; Singh, P.K. Bacterial biofilms: An emerging link to disease pathogenesis. Annu. Rev. Microbiol. 2003, 57, 677–701. [Google Scholar] [CrossRef]
- O’Toole, G.; Kaplan, H.; Kolter, R. Biofilm Formation as Microbial Development. Annu. Rev. Microbiol. 2000, 54, 49–79. [Google Scholar] [CrossRef]
- Mah, T.F.; Pitts, B.; Pellock, B.; Walker, G.C.; Stewart, P.S.; O’Toole, G.A. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 2003, 426, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol. 2007, 5, 48–56. [Google Scholar] [CrossRef]
- Conlon, B.P. Staphylococcus aureus chronic and relapsing infections: Evidence of a role for persister cells: An investigation of persister cells, their formation and their role in S. aureus disease. Bioessays 2014, 36, 991–996. [Google Scholar] [CrossRef]
- Khan, F.; Pham, D.T.N.; Tabassum, N.; Oloketuyi, S.F.; Kim, Y.M. Treatment strategies targeting persister cell formation in bacterial pathogens. Crit. Rev. Microbiol. 2020, 46, 665–688. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms and device-associated infections. Emerg. Infect. Dis. 2001, 7, 277–281. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcus epidermidis—The ‘accidental’ pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567. [Google Scholar] [CrossRef]
- Khan, F. Strategies for Controlling Biofilm-forming Microbial Pathogens on Biotic and Abiotic Surfaces. Curr. Drug Targets 2022, 23, 956–959. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Hu, F.Z.; Gieseke, A.; Nistico, L.; Nguyen, D.; Hayes, J.; Forbes, M.; Greenberg, D.P.; Dice, B.; Burrows, A.; et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA 2006, 296, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Schulze, A.; Mitterer, F.; Pombo, J.P.; Schild, S. Biofilms by bacterial human pathogens: Clinical relevance—Development, composition and regulation—Therapeutical strategies. Microb. Cell 2021, 8, 28–56. [Google Scholar] [CrossRef]
- Zaki, F.R.; Monroy, G.L.; Shi, J.; Sudhir, K.; Boppart, S.A. Texture-based speciation of otitis media-related bacterial biofilms from optical coherence tomography images using supervised classification. J. Biophotonics 2024, e202400075. [Google Scholar] [CrossRef]
- Voinescu, A.; Licker, M.; Muntean, D.; Musuroi, C.; Musuroi, S.I.; Izmendi, O.; Vulpie, S.; Jumanca, R.; Munteanu, M.; Cosnita, A. A Comprehensive Review of Microbial Biofilms on Contact Lenses: Challenges and Solutions. Infect. Drug Resist. 2024, 17, 2659–2671. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-associated infections, medical devices and biofilms: Risk, tolerance and control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Tabassum, N.; Kim, Y.M. A strategy to control colonization of pathogens: Embedding of lactic acid bacteria on the surface of urinary catheter. Appl. Microbiol. Biotechnol. 2020, 104, 9053–9066. [Google Scholar] [CrossRef]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef]
- Mah, T.F. Biofilm-specific antibiotic resistance. Future Microbiol. 2012, 7, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Neu, T.R.; Wozniak, D.J. The EPS matrix: The “house of biofilm cells”. J. Bacteriol. 2007, 189, 7945–7947. [Google Scholar] [CrossRef]
- Chen, Y.; Kolodkin-Gal, I. Host-Biofilm Interactions. Microorganisms 2022, 10, 1641. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.W.; Mah, T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef] [PubMed]
- Mubeen, B.; Ansar, A.N.; Rasool, R.; Ullah, I.; Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Alzarea, S.I.; Nadeem, M.S.; Kazmi, I. Nanotechnology as a novel approach in combating microbes providing an alternative to antibiotics. Antibiotics 2021, 10, 1473. [Google Scholar] [CrossRef]
- Anderson, M.; Panteli, D.; van Kessel, R.; Ljungqvist, G.; Colombo, F.; Mossialos, E. Challenges and opportunities for incentivising antibiotic research and development in Europe. Lancet Reg. Health Eur. 2023, 33, 100705. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Jensen, P.; Fiandaca, M.J.; Pedersen, J.; Hansen, C.R.; Andersen, C.B.; Pressler, T.; Givskov, M.; Høiby, N. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr. Pulmonol. 2009, 44, 547–558. [Google Scholar] [CrossRef]
- Kampouraki, Z.C.; Petala, M.; Zacharias, K.; Konstantinidis, A.; Zabulis, X.; Karamaounas, P.; Kostoglou, M.; Karapantsios, T.D. Highly sensitive resistance spectroscopy technique for online monitoring of biofilm growth on metallic surfaces. Environ. Res. 2024, 240, 117401. [Google Scholar] [CrossRef]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Bahrami, R.; Bahador, A. Revolution of artificial intelligence in antimicrobial, anti-biofilm, and anti-inflammatory techniques: Smart photo-sonodynamic appliance in the internet of dental things (IoDT). Med. Hypotheses 2024, 184, 111270. [Google Scholar] [CrossRef]
- Ray, R.R.; Pattnaik, S. Technological advancements for the management of oral biofilm. Biocatal. Agric. Biotechnol. 2024, 56, 103017. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhai, Y.; Jeong, K.C. Advancing understanding of microbial biofilms through machine learning-powered studies. Food Sci. Biotechnol. 2023, 32, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Parul; Singh, A.P. Potential Use of Biotechnological Tools to Eradicate Microbial Biofilms. In Microbial Biotechnology in the Food Industry: Advances, Challenges, and Potential Solutions; Ahmad, F., Mohammad, Z.H., Ibrahim, S.A., Zaidi, S., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 447–470. [Google Scholar]
- Wang, X.; Chen, C.; Hu, J.; Liu, C.; Ning, Y.; Lu, F. Current strategies for monitoring and controlling bacterial biofilm formation on medical surfaces. Ecotoxicol. Environ. Saf. 2024, 282, 116709. [Google Scholar] [CrossRef] [PubMed]
- Bridier, A.; Sanchez-Vizuete, P.; Guilbaud, M.; Piard, J.C.; Naïtali, M.; Briandet, R. Biofilm-associated persistence of food-borne pathogens. Food Microbiol. 2015, 45, 167–178. [Google Scholar] [CrossRef]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial Biofilms in the Food Industry-A Comprehensive Review. Int. J. Environ. Res. Public Health 2021, 18, 2014. [Google Scholar] [CrossRef]
- Rani Suriani, A.; Pitts, B.; Beyenal, H.; Veluchamy Raaja, A.; Lewandowski, Z.; Davison William, M.; Buckingham-Meyer, K.; Stewart Philip, S. Spatial Patterns of DNA Replication, Protein Synthesis, and Oxygen Concentration within Bacterial Biofilms Reveal Diverse Physiological States. J. Bacteriol. 2007, 189, 4223–4233. [Google Scholar] [CrossRef]
- Jeong, G.-J.; Rather, M.A.; Khan, F.; Tabassum, N.; Mandal, M.; Kim, Y.-M. pH-responsive polymeric nanomaterials for the treatment of oral biofilm infections. Colloids Surf. B Biointerfaces 2024, 234, 113727. [Google Scholar] [CrossRef]
- Marić, S.; Jasmina, V.; Marić, S.; Vraneš, J. Characteristics and significance of microbial biofilm formation. Period. Biol. 2007, 109, 115–121. [Google Scholar]
- Stone, P.W. Economic burden of healthcare-associated infections: An American perspective. Expert Rev. Pharmacoecon Outcomes Res. 2009, 9, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Gold, J.A.; Adjei, S.; Gundlapalli, A.V.; Huang, Y.-L.A.; Chiller, T.; Benedict, K.; Toda, M. Increased hospitalizations involving fungal infections during COVID-19 pandemic, United States, January 2020–December 2021. Emerg. Infect. Dis. 2023, 29, 1433. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef] [PubMed]
- Mirghani, R.; Saba, T.; Khaliq, H.; Mitchell, J.; Do, L.; Chambi, L.; Diaz, K.; Kennedy, T.; Alkassab, K.; Huynh, T.; et al. Biofilms: Formation, drug resistance and alternatives to conventional approaches. AIMS Microbiol. 2022, 8, 239–277. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef]
- Shineh, G.; Mobaraki, M.; Perves Bappy, M.J.; Mills, D.K. Biofilm Formation, and Related Impacts on Healthcare, Food Processing and Packaging, Industrial Manufacturing, Marine Industries, and Sanitation—A Review. Appl. Microbiol. 2023, 3, 629–665. [Google Scholar] [CrossRef]
- Damyanova, T.; Dimitrova, P.D.; Borisova, D.; Topouzova-Hristova, T.; Haladjova, E.; Paunova-Krasteva, T. An Overview of Biofilm-Associated Infections and the Role of Phytochemicals and Nanomaterials in Their Control and Prevention. Pharmaceutics 2024, 16, 162. [Google Scholar] [CrossRef]
- Mishra, A.; Aggarwal, A.; Khan, F. Medical Device-Associated Infections Caused by Biofilm-Forming Microbial Pathogens and Controlling Strategies. Antibiotics 2024, 13, 623. [Google Scholar] [CrossRef]
- Khan, J.; Tarar, S.M.; Gul, I.; Nawaz, U.; Arshad, M. Challenges of antibiotic resistance biofilms and potential combating strategies: A review. 3 Biotech 2021, 11, 169. [Google Scholar] [CrossRef]
- Hofer, U. The cost of biofilms. Nat. Rev. Microbiol. 2022, 20, 445. [Google Scholar] [CrossRef]
- Highmore, C.J.; Melaugh, G.; Morris, R.J.; Parker, J.; Direito, S.O.L.; Romero, M.; Soukarieh, F.; Robertson, S.N.; Bamford, N.C. Translational challenges and opportunities in biofilm science: A BRIEF for the future. npj Biofilms Microbiomes 2022, 8, 68. [Google Scholar] [CrossRef]
- World Health Organization. Global Spending on Health: A World in Transition; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Jennings, L.K.; Dreifus, J.E.; Reichhardt, C.; Storek, K.M.; Secor, P.R.; Wozniak, D.J.; Hisert, K.B.; Parsek, M.R. Pseudomonas aeruginosa aggregates in cystic fibrosis sputum produce exopolysaccharides that likely impede current therapies. Cell Rep. 2021, 34, 108782. [Google Scholar] [CrossRef] [PubMed]
- Cámara, M.; Green, W.; MacPhee, C.E.; Rakowska, P.D.; Raval, R.; Richardson, M.C.; Slater-Jefferies, J.; Steventon, K.; Webb, J.S. Economic significance of biofilms: A multidisciplinary and cross-sectoral challenge. npj Biofilms Microbiomes 2022, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, R.; Jeckel, H.; Jelli, E.; Singh, K.; Vaidya, S.; Bayer, M.; Vidakovic, L.; Díaz-Pascual, F.; Fong, J.; Dragoš, A.; et al. BiofilmQ, a software tool for quantitative image analysis of microbial biofilm communities. bioRxiv 2019, 735423. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Bogachev, M.I.; Volkov, V.Y.; Markelov, O.A.; Trizna, E.Y.; Baydamshina, D.R.; Melnikov, V.; Murtazina, R.R.; Zelenikhin, P.V.; Sharafutdinov, I.S.; Kayumov, A.R. Fast and simple tool for the quantification of biofilm-embedded cells sub-populations from fluorescent microscopic images. PLoS ONE 2018, 13, e0193267. [Google Scholar] [CrossRef] [PubMed]
- Ragi, S.; Rahman, M.H.; Duckworth, J.; Jawaharraj, K.; Chundi, P.; Gadhamshetty, V. Artificial Intelligence-Driven Image Analysis of Bacterial Cells and Biofilms. IEEE/ACM Trans. Comput. Biol. Bioinform. 2023, 20, 174–184. [Google Scholar] [CrossRef]
- Tabassum, N.; Khan, F.; Jeong, G.J.; Jo, D.M.; Kim, Y.M. Silver nanoparticles synthesized from Pseudomonas aeruginosa pyoverdine: Antibiofilm and antivirulence agents. Biofilm 2024, 7, 100192. [Google Scholar] [CrossRef]
- Alhede, M.; Qvortrup, K.; Liebrechts, R.; Høiby, N.; Givskov, M.; Bjarnsholt, T. Combination of microscopic techniques reveals a comprehensive visual impression of biofilm structure and composition. FEMS Immunol. Med. Microbiol. 2012, 65, 335–342. [Google Scholar] [CrossRef]
- Hung, C.; Zhou, Y.; Pinkner, J.S.; Dodson, K.W.; Crowley, J.R.; Heuser, J.; Chapman, M.R.; Hadjifrangiskou, M.; Henderson, J.P.; Hultgren, S.J. Escherichia coli biofilms have an organized and complex extracellular matrix structure. mBio 2013, 4, 10–1128. [Google Scholar] [CrossRef]
- Relucenti, M.; Familiari, G.; Donfrancesco, O.; Taurino, M.; Li, X.; Chen, R.; Artini, M.; Papa, R.; Selan, L. Microscopy Methods for Biofilm Imaging: Focus on SEM and VP-SEM Pros and Cons. Biology 2021, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Achinas, S.; Yska, S.; Charalampogiannis, N.; Krooneman, J.; Euverink, G.-J. A Technological Understanding of Biofilm Detection Techniques: A Review. Materials 2020, 13, 3147. [Google Scholar] [CrossRef]
- Sazanova, K.; Shchiparev, S.; Vlasov, D. Formation of organic acids by fungi isolated from the surface of stone monuments. Microbiology 2014, 83, 516–522. [Google Scholar] [CrossRef]
- Nouri, H.; Beecham, S.; Anderson, S.; Nagler, P. High Spatial Resolution WorldView-2 Imagery for Mapping NDVI and Its Relationship to Temporal Urban Landscape Evapotranspiration Factors. Remote Sens. 2014, 6, 580–602. [Google Scholar] [CrossRef]
- Bakke, R.; Kommedal, R.; Kalvenes, S. Quantification of Biofilm Accumulation by an Optical Approach. J. Microbiol. Methods 2001, 44, 13–26. [Google Scholar] [CrossRef]
- Grishkin, V.; Iakushkin, O.; Stepenko, N. Biofouling detection based on image processing technique. In Proceedings of the 2017 Computer Science and Information Technologies (CSIT), Yerevan, Armenia, 25–29 September 2017; pp. 158–161. [Google Scholar]
- Stewart, G.N. The charges produced by the growth of bacteria in the molecular concentration and electrical conductivity of culture media. J. Exp. Med. 1899, 4, 235–243. [Google Scholar] [CrossRef]
- Cady, P.; Dufour, S.W.; Lawless, P.; Nunke, B.; Kraeger, S.J. Impedimetric screening for bacteriuria. J. Clin. Microbiol. 1978, 7, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Oliver, L.M.; Dunlop, P.S.; Byrne, J.A.; Blair, I.S.; Boyle, M.; McGuigan, K.G.; McAdams, E.T. An impedimetric sensor for monitoring the growth of Staphylococcus epidermidis. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 535–538. [Google Scholar]
- Costa, A.C.; Pereira, C.A.; Freire, F.; Junqueira, J.C.; Jorge, A.O. Methods for obtaining reliable and reproducible results in studies of Candida biofilms formed in vitro. Mycoses 2013, 56, 614–622. [Google Scholar] [CrossRef]
- Peeters, E.; Nelis, H.J.; Coenye, T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods 2008, 72, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Zambrano, L.J.; Escribano, P.; Bouza, E.; Guinea, J. Production of biofilm by Candida and non-Candida spp. isolates causing fungemia: Comparison of biomass production and metabolic activity and development of cut-off points. Int. J. Med. Microbiol. 2014, 304, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Gabrielson, J.; Hart, M.; Jarelöv, A.; Kühn, I.; McKenzie, D.; Möllby, R. Evaluation of redox indicators and the use of digital scanners and spectrophotometer for quantification of microbial growth in microplates. J. Microbiol. Methods 2002, 50, 63–73. [Google Scholar] [CrossRef]
- Clayborn, J.; Adams, J.; Baker, A.; Ricke, S. Assessment of Salmonella spp. Attachment to Reusable Plastic Containers Based on Scanning Electron Microscopy and BAX® PCR. J. Food Res. 2015, 4, 166. [Google Scholar] [CrossRef]
- Chatterjee, S.; Biswas, N.; Datta, A.; Dey, R.; Maiti, P. Atomic force microscopy in biofilm study. Microscopy 2014, 63, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Merino, L.; Procura, F.; Trejo, F.M.; Bueno, D.J.; Golowczyc, M.A. Biofilm formation by Salmonella sp. in the poultry industry: Detection, control and eradication strategies. Food Res. Int. 2019, 119, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Tabassum, N.; Jeong, G.J.; Jung, W.K.; Kim, Y.M. Inhibition of Mixed Biofilms of Candida albicans and Staphylococcus aureus by β-Caryophyllene-Gold Nanoparticles. Antibiotics 2023, 12, 726. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hébraud, M.; Jaglic, Z.; et al. Critical review on biofilm methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef]
- Wilson, C.; Lukowicz, R.; Merchant, S.; Valquier-Flynn, H.; Caballero, J.; Sandoval, J.; Okuom, M.; Huber, C.; Brooks, T.D.; Wilson, E.; et al. Quantitative and Qualitative Assessment Methods for Biofilm Growth: A Mini-review. Res. Rev. J. Eng. Technol. 2017, 6, 1–25. [Google Scholar]
- Van den Driessche, F.; Rigole, P.; Brackman, G.; Coenye, T. Optimization of resazurin-based viability staining for quantification of microbial biofilms. J. Microbiol. Methods 2014, 98, 31–34. [Google Scholar] [CrossRef]
- Ariafar, M.N.; Iğci, N.; Akçelik, M.; Akçelik, N. Investigation of the effect of different environmental conditions on biofilm structure of Salmonella enterica serotype Virchow via FTIR spectroscopy. Arch. Microbiol. 2019, 201, 1233–1248. [Google Scholar] [CrossRef]
- Serra, D.; Bosch, A.; Russo, D.M.; Rodríguez, M.E.; Zorreguieta, A.; Schmitt, J.; Naumann, D.; Yantorno, O. Continuous nondestructive monitoring of Bordetella pertussis biofilms by Fourier transform infrared spectroscopy and other corroborative techniques. Anal. Bioanal. Chem. 2007, 387, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Aybar, P.; Lopez, S.; Molina, S.; Jara, R.; Sesto, E.; Valdez, J.; Ben Altabef, A.; Vernieri, A. FTIR spectroscopy of chronic venous leg ulcer exudates: An approach to spectral healing marker identification. Analyst 2018, 143, 1583–1592. [Google Scholar]
- Suci, P.A.; Geesey, G.G.; Tyler, B.J. Integration of Raman microscopy, differential interference contrast microscopy, and attenuated total reflection Fourier transform infrared spectroscopy to investigate chlorhexidine spatial and temporal distribution in Candida albicans biofilms. J. Microbiol. Methods 2001, 46, 193–208. [Google Scholar] [CrossRef]
- Rajaraman, V. JohnMcCarthy—Father of artificial intelligence. Resonance 2014, 19, 198–207. [Google Scholar] [CrossRef]
- Searle, J.R. Minds, brains, and programs. Behav. Brain Sci. 1980, 3, 417–424. [Google Scholar] [CrossRef]
- Zhang, Z.; Sejdić, E. Radiological images and machine learning: Trends, perspectives, and prospects. Comput. Biol. Med. 2019, 108, 354–370. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, K.L.-C.; Lo, C.-M.; Hsiao, C.-J. Computer-aided grading of gliomas based on local and global MRI features. Comput. Methods Programs Biomed. 2017, 139, 31–38. [Google Scholar] [CrossRef]
- Goyal, M.; Knackstedt, T.; Yan, S.; Hassanpour, S. Artificial intelligence-based image classification methods for diagnosis of skin cancer: Challenges and opportunities. Comput. Biol. Med. 2020, 127, 104065. [Google Scholar] [CrossRef]
- Alzubaidi, L.; Zhang, J.; Humaidi, A.J.; Al-Dujaili, A.; Duan, Y.; Al-Shamma, O.; Santamaría, J.; Fadhel, M.A.; Al-Amidie, M.; Farhan, L. Review of deep learning: Concepts, CNN architectures, challenges, applications, future directions. J. Big Data 2021, 8, 53. [Google Scholar] [CrossRef]
- Nannavecchia, A.; Girardi, F.; Fina, P.R.; Scalera, M.; Dimauro, G. Personal Heart Health Monitoring Based on 1D Convolutional Neural Network. J. Imaging 2021, 7, 26. [Google Scholar] [CrossRef]
- Aggarwal, A.; Agarwal, R. Optimal determination of wavelet for football player EEG using SVM classifier. Biomed. Res. 2019, 29, 218–226. [Google Scholar] [CrossRef]
- Cao, C.; Liu, F.; Tan, H.; Song, D.; Shu, W.; Li, W.; Zhou, Y.; Bo, X.; Xie, Z. Deep Learning and Its Applications in Biomedicine. Genom. Proteom. Bioinform. 2018, 16, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Im, H.R.; Im, S.J.; Nguyen, D.V.; Jeong, S.P.; Jang, A. Real-time diagnosis and monitoring of biofilm and corrosion layer formation on different water pipe materials using non-invasive imaging methods. Chemosphere 2024, 361, 142577. [Google Scholar] [CrossRef]
- Ruhal, R.; Kataria, R. Biofilm patterns in gram-positive and gram-negative bacteria. Microbiol. Res. 2021, 251, 126829. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar]
- Abeyrathna, D.; Ashaduzzaman, M.; Malshe, M.; Kalimuthu, J.; Gadhamshetty, V.; Chundi, P.; Subramaniam, M. An AI-based approach for detecting cells and microbial byproducts in low volume scanning electron microscope images of biofilms. Front. Microbiol. 2022, 13, 996400. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.H.; Azam, M.A.; Hossen, M.A.; Ragi, S.; Venkataramana, G. BiofilmScanner: A Computational Intelligence Approach to Obtain Bacterial Cell Morphological Attributes from Biofilm Image. arXiv 2023, arXiv:2302.09629. [Google Scholar]
- Maglietta, R.; Carlucci, R.; Fanizza, C.; Dimauro, G.; Maglietta, R. Machine Learning and Image Processing Methods for Cetacean Photo Identification: A Systematic Review. IEEE Access 2022, 10, 80195–80207. [Google Scholar] [CrossRef]
- Ding, H.; Yang, Y.; Li, X.; Cheung, G.S.; Matinlinna, J.P.; Burrow, M.; Tsoi, J.K. A simple AI-enabled method for quantifying bacterial adhesion on dental materials. Biomater. Investig. Dent. 2022, 9, 75–83. [Google Scholar] [CrossRef]
- Dimauro, G.; Deperte, F.; Maglietta, R.; Bove, M.; La Gioia, F.; Renò, V.; Simone, L.; Gelardi, M. A Novel Approach for Biofilm Detection Based on a Convolutional Neural Network. Electronics 2020, 9, 881. [Google Scholar] [CrossRef]
- El-Naggar, N.; Dalal, S.; Zweil, A.; Eltarahony, M. Artificial intelligence-based optimization for chitosan nanoparticles biosynthesis, characterization and in-vitro assessment of its anti-biofilm potentiality. Sci. Rep. 2023, 13, 26. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, H.; Ye, T.; Juhas, M. Deep Learning for Imaging and Detection of Microorganisms. Trends Microbiol. 2021, 29, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Qamar, A.; Kerdi, S.; Amin, N.; Zhang, X.; Vrouwenvelder, J.; Ghaffour, N. A deep neural networks framework for in-situ biofilm thickness detection and hydrodynamics tracing for filtration systems. Sep. Purif. Technol. 2022, 301, 121959. [Google Scholar] [CrossRef]
- Cascarano, G.; Debitonto, F.; Lemma, R.; Brunetti, A.; Buongiorno, D.; De Feudis, I.; Guerriero, A.; Venere, U.; Matino, S.; Rocchetti, M.; et al. A neural network for glomerulus classification based on histological images of kidney biopsy. BMC Med. Inform. Decis. Mak. 2021, 21, 300. [Google Scholar] [CrossRef] [PubMed]
- Vyas, N.; Sammons, R.; Addison, O.; Dehghani, H.; Walmsley, A. A quantitative method to measure biofilm removal efficiency from complex biomaterial surfaces using SEM and image analysis. Sci. Rep. 2016, 6, 32694. [Google Scholar] [CrossRef]
- Rajput, A.; Bhamare, K.T.; Thakur, A.; Kumar, M. Anti-Biofilm: Machine Learning Assisted Prediction of IC50 Activity of Chemicals Against Biofilms of Microbes Causing Antimicrobial Resistance and Implications in Drug Repurposing. J. Mol. Biol. 2023, 435, 168115. [Google Scholar] [CrossRef]
- Zou, H.; Sopasakis, A.; Maillard, F.; Karlsson, E.; Duljas, J.; Silwer, S.; Ohlsson, P.; Hammer, E.C. Bacterial community characterization by deep learning aided image analysis in soil chips. Ecol. Inform. 2024, 81, 102562. [Google Scholar] [CrossRef]
- Buetti-Dinh, A.; Galli, V.; Bellenberg, S.; Ilie, O.; Herold, M.; Christel, S.; Boretska, M.; Pivkin, I.; Wilmes, P.; Sand, W.; et al. Deep Neural Networks Outperform Human Expert’s Capacity in Characterizing Bioleaching Bacterial Biofilm Composition. Biotechnol. Rep. 2019, 22, e00321. [Google Scholar] [CrossRef]
- Lee, A.; Park, S.; Yoo, J.; Kang, J.; Lim, J.; Seo, Y.-W.; Kim, B.; Kim, G. Detecting Bacterial Biofilms Using Fluorescence Hyperspectral Imaging and Various Discriminant Analyses. Sensors 2021, 21, 2213. [Google Scholar] [CrossRef]
- Artini, M.; Papa, R.; Sapienza, F.; Božović, M.; Vrenna, G.; Tuccio Guarna Assanti, V.; Sabatino, M.; Garzoli, S.; Fiscarelli, E.V.; Ragno, R.; et al. Essential Oils Biofilm Modulation Activity and Machine Learning Analysis on Pseudomonas aeruginosa Isolates from Cystic Fibrosis Patients. Microorganisms 2022, 10, 887. [Google Scholar] [CrossRef]
- Papa, R.; Garzoli, S.; Vrenna, G.; Sabatino, M.; Sapienza, F.; Relucenti, M.; Donfrancesco, O.; Fiscarelli, E.V.; Artini, M.; Selan, L.; et al. Essential Oils Biofilm Modulation Activity, Chemical and Machine Learning Analysis. Application on Staphylococcus aureus Isolates from Cystic Fibrosis Patients. Int. J. Mol. Sci. 2020, 21, 9258. [Google Scholar] [CrossRef]
- Rickert, C.A.; Hayta, E.N.; Selle, D.M.; Kouroudis, I.; Harth, M.; Gagliardi, A.; Lieleg, O. Machine Learning Approach to Analyze the Surface Properties of Biological Materials. ACS Biomater. Sci. Eng. 2021, 7, 4614–4625. [Google Scholar] [CrossRef]
- Srivastava, G.N.; Malwe, A.S.; Sharma, A.K.; Shastri, V.; Hibare, K.; Sharma, V.K. Molib: A machine learning based classification tool for the prediction of biofilm inhibitory molecules. Genomics 2020, 112, 2823–2832. [Google Scholar] [CrossRef] [PubMed]
- Olcay, B.; Ozdemir, G.D.; Özdemir, M.; Ercan, U.; Güren, O.; Karaman, O. Prediction of the synergistic effect of antimicrobial peptides and antimicrobial agents via supervised machine learning. BMC Biomed. Eng. 2024, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Vishnepolsky, B.; Gabrielian, A.; Rosenthal, A.; Hurt, D.E.; Tartakovsky, M.; Managadze, G.; Grigolava, M.; Makhatadze, G.I.; Pirtskhalava, M. Predictive Model of Linear Antimicrobial Peptides Active against Gram-Negative Bacteria. J. Chem. Inf. Model. 2018, 58, 1141–1151. [Google Scholar] [CrossRef]
- Jelli, E.; Ohmura, T.; Netter, N.; Abt, M.; Jiménez-Siebert, E.; Neuhaus, K.; Rode, D.; Nadell, C.; Drescher, K. Single-cell segmentation in bacterial biofilms with an optimized deep learning method enables tracking of cell lineages and measurements of growth rates. Mol. Microbiol. 2023, 119, 659–676. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Pereira, J.E.; Maltez, L.; Poeta, P.; Igrejas, G. Influence of Environmental Factors on Biofilm Formation of Staphylococci Isolated from Wastewater and Surface Water. Pathogens 2022, 11, 1069. [Google Scholar] [CrossRef]
- Hermanowicz, S.W. A simple 2D biofilm model yields a variety of morphological features. Math. Biosci. 2001, 169, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tango, C.N.; Akkermans, S.; Hussain, M.S.; Khan, I.; Van Impe, J.; Jin, Y.-G.; Oh, D.H. Modeling the effect of pH, water activity, and ethanol concentration on biofilm formation of Staphylococcus aureus. Food Microbiol. 2018, 76, 287–295. [Google Scholar] [CrossRef]
- Vaezi, S.; Poorazizi, E.; Tahmourespour, A.; Aminsharei, F. Application of artificial neural networks to describe the combined effect of pH, time, NaCl and ethanol concentrations on the biofilm formation of Staphylococcus aureus. Microb. Pathog. 2020, 141, 103986. [Google Scholar] [CrossRef]
- Oliveira, V.; Sousa, V.; Dias-Ferreira, C. Artificial neural network modelling of the amount of separately-collected household packaging waste. J. Clean. Prod. 2019, 210, 401–409. [Google Scholar] [CrossRef]
- Kuroda, S.; Okuda, H.; Ishida, W.; Koseki, S. Modeling growth limits of Bacillus spp. spores by using deep-learning algorithm. Food Microbiol. 2018, 78, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Mateo, F.; Gadea, R.; Mateo, E.; Jiménez, M. Multilayer perceptron neural networks and radial-basis function networks as tools to forecast accumulation of deoxynivalenol in barley seeds contaminated with Fusarium culmorum. Food Control 2011, 22, 88–95. [Google Scholar] [CrossRef]
- Panagou, E.; Mohareb, F.; Argyri, A.; Bessant, C.; Nychas, G.-J. A Comparison of Artificial Neural Networks and Partial Least Squares Modelling for the Rapid Detection of the Microbial Spoilage of Beef Fillets Based on Fourier Transform Infrared Spectral Fingerprints. Food Microbiol. 2011, 28, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Geeraerd, A.H.; Herremans, C.H.; Cenens, C.; Van Impe, J.F. Application of artificial neural networks as a non-linear modular modeling technique to describe bacterial growth in chilled food products. Int. J. Food Microbiol. 1998, 44, 49–68. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, R.; Bhatia, R.; Toor, A.P.; Setia, H. Predicting microbial response to anthropogenic environmental disturbances using artificial neural network and multiple linear regression. Int. J. Cogn. Comput. Eng. 2021, 2, 65–70. [Google Scholar] [CrossRef]
- Kavuncuoglu, H.; Kavuncuoğlu, E.; Ilter, S.; Benli, B.; Sagdic, O.; Yalcin, H. Prediction of the antimicrobial activity of walnut (Juglans regia L.) kernel aqueous extracts using artificial neural network and multiple linear regression. J. Microbiol. Methods 2018, 148, 78–86. [Google Scholar] [CrossRef]
- Hiura, S.; Koseki, S.; Koyama, K. Prediction of population behavior of Listeria monocytogenes in food using machine learning and a microbial growth and survival database. Sci. Rep. 2021, 11, 10613. [Google Scholar] [CrossRef]
- Upadhyay, A.; Upadhyay, A.; Sarangi, P.; Chawade, A.; Pareek, N.; Tripathi, D.; Vivekanand, V. Machine learning approach for microbial growth kinetics analysis of acetic acid-producing bacteria isolated from organic waste. Biochem. Eng. J. 2024, 202, 109164. [Google Scholar] [CrossRef]
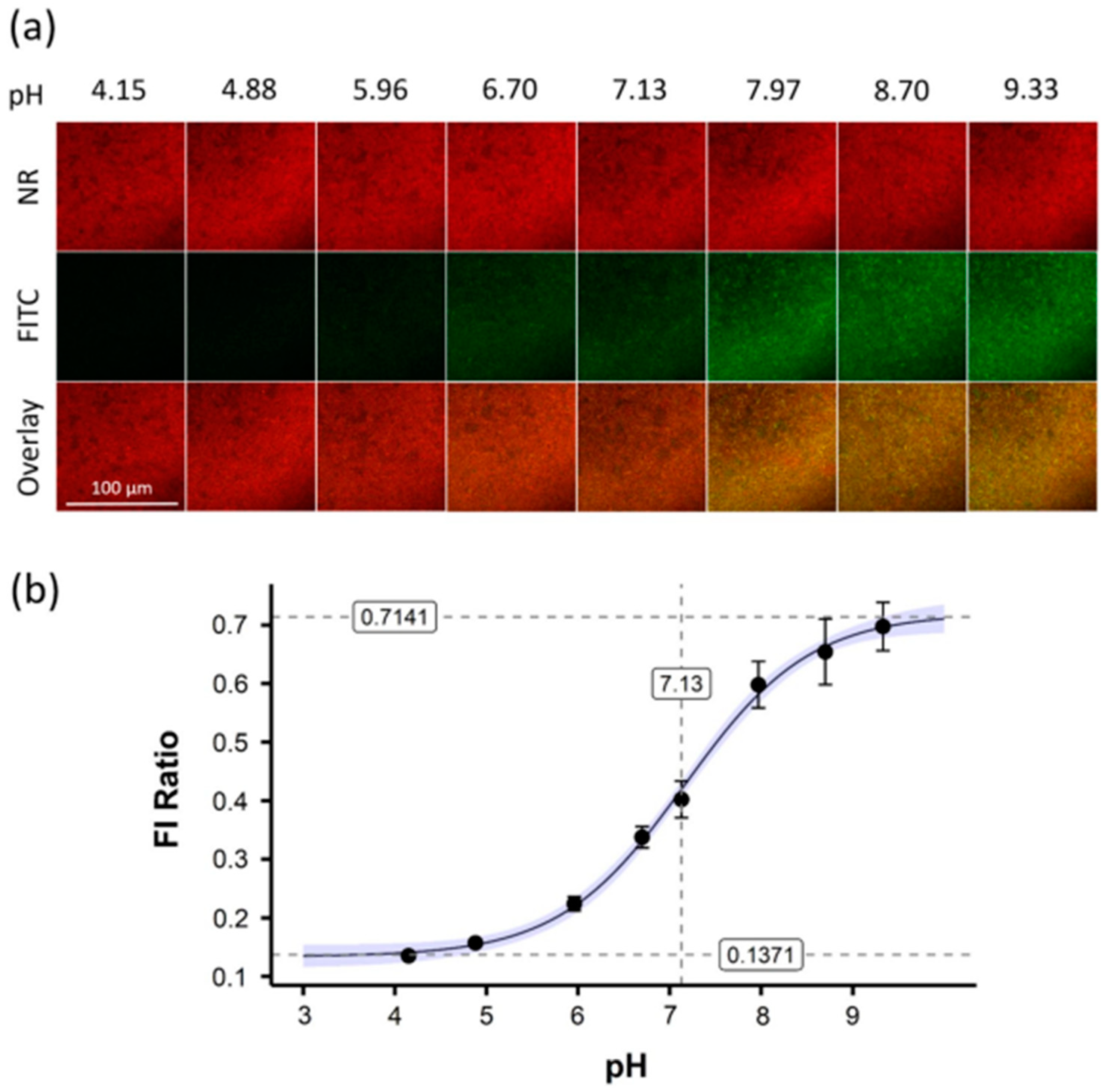
- Kromer, C.; Schwibbert, K.; Gadicherla, A.K.; Thiele, D.; Nirmalananthan-Budau, N.; Laux, P.; Resch-Genger, U.; Luch, A.; Tschiche, H.R. Monitoring and imaging pH in biofilms utilizing a fluorescent polymeric nanosensor. Sci. Rep. 2022, 12, 9823. [Google Scholar] [CrossRef]





| Name of Pathogens | Surfaces | Name of AI Model | Application of Models in Biofilm Detection | References |
|---|---|---|---|---|
| Desulfovibrio alaskensis G20 | Mild steel | M-RCNN | 227 times faster than conventional methods | [72] |
| Desulfovibrio alaskensis G20 | Mild steel | M-RCNN | 1.06 times faster then ImageJ | [111] |
| Desulfovibrio alaskensis G20 | Steel | CNN-YOLACT | 2.1 times faster then M-RCNN | [113] |
| Pseudomonas aeruginosa, Staphylococcus aureus, and Candida albicans | Olea europaea leaves | ANN | NA | [117] |
| Escherichia coli and Salmonella typhimurium | NA | k-NN, LDA | k-NN better accuracy 90% | [125] |
| S. aureus | Polystyrene plate | RF, LR, SVM, GB, DT, k-NN | GB performed best | [126] |
| P. aeruginosa | Polystyrene plate | RF, LR, SVM, GB, DT, k-NN | GB performed best | [125] |
| Bacillus subtilis | MSgg agar | k-NN, GNB, LR, RF | k-NN performed best | [127] |
| S. aureus, Acinetobacter baumannii, P. aeruginosa, Stenotrophomonas maltophilia and E. coli | NA | PCA | 95% ACCURACY | [106] |
| P. aeruginosa | NA | oLGBMC | 76.92% ACCURACY | [131] |
| P. aeruginosa | Metallic (cast iron) and non-metallic (PVC) | ANN, CNN | high correlation coefficients (0.98 and 0.91) in predicting biofilm thickness for cast iron and PVC | [109] |
| Haemophilus influenzae, Streptococcus pneumoniae, Moraxella catarrhalis, P. aeruginosa, and S. aureus | Poly-D-lysine coated chamber slide/glass-bottom dishes (in vitro) and middle ear mucosal surface (in vivo) | VM-RBF, RF, and XGBoost | VM-RBF classifier achieved more than 92% sensitivity, XGBoost shows 90% and 97% sensitivities for the in vitro and in vivo datasets | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, A.; Tabassum, N.; Aggarwal, A.; Kim, Y.-M.; Khan, F. Artificial Intelligence-Driven Analysis of Antimicrobial-Resistant and Biofilm-Forming Pathogens on Biotic and Abiotic Surfaces. Antibiotics 2024, 13, 788. https://doi.org/10.3390/antibiotics13080788
Mishra A, Tabassum N, Aggarwal A, Kim Y-M, Khan F. Artificial Intelligence-Driven Analysis of Antimicrobial-Resistant and Biofilm-Forming Pathogens on Biotic and Abiotic Surfaces. Antibiotics. 2024; 13(8):788. https://doi.org/10.3390/antibiotics13080788
Chicago/Turabian StyleMishra, Akanksha, Nazia Tabassum, Ashish Aggarwal, Young-Mog Kim, and Fazlurrahman Khan. 2024. "Artificial Intelligence-Driven Analysis of Antimicrobial-Resistant and Biofilm-Forming Pathogens on Biotic and Abiotic Surfaces" Antibiotics 13, no. 8: 788. https://doi.org/10.3390/antibiotics13080788
APA StyleMishra, A., Tabassum, N., Aggarwal, A., Kim, Y.-M., & Khan, F. (2024). Artificial Intelligence-Driven Analysis of Antimicrobial-Resistant and Biofilm-Forming Pathogens on Biotic and Abiotic Surfaces. Antibiotics, 13(8), 788. https://doi.org/10.3390/antibiotics13080788







