Antibacterial Activity and Prebiotic Properties of Six Types of Lamiaceae Honey
Abstract
:1. Introduction
2. Results and Discussion
2.1. Action of the Honey on Pathogenic Strains
2.1.1. Minimal Inhibitory Concentration
2.1.2. Hydrophobicity
2.1.3. Hemolytic Activity of Pathogens Grown in the Presence of Honey
2.1.4. Antibiofilm Activity of the Honey
Action of the Honey on Bacterial Biofilm
Action of the Honey on Sessile Bacterial Metabolism
Evaluation of the Amount of Extracellular Bacterial DNA and Proteins
2.2. Prebiotic Effect of the Honey
2.2.1. Effect of Honey on the Probiotics’ Growth
2.2.2. Hydrophobicity of Probiotics
2.2.3. Antioxidative Properties of Probiotics
2.2.4. Cytotoxic Activity of Probiotics
2.2.5. Antibiofilm Activity of Probiotics
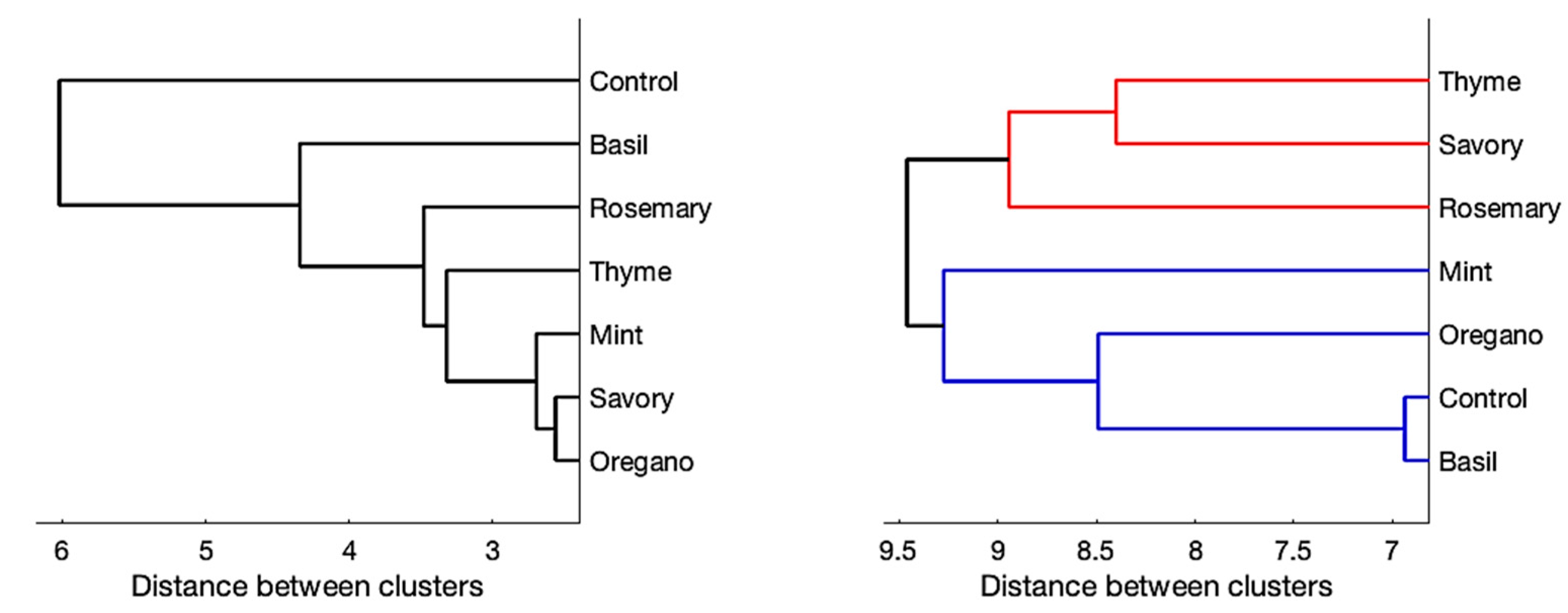
2.3. Conclusions
3. Materials and Methods
3.1. Microbial Adhesion to Solvent
3.2. Minimal Inhibitory Concentration (MIC)
3.3. Hemolytic Activity Exhibited by the Pathogenic Strains in the Presence of the Honey
3.4. Antibiofilm Activity Exhibited by the Honey
3.4.1. The Action of Honey on Immature Biofilm
3.4.2. The Action of the Honey on Mature Biofilm
3.4.3. The Action of the Honey on the Bacterial Sessile Cells’ Metabolism
3.5. Evaluation of Nuclear and Protein Amount in the Culture Supernatants
3.6. Prebiotic Effect of Honey
3.6.1. Probiotic Adhesion to the Solvent
3.6.2. Antioxidant Activity of Probiotics Grown in the Presence of Honey
Reducing Power Capacity
The Anti-Lipid Peroxidation Activity (ILAP, Inhibition of Linoleic Acid Peroxidation)
Hydroxyl Radical Scavenging Activity
3.7. Antibiofilm Activity of the Supernatants of the LAB Grown in the Presence of the Honey
3.8. In Vitro Cytotoxicity Assay of Probiotics and Honey for MCF-7, and Caco-2 Cells
3.8.1. Cell Cultures
3.8.2. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Assay
3.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, R.; Kumar, S.; Kanwar, S.S. Biomedical Perspectives of Herbal Honey. In Biomedical Perspectives of Herbal Honey; Springer Nature Singapore: Singapore, 2024; pp. 89–167. [Google Scholar]
- Mohan, A.; Quek, S.Y.; Gutierrez-Maddox, N.; Gao, Y.; Shu, Q. Effect of honey in improving the gut microbial balance. Food Qual. Saf. 2017, 1, 107–115. [Google Scholar] [CrossRef]
- Jones, R.M.; Luo, L.; Ardita, C.S.; Richardson, A.N.; Kwon, Y.M.; Mercante, J.W.; Alam, A.; Gates, C.L.; Wu, H.; Swanson, P.A.; et al. Symbiotic lactobacilli stimulate gut epithelial proliferation via Nox-mediated generation of reactive oxygen species. EMBO J. 2013, 23, 3017–3028. [Google Scholar] [CrossRef]
- Kang, T.S.; Korber, D.R.; Tanaka, T. Influence of oxygen on NADH recycling and oxidative stress resistance systems in Lactobacillus panis PM1. Amb Express 2013, 3, 10. [Google Scholar]
- Lin, X.; Xia, Y.; Yang, Y.; Wang, G.; Zhou, W.; Ai, L. Probiotic characteristics of Lactobacillus plantarum AR113 and its molecular mechanism of antioxidant. LWT 2020, 126, 109278. [Google Scholar] [CrossRef]
- Romário-Silva, D.; Alencar, S.M.; Bueno-Silva, B.; Sardi, J.d.C.O.; Franchin, M.; Carvalho, R.D.P.d.; Ferreira, T.E.d.S.A.; Rosalen, P.L. Antimicrobial Activity of Honey against Oral Microorganisms: Current Reality, Methodological Challenges and Solutions. Microorganisms 2022, 10, 2325. [Google Scholar] [CrossRef] [PubMed]
- Edo, G.I.; Onoharigho, F.O.; Akpoghelie, P.O.; Akpoghelie, E.O.; Agbo, J.J.; Agoh, E.; Lawal, R.A. Natural honey (raw honey): Insights on quality, composition, economic and health effects: A comprehensive review. Food Sci. Eng. 2023, 4, 265–293. [Google Scholar]
- Khataybeh, B.; Jaradat, Z.; Ababneh, Q. Anti-bacterial, anti-biofilm and anti-quorum sensing activities of honey: A review. J. Ethnopharmacol. 2023, 317, 116830. [Google Scholar] [CrossRef]
- Molan, P.C. The Antibacterial Activity of Honey. Bee World 1992, 73, 5–28. [Google Scholar] [CrossRef]
- WHO. A Global Health Guardian: Climate Change, Air Pollution and Antimicrobial Resistance; WHO: Geneve, Switzerland, 2017. [Google Scholar]
- Alzahrani, H.; Alsabehi, R.; Boukraâ, L.; Abdellah, F.; Bellik, Y.; Bakhotmah, B. Antibacterial and Antioxidant Potency of Floral Honeys from Different Botanical and Geographical Origins. Molecules 2012, 17, 10540–10549. [Google Scholar] [CrossRef]
- Khan, S.U.; Anjum, S.I.; Rahman, K.; Ansari, M.J.; Khan, W.U.; Kamal, S.; Khattak, B.; Muhammad, A.; Khan, H.U. Honey: Single food stuff comprises many drugs. Saudi J. Biol. Sci. 2018, 25, 320–325. [Google Scholar] [CrossRef]
- Merckoll, P.; Jonassen, T.Ø.; Vad, M.E.; Jeansson, S.L.; Melby, K.K. Bacteria, biofilm and honey: A study of the effects of honey on ‘planktonic’ and biofilm-embedded chronic wound bacteria. Scand. J. Infect. Dis. 2009, 41, 341–347. [Google Scholar] [CrossRef]
- Nishio, E.K.; Ribeiro, J.M.; de Oliveira, A.G.; de Jesus Andrade, C.G.T.; Proni, E.A.; Kobayashi, R.K.T.; Nakazato, G. Antibacterial synergic effect of honey from two stingless bees: Scaptotrigona bipunctata Lepeletier, 1836, and S. postica Latreille, 1807. Sci. Rep. 2016, 6, 21641. [Google Scholar] [CrossRef] [PubMed]
- Bendahbia, K.; Abidli, Z.; Zaazaa, L.; Hmimou, S.; Boukhorb, S.; Jadda, S.; Haimer, A.; Mokhtari, A.; Soulaymani, A.; Khadmaoui, A. Antimicrobial activity of thyme honey from Morocco against pathogenic bacteria. J. Hyg. Eng. Des. 2020, 32, 23–27. [Google Scholar]
- Paton, A.J.; Harley, R.M.; Harley, M.M. Ocinum: An Overwiew of Classification and Relationships; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Hussain, A.; Anwar, F.; Sherazi, S.T.; Przybylski, R. Chemical Composition, Antioxidant and Antimicrobial Activities of Basil (Ocimum basilicum) Essential oils Depends on Seasonal Variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Euro+Med. The Euro+Med PlantBase—The Information Resource for Euro-Mediterranean Plant Diversity. Available online: https://www.emplantbase.org/home.html (accessed on 26 August 2024).
- Lukas, B.; Schmiderer, C.; Franz, C.; Novak, J. Composition of Essential Oil Compounds from Different Syrian Populations of Origanum syriacum L. (Lamiaceae). J. Agric. Food Chem. 2009, 57, 1362–1365. [Google Scholar] [CrossRef]
- Fratianni, F.; De Martino, L.; Melone, A.; De Feo, V.; Coppola, R.; Nazzaro, F. Preservation of Chicken Breast Meat Treated with Thyme and Balm Essential Oils. J. Food Sci. 2010, 75, M528–M535. [Google Scholar] [CrossRef] [PubMed]
- Mendes, F.S.F.; Garcia, L.M.; da Silva Moraes, T.; Casemiro, L.A.; de Alcântara, C.B.; Ambrósio, S.R.; Veneziani, R.C.S.; Miranda, M.L.D.; Martins, C.H.G. Antibacterial activity of salvia officinalis L. against periodontopathogens: An in vitro study. Anaerobe 2020, 63, 102194. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Zedan, H.; Ibrahim, Y. Quorum sensing inhibitory effect of bergamot oil and aspidosperma extract against Chromobacterium violaceum and Pseudomonas aeruginosa. Arch. Microbiol. 2021, 203, 4663–4675. [Google Scholar] [CrossRef]
- Kaur, A.P.; Bhardwaj, S.; Dhanjal, D.S.; Nepovimova, E.; Cruz-Martins, N.; Kucˇa, K.; Chopra, C.; Singh, R.; Kumar, H.; Șen, F.; et al. Plant Prebiotics and Their Role in the Amelioration of Diseases. Biomolecules 2021, 11, 440. [Google Scholar] [CrossRef]
- Chassagne, F.; Samarakoon, T.; Porras, G.; Lyles, J.T.; Dettweiler, M.; Marquez, L.; Salam, A.M.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. A Systematic Review of Plants with Antibacterial Activities: A Taxonomic and Phylogenetic Perspective. Front. Pharmacol. 2021, 11, 586548. [Google Scholar] [CrossRef]
- Boukraâ, L.; Alzahrani, H.A.; Abdellah, F.; Bakhotmah, B.; Hammoudi, S.M. Synergistic Effect of Monofloral Honeys and Essential Oils against Pseudomonas aeruginosa. Br. Microbiol. Res. J. 2013, 3, 564–573. [Google Scholar] [CrossRef]
- Imtara, H.; Elamine, Y.; Lyoussi, B. Honey Antibacterial Effect Boosting Using Origanum vulgare L. Essential Oil. Evid. Based Complement. Altern. Med. 2018, 2018, 7842583. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, F.; De Giulio, B.; d’Acierno, A.; Amato, G.; De Feo, V.; Coppola, R.; Nazzaro, F. In Vitro Prebiotic Effects and Antibacterial Activity of Five Leguminous Honeys. Foods 2023, 12, 3338. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Bacterial Priority Pathogens List; WHO: Geneve, Switzerland, 2024. [Google Scholar]
- Fratianni, F.; Amato, G.; d’Acierno, A.; Ombra, M.N.; De Feo, V.; Coppola, R.; Nazzaro, F. In vitro prospective healthy and nutritional benefits of different Citrus monofloral honeys. Sci. Rep. 2023, 13, 1088. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L. Host-Pathogen Interactions: Redefining the Basic Concepts of Virulence and Pathogenicity. Infect. Immun. 1999, 67, 3703–3713. [Google Scholar] [CrossRef]
- Berne, C.; Ellison, C.; Ducret, A.; Brun, Y. Bacterial adhesion at the single-cell level. Nat. Rev. Microbiol. 2018, 16, 1. [Google Scholar] [CrossRef]
- Wang, Z.; Elimelech, M.; Lin, S. Environmental Applications of Interfacial Materials with Special Wettability. Environ. Sci. Technol. 2016, 50, 2132–2150. [Google Scholar] [CrossRef]
- Maikranz, E.; Spengler, C.; Thewes, N.; Thewes, A.; Nolle, F.; Jung, P.; Bischoff, M.; Santen, L.; Jacobs, K. Different binding mechanisms of Staphylococcus aureus to hydrophobic and hydrophilic surfaces. Nanoscale 2020, 12, 19267–19275. [Google Scholar] [CrossRef] [PubMed]
- Kwakman, P.H.S.; Velde, A.A.t.; de Boer, L.; Speijer, D.; Christina Vandenbroucke-Grauls, M.J.; Zaat, S.A.J. How honey kills bacteria. FASEB J. 2010, 24, 2576–2582. [Google Scholar] [CrossRef]
- Shen, L.; Liu, D.; Li, M.; Jin, F.; Din, M.; Parnell, L.D.; Lai, C.Q. Mechanism of Action of Recombinant Acc-Royalisin from Royal Jelly of Asian Honeybee against Gram-Positive Bacteria. PLoS ONE 2012, 7, e47194. [Google Scholar] [CrossRef]
- Aygül, A.; S¸erbetçi, T. The antibacterial and antivirulent potential of Hypericum lydium against Staphylococcus aureus: Inhibition of growth, biofilm formation, and hemolytic activity. Eur. J. Integr. Med. 2020, 35, 101061. [Google Scholar] [CrossRef]
- Hodille, E.; Rose, W.; Diep, B.A.; Goutelle, S.; Lina, G.; Dumitrescu, O. The Role of Antibiotics in Modulating Virulence in Staphylococcus aureus. Clin. Microbiol. Rev. 2017, 30, 887–917. [Google Scholar] [CrossRef] [PubMed]
- Tayabali, A.F.; Nguyen, K.C.; Shwed, P.S.; Crosthwait, J.; Coleman, G.; Seligy, V.L. Comparison of the Virulence Potential of Acinetobacter Strains from Clinical and Environmental Sources. PLoS ONE 2012, 7, e37024. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Zhou, M.; Liu, Z.; Chen, Y.; Li, R. Inhibition effects of low concentrations of epigallocatechin gallate on the biofilm formation and hemolytic activity of Listeria monocytogenes. Food Control 2018, 85, 119–126. [Google Scholar] [CrossRef]
- Ghosh, D.; Mangar, P.; Choudhury, A.; Kumar, A.; Saha, A.; Basu, P.; Saha, D. Characterization of a hemolytic and antibiotic- resistant Pseudomonas aeruginosa strain S3 pathogenic to fish isolated from Mahananda River in India. PLoS ONE 2024, 19, e0300134. [Google Scholar] [CrossRef]
- Cané, L.; Saffioti, N.A.; Genetet, S.; Daza Millone, M.A.; Ostuni, M.A.; Schwarzbaum, P.J.; Mouro-Chanteloup, I.; Herlax, V. Alpha hemolysin of E. coli induces hemolysis of human erythrocytes independently of toxin interaction with membrane proteins. Biochimie 2024, 216, 3–13. [Google Scholar] [CrossRef]
- Ramón Sierra, J.; Martínez Guevara, J.; Pool-Yam, L.; Magaña-Ortiz, D.; Yam, A.; Ortiz-Vázquez, E. Effects of phenolic and protein extracts from Melipona beecheii honey on pathogenic strains of Escherichia coli and Staphylococcus aureus. Food Sci. Biotechnol. 2020, 29, 1013–1021. [Google Scholar] [CrossRef]
- Brown, H.L.; Metters, G.; Hitchings, M.D.; Wilkinson, T.S.; Sousa, L.; Cooper, J.; Dance, H.; Atterbury, R.J.; Jenkins, R. Antibacterial and Antivirulence Activity of Manuka Honey against Genetically Diverse Staphylococcus pseudintermedius Strains. Appl. Environ. Microbiol. 2020, 86, e01768-20. [Google Scholar] [CrossRef]
- Jia, Y.; Guan, Z.; Liu, C.; Huang, M.; Li, J.; Feng, J.; Shen, B.; Yang, G. Staphylococcus aureus-hemolysin causes skin inflammation by acting as an agonist of epidermal growth factor receptor. Microbiol. Spectr. 2024, 12, e02227-23. [Google Scholar] [CrossRef]
- Hu, D.L.; Ono, H.K.; Li, S.; Fang, R. Exotoxins of Staphylococcus aureus. In Staphylococcus aureus: Interplay between Bacteria and Hosts; Nakane, A., Asano, K., Eds.; Springer Nature Singapore: Singapore, 2024; pp. 81–117. [Google Scholar] [CrossRef]
- Jadaun, V.; Prateeksha; Singh, B.R.; Paliya, B.S.; Upreti, D.K.; Rao, C.V.; Rawat, A.K.S.; Singh, B.N. Honey enhances the anti-quorum sensing activity and anti-biofilm potential of curcumin. RSC Adv. 2015, 5, 71060–71070. [Google Scholar] [CrossRef]
- Salosso, Y.; Tjendanawangi, A.; Lopez, S.; Pasaribu, W. Effect of The Combination of Kefa Forest Honey and Euphorbia hirta as a Curative agent of Vibrio alginolyticus in the Hybrid Grouper Epinephelus fuscoguttatus. IOP Conf. Ser. Earth Environ. Sci. 2023, 1147, 012006. [Google Scholar] [CrossRef]
- Roby, M.; Fathy, Y.; Esmail, A.H.; Mohdaly, A.; Hassanien, M. Evaluation of Egyptian honeys and their floral origins: Phenolic compounds, antioxidant activities, and antimicrobial characteristics. Environ. Sci. Pollut. Res. 2020, 27, 20748–20756. [Google Scholar] [CrossRef]
- Abbas, H.A. Comparative Antibacterial and Antibiofilm Activities of Manuka Honey and Egyptian Clover Honey. Asian J. Appl. Sci. 2014, 2, 984. [Google Scholar]
- Balázs, V.L.; Nagy-Radványi, L.; Bencsik-Kerekes, E.; Koloh, R.; Szabó, D.; Kocsis, B.; Farkas, Á. Antibacterial and antibiofilm effect of unifloral honeys against bacteria isolated from chronic wound infections. Microorganisms 2023, 11, 509. [Google Scholar] [CrossRef] [PubMed]
- Yamani, H.A.; Ali, W.M. Medicinal plant (Ocimum basilicum) based enhancement of honey bioactivity. S. Afr. J. Ofbotany 2023, 163, 427–432. [Google Scholar] [CrossRef]
- Russo, N.; Di Rosa, A.R.; Pino, A.; Mazzeo, G.; Liotta, L.; Caggia, C.; Randazzo, C.L. Assessment of sensory properties and in vitro antimicrobial activity of monofloral Sicilian honey. Food Biosci. 2023, 52, 102449. [Google Scholar] [CrossRef]
- Schell, K.; Fernandes, K.; Shanahan, E.; Wilson, I.; Blair, S.; Carter, D.; Cokcetin, N. The Potential of Honey as a Prebiotic Food to Re-engineer the Gut Microbiome Toward a Healthy State. Front. Nutr. 2022, 9, 957932. [Google Scholar] [CrossRef]
- Chettri, U.; Kumari, S. A Review on Prebiotic Importance of Stingless Bee Honey and its Ethnomedicinal and Therapeutic Potential. Int. J. Pharm. Sci. Rev. Res. 2020, 63, 150–156. [Google Scholar]
- de Melo, F.H.C.; Menezes, F.N.D.D.; de Sousa, J.M.B.; dos Santos Lima, M.; da Silva Campelo Borges, G.; de Souza, E.L.; Magnani, M. Prebiotic activity of monofloral honeys produced by stingless bees in the semi-arid region of Brazilian Northeastern toward Lactobacillus acidophilus LA-05 and Bifidobacterium lactis BB-12. Food Res. Int. 2020, 128, 108809. [Google Scholar] [CrossRef]
- Das, A.; Datta, S.; Mukherjee, S.; Bose, S.; Ghosh, S.; Dhar, P. Evaluation of antioxidative, antibacterial and probiotic growth stimulatory activities of Sesamum indicum honey containing phenolic compounds and lignans. LWT Food Sci. Technol. 2015, 61, 244–250. [Google Scholar] [CrossRef]
- Shamala, T.; Jyothi, Y.P.S.; Saibaba, P. Stimulatory effect of honey on multiplication of lactic acid bacteria under in vitro and in vivo conditions. Lett. Appl. Microbiol. 2000, 30, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Khozeimeh, F.; Golestannejad, Z.; Tofighi, M.; Ayen, A.; Doostmohamadi, M.; Gavanji, S.; Bakhtari, A. Antibacterial Activity of Milk Vetch Flower Honey against Four Bacteria of Human Oral Flora: Streptococcus mutans, Lactobacillus casei, Lactobasillus rhamnosus and Lactobasillus plantarum. Annu. Res. Rev. Biol. 2014, 4, 3335. [Google Scholar] [CrossRef]
- Fratianni, F.; Ombra, M.N.; d’Acierno, A.; Caputo, L.; Amato, G.; De Feo, V.; Coppola, R.; Nazzaro, F. Polyphenols Content and In Vitro α-Glycosidase Activity of Different Italian Monofloral Honeys, and Their Effect on Selected Pathogenic and Probiotic Bacteria. Microorganisms 2021, 9, 1694. [Google Scholar] [CrossRef]
- Jacobsen, C.; Rosenfeldt, V.; Hayford, A.; Møller, P.; Michaelsen, K.; Paerregaard, A.; Sandström, B.; Tvede, M.; Jakobsen, M. Screening of Probiotic Activities of Forty-Seven Strains of Lactobacillus spp. by In Vitro Techniques and Evaluation of the Colonization Ability of Five Selected Strains in Humans. Appl. Environ. Microbiol. 1999, 65, 4949–4956. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, H.U.; Erden, Y.; Ozel, H.B. In vitro cytotoxic effects of lactobacilli grown with lime honey on human breast and colon cancer cells. Food Biosci. 2021, 41, 101020. [Google Scholar] [CrossRef]
- Firuzi, O.; Miri, R.; Tavakkoli, M.; Saso, L. Antioxidant Therapy: Current Status and Future Prospects. Curr. Med. Chem. 2011, 18, 3871–3888. [Google Scholar] [CrossRef]
- Hoffmann, A.; Kleniewska, P.; Pawliczak, R. Antioxidative activity of probiotics. Arch. Med. Sci. 2021, 17, 792–804. [Google Scholar] [CrossRef]
- Kullisaar, T.; Zilmer, M.; Mikelsaar, M.; Vihalemm, T.; Annuk, H.; Kairane, C.; Kilk, A. Two antioxidative lactobacilli strains as promising probiotics. Int. J. Food Microbiol. 2002, 72, 215–224. [Google Scholar] [CrossRef]
- Amaretti, A.; Nunzio, M.; Pompei, A.; Raimondi, S.; Rossi, M.; Bordoni, A. Antioxidant properties of potentially probiotic bacteria: In vitro and in vivo activities. Appl. Microbiol. Biotechnol. 2012, 97, 809–817. [Google Scholar] [CrossRef]
- Won, G.; Choi, S.I.; Park, N.; Kim, J.E.; Kang, C.H.; Kim, G.H. In Vitro Antidiabetic, Antioxidant Activity, and Probiotic Activities of Lactiplantibacillus plantarum and Lacticaseibacillus paracasei Strains. Curr. Microbiol. 2021, 78, 3181–3191. [Google Scholar] [CrossRef]
- Chua, L.S.; Rahaman, N.; Adnan, N.; Tan, E.T.T. Antioxidant Activity of Three Honey Samples in relation with Their Biochemical Components. J. Anal. Methods Chem. 2013, 2013, 313798. [Google Scholar] [CrossRef] [PubMed]
- Abu-Farich, B.; Hamarsheh, H.; Masalha, M.; Aboulghazi, A.; Kmail, A.; Ouassete, M.; Imtara, H.; Badiaa, L.; Saad, B. Polyphenol Contents, Antibacterial and Antioxidant Effects of Four Palestinian Honey Samples, and their Anticancer Effects on Human Breast Cancer Cells. J. Pure Appl. Microbiol. 2024, 18, 1372–1385. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernández, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Amici, A.; Quiles, J.L.; Battino, M. The inhibitory effect of Manuka honey on human colon cancer HCT-116 and LoVo cell growth. Part 1: The suppression of cell proliferation, promotion of apoptosis and arrest of the cell cycle. Food Funct. 2018, 9, 2145–2157. [Google Scholar] [CrossRef]
- Qanash, H.; Bazaid, A.S.; Binsaleh, N.K.; Patel, M.; Althomali, O.W.; Sheeha, B.B. In Vitro Antiproliferative Apoptosis Induction and Cell Cycle Arrest Potential of Saudi Sidr Honey against Colorectal Cancer. Nutrients 2023, 15, 3448. [Google Scholar] [CrossRef]
- Escriche, I.; Kadar, M.; Juan, M.; Domenech, E. Suitability of antioxidant capacity, flavonoids and phenolic acids for floral authentication of honey. Impact of industrial thermal treatment. Food Chem. 2014, 142, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Aragón, F.; Carino, S.; Perdigón, G.; LeBlanc, A. The administration of milk fermented by the probiotic Lactobacillus casei CRL 431 exerts an immunomodulatory effect against a breast tumor in a mouse model. Immunobiology 2014, 219, 457–464. [Google Scholar] [CrossRef]
- Riaz Rajoka, M.S.; Zhao, H.; Lu, Y.; Lian, Z.; Li, N.; Hussain, N.; Shao, D.; Jin, M.; Li, Q.; Shi, J. Anticancer potential against cervix cancer (HeLa) cell line of probiotic Lactobacillus casei and Lactobacillus paracasei strains isolated from human breast milk. Food Funct. 2018, 9, 2705–2715. [Google Scholar] [CrossRef]
- Chondrou, P.; Karapetsas, A.; Kiousi, D.E.; Tsela, D.; Tiptiri, A.; Anestopoulos, I.; Kotsianidis, I.; Bezirtzoglou, E.; Pappa, A.; Galani, A. Lactobacillus paracasei K5 displays adhesion, anti-proliferative activity and apoptotic effects in human colon cancer cells. Benef. Microbes 2018, 9, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Zhang, L.W.; Fan, R.B.; Han, X.; Yi, H.X.; Zhang, L.L.; Xue, C.H.; Li, H.B.; Zhang, Y.H.; Shigwedha, N. Induction of HT-29 cells apoptosis by lactobacilli isolated from fermented products. Res. Microbiol. 2014, 165, 202–214. [Google Scholar] [CrossRef]
- Baazeem, A.; Helal, M.; Sami, R.; Alshehry, G.; Algarni, E.; Hilary, U.; Baakdah, F.; Alharthy, S.A.; Mahmoud Johari, D. Positive effects of dietary honey and aflatoxin B1 on serum enzymes, superoxide dismutase activity, β-glucuronidase enzyme activity, and colonic probiotic bacteria on rats. Mater. Express 2024, 14, 60–65. [Google Scholar] [CrossRef]
- Masad, R.J.; Idriss, I.; Mohamed, Y.A.; Al-Sbiei, A.; Bashir, G.; Al-Marzooq, F.; Altahrawi, A.; Fernandez-Cabezudo, M.J.; Al-Ramadi, B.K. Oral administration of Manuka honey induces IFNγ-dependent resistance to tumor growth that correlates with beneficial modulation of gut microbiota composition. Front. Immunol. 2024, 15, 1354297. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, H.U. Probiotic bacteria grown with chestnut honey enhance in vitro cytotoxicity on breast and colon cancer cells. Arch. Biol. Sci. 2020, 2, 329–338. [Google Scholar] [CrossRef]
- Moradi, M.; Molaei, R.; Guimarães, J.T. A review on preparation and chemical analysis of postbiotics from lactic acid bacteria. Enzym. Microb. Technol. 2021, 143, 109722. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Arioli, S.; Behare, P.; Belzer, C.; Berni Canani, R.; Chatel, J.M.; D’Auria, E.; de Freitas, M.Q.; Elinav, E.; Esmerino, E.A.; et al. Postbiotics—When simplification fails to clarify. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 825–826. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cellgrowth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef]
- Antunes, L.C.S.; Imperi, F.; Carattoli, A.; Visca, P. Deciphering the Multifactorial Nature of Acinetobacter baumannii Pathogenicity. PLoS ONE 2011, 6, e22674. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, X.; Bai, T.; Zheng, X.; Yang, L.; Li, Q.; Wang, X. Lysine Inhibits Hemolytic Activity of Staphylococcus aureus and Its Application in Food Model Contaminated with Staphylococcus aureus. Toxins 2022, 14, 867. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, L.; He, Q.; Arabi, S.A.; Zhao, H.; Chen, W.; Ye, X.; Liu, D. Synergistic antibacterial effects of ultrasound and thyme essential oils nanoemulsion against Escherichia coli O157:H7. Ultrason. Sonochemistry 2020, 66, 104988. [Google Scholar] [CrossRef]
- Lin, M.L.; Chang, F.J. Antioxidative effect of intestinal bacteria Bifidobacterium longum ATCC 15708 and Lactobacillus acidophilus ATCC 4356. Dig. Dis. Sci. 2000, 45, 1617–1622. [Google Scholar] [CrossRef]
- Guo, T.; Wei, L.; Sun, J.; lin Hou, C.; Fan, L. Antioxidant activities of extract and fractions from Tuber indicum Cooke & Massee. Food Chem. 2011, 127, 1634–1640. [Google Scholar] [CrossRef]
| MIC | AB | EC | LM | PA | SA |
|---|---|---|---|---|---|
| B | 38.0 ± 2.0 a | >50 b | >50 b | >50 b | 34.0 ± 1.0 a |
| M | 38.0 ± 1.0 a | >50 b | >50 b | >50 b | 34.0 ± 1.0 a |
| O | 40.0 ± 3.0 a | >50 b | >50 b | >50 b | 34.0 ± 2.0 a |
| R | 36.0 ± 2.0 a | >50 b | >50 b | 38.0 ± 2.0 a | 36.0 ± 1.0 nd |
| S | 38.0 ± 1.0 a | >50 b | 38 ± 2.0 nd | >50 b | 36.0 ± 3.0 nd |
| T | 38.0 ± 3.0 a | 38.0 ± 2.0 a | >50 b | >50 b | 36.0 ± 1.0 nd |
| C | 30.0 ± 1.0 | 30.0 ± 2.0 | 30.0 ± 2.0 | 28.0 ± 2.0 | 34.0 ± 1.0 |
| AB | EC | LM | PA | SA | |
|---|---|---|---|---|---|
| B | 8.6 ± 1.1 nd | 0.0 ± 0.0 a | 0.0 ± 0.0 b | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| M | 2.0 ± 0.1 a | 0.0 ± 0.0 a | 0.0 ± 0.0 b | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| O | 4.76± 0.7 a | 0.0 ± 0.0 a | 8.3 ± 0.2 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| R | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 b | 0.0 ± 0.0 a | 6.0 ± 0.5 a |
| S | 4.7 ± 0.1 a | 0.0 ± 0.0 a | 0.0 ± 0.0 b | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| T | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 b | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| C | 8.2 ± 0.6 | 16.7 ± 1.7 | 19.7 ± 1.6 | 11.8 ± 1.3 | 14.7 ± 1.1 |
| AB | EC | LM | PA | SA | |
|---|---|---|---|---|---|
| B | 29.3 ± 1.4 b | 7.0 ± 1.0 a | 15.3 ± 1.1 a | 23.4 ± 2.0 nd | 3.1 ± 0.1 nd |
| M | 38.3 ± 2.4 a | 2.8 ± 0.2 a | 4.5 ± 0.4 nd | 20.0 ± 1.6 nd | 7.0 ± 1.0 nd |
| O | 37.6 ± 2.2 a | 7.9 ± 1.0 a | 1.2 ± 0.1 nd | 10.4 ± 0.6 a | 4.5 ± 0.3 nd |
| R | 40.1 ± 2.3 nd | 8.1 ± 0.6 a | 2.9 ± 0.3 nd | 19.6 ± 1.7 nd | 17.7 ± 1.6 a |
| S | 32.5 ± 2.4 a | 10.2 ± 1.0 nd | 1.9 ± 0.2 nd | 22.5 ± 1.7 nd | 5.1 ± 1.5 nd |
| T | 44.6 ± 3.3 nd | 12.9 ± 1.1 nd | 1.5 ± 0.2 nd | 9.0 ± 0.6 a | 5.1 ± 0.1 nd |
| C | 42.5 ± 3.6 | 11.6 ± 1.1 | 3.7 ± 0.5 | 23.6 ± 1.6 | 7.2 ± 0.5 |
| T | AB | EC | LM | PA | SA | |
|---|---|---|---|---|---|---|
| B | 0 | 0.00 ± 0.00 a | 0.048 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 a |
| M | 0 | 0.00 ± 0.00 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.35 ± 0.04 b | 0.00 ± 0.00 a |
| O | 0 | 0.58 ± 0.02 a | 0.06 ± 0.0 b | 0.02 ± 0.001 b | 0.21 ± 0.04 b | 0.35 ± 0.06 a |
| R | 0 | 0.011 ± 0.03 a | 0.39 ± 0.04 b | 0.78 ± 0.07 b | 0.70 ± 0.08 b | 0.11 ± 0.05 a |
| S | 0 | 0.00 ± 0.00 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.58 ± 0.03 b | 0.00 ± 0.00 a |
| T | 0 | 0.12 ± 0.02 a | 0.07 ± 0.01 b | 0.85 ± 0.06 b | 0.61 ± 0.05 b | 0.00 ± 0.00 a |
| B | 24 | 1.05 ± 0.32 a | 0.085 ± 0.00 b | 0.00 ± 0.00 b | 0.54 ± 0.04 b | 0.43 ± 0.1 a |
| M | 24 | 0.00 ± 0.00 a | 0.00 ± 0.00 b | 0.60 ± 0.1 b | 0.00 ± 0.00 b | 0.51 ± 0.12 nd |
| O | 24 | 1.24 ± 0.12 nd | 4.98 ± 0.42 b | 5.02 ± 0.32 b | 0.56 ± 0.04 b | 0.55 ± 0.12 nd |
| R | 24 | 1.35 ± 0.53 nd | 3.89 ± 0.44 a | 4.99 ± 0.17 b | 0.62 ± 0.06 b | 0.11 ± 0.03 a |
| S | 24 | 0.09 ± 0.02 a | 3.88 ± 0.56 a | 5.08 ± 0.23 b | 0.59 ± 0.04 b | 0.13 ± 0.02 a |
| T | 24 | 0.59 ± 0.12 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 a |
| C | 1.18 ± 0.00 | 2.38 ± 0.00 | 2.23 ± 0.02 | 2.75 ± 0.01 | 0.59 ± 0.003 |
| T | AB | EC | LM | PA | SA | |
|---|---|---|---|---|---|---|
| B | 0 | 1.60 ± 0.23 a | 0.048 ± 0.03 nd | 0.00 ± 0.00 a | 7.50 ± 0.66 c | 0.00 ± 0.00 a |
| M | 0 | 0.49 ± 0.00 a | 8.17 ± 0.80 d | 7.57 ± 0.00 c | 0.03 ± 0.01 a | 0.07 ± 0.02 a |
| O | 0 | 0.54 ± 0.11 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.03 ± 0.00 a | 0.00 ± 0.00 a |
| R | 0 | 7.39 ± 0.31 c | 0.65 ± 0.21 a | 2.19 ± 0.17 b | 0.36 ± 0.06 a | 0.00 ± 0.00 a |
| S | 0 | 0.68 ± 0.22 a | 0.60 ± 0.14 a | 0.09 ± 0.01 a | 0.58 ± 0.11 a | 0.06 ± 0.02 a |
| T | 0 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 1.15 ± 0.22 b | 0.07 ± 0.02 a |
| B | 24 | 0.00 ± 0.00 a | 0.12 ± 0.01 a | 0.092 ± 0.05 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| M | 24 | 0.66 ± 0.06 a | 0.03 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| O | 24 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.45 ± 0.03 a |
| R | 24 | 0.21 ± 0.02 a | 0.12 ± 0.02 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| S | 24 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| T | 24 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| C | 1.02 ± 0.00 | 0.24 ± 0.02 | 0.28 ± 0.00 | 0.15 ± 0.00 | 1.76 ± 0.17 |
| LC | LG | LPC | LR | |
|---|---|---|---|---|
| B | 0.833 ± 0.15 a | 0.904 ± 0.12 nd | 0.722 ± 0.12 a | 0.822 ± 0.09 a |
| M | 0.783 ± 0.08 nd | 0.887 ± 0.06 a | 0.744 ± 0.06 nd | 0.821 ± 0.07 a |
| O | 0.91 ± 0.10 a | 0.925 ± 0.11 nd | 0.733 ± 0.057 a | 0.776 ± 0.07 nd |
| R | 0.965 ± 0.07 a | 0.971 ± 0.02 a | 0.725 ± 0.015 a | 0.816 ± 0.12 a |
| S | 0.907 ± 0.06 a | 0.974 ± 0.06 a | 0.736 ± 0.022 a | 0.831 ± 0.02 a |
| T | 0.883 ± 0.01 a | 0.907 ± 0.02 nd | 0.742 ± 0.017 nd | 0.835 ± 0.01 a |
| C | 0.765 ± 0.02 | 0.927 ± 0.02 | 0.781 ± 0.05 | 0.749 ± 0.04 |
| LC | LG | LPC | LR | |
|---|---|---|---|---|
| B | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 13.42 ± 0.22 b | 1.3 ± 0.05 a |
| M | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| O | 0.00 ± 0.00 a | 27.26 ± 1.14 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| R | 9.28 ± 0.11b | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| S | 0.00 ± 0.00 a | 1.78 ± 0.12 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| T | 2.41 ± 0.01a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| C | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| LC | LG | LPC | LR | ||
|---|---|---|---|---|---|
| ILAP (%) | B | 7.6 ± 1.6 a | 17.4 ± 1.0 a | 18.3 ± 1.7 a | 11.7 ± 1.2 b |
| M | 7.5 ± 0.9 a | 17.0 ± 1.8 a | 15.9 ± 1.6 a | 8.8 ± 1.2 b | |
| O | 5.0 ± 0.6 a | 0.0 ± 0.0 b | 15.7 ± 2.4 a | 6.6 ± 1.5 a | |
| R | 9.2 ± 0.9 a | 4.5 ± 0.6 a | 0.0 ± 0.0 b | 12.4 ± 2.1 b | |
| S | 10.3 ± 1.7 a | 8.3 ± 2.2 a | 0.0 ± 0.0 b | 3.9 ± 0.9 a | |
| T | 5.3 ± 0.7 a | 9.7 ± 0.5 a | 3.2 ± 1.3 a | 0.4 ± 0.1 a | |
| C | 15.8 ± 1.4 | 11.8 ± 0.3 | 11.9 ± 0.5 | 1.30 ± 0.1 | |
| OHRS (%) | B | 46.1 ± 1.6 a | 61.3 ± 2.1 c | 37.9 ± 0.4 b | 57.5 ± 0.6 c |
| M | 38.0 ± 1.0 nd | 42.9 ± 1.4 b | 49.3 ± 0.8 c | 39.5 ± 0.0 b | |
| O | 27.4 ± 0.3 a | 24.5 ± 0.9 a | 24.7 ± 0.4 a | 30.7 ± 1.8 a | |
| R | 31.7 ± 1.8 a | 40.2 ± 0.2 a | 53.3 ± 0.4 c | 47.0 ± 8.0 b | |
| S | 30.4 ± 0.9 a | 25.7 ± 6.1 a | 30.6 ± 0.2 a | 39.4 ± 1.1 b | |
| T | 38.7 ± 4.6 nd | 59.0 ± 0.5 c | 62.9 ± 1.4 c | 65.8 ± 1.1 c | |
| C | 36.0 ± 2.8 | 33.0 ± 2.2 | 27.4 ± 0.0 | 26.2 ± 0.6 | |
| CFS (mM CystEq) | B | 0.86 ± 0.06 a | 2.06 ± 0.06 a | 2.11 ± 0.1 a | 2.01 ± 0.04 a |
| M | 0.83 ± 0.13 a | 1.99 ± 0.06 a | 1.17 ± 0.05 a | 2.67 ± 0.21 b | |
| O | 1.77 ± 0.15 nd | 1.28 ± 0.03 a | 1.47 ± 0.1 a | 2.27 ± 0.11 b | |
| R | 1.43 ± 0.05 nd | 2.22 ± 0.71 a | 0.22 ± 0.11 a | 3.20 ± 0.09 c | |
| S | 1.63 ± 0.15 nd | 2.62 ± 0.17 nd | 1.31 ± 0.11 nd | 2.8 ± 0.11 c | |
| T | 1.23 ± 0.02 nd | 1.63 ± 0.02 a | 0.25 ± 0.03 a | 1.22 ± 0.02 a | |
| C | 1.58 ± 0.09 | 2.58 ± 0.11 | 1.38 ± 0.06 | 1.02 ± 0.06 |
| Antimicrobial Activity | ||||||||
|---|---|---|---|---|---|---|---|---|
| hydrophobicity | hemolytic activity | CV0 | MTT 0 | CV 24 | MTT24 | |||
| basil | ++++ | +++ | ++ | ++ | ++++ | − | ||
| mint | ++++ | ++++ | ++ | ++ | +++++ | − | ||
| oregano | +++ | +++++ | ++ | ++ | ++++ | − | ||
| rosemary | +++++ | ++++ | ++++ | ++ | +++++ | − | ||
| savory | ++++ | ++++ | +++ | ++ | ++ | − | ||
| thyme | +++++ | +++ | ++ | ++ | +++++ | − | ||
| probiotic activity | ||||||||
| hydrophobicity | growth | CV0 | MTT 0 | antioxidant ILAP | antioxidant OHRS | antioxidant CFS | cytotoxic activity | |
| basil | ++ | ++ | + | +++++ | +++ | +++++ | ++ | − |
| mint | - | ++ | +++++ | +++++ | +++ | +++ | + | ++++ |
| oregano | + | + | ++ | +++++ | ++ | − | +++ | ++++ |
| rosemary | + | − | +++ | ++++ | + | +++ | + | ++++ |
| savory | - | ++ | ++ | +++ | + | ++ | +++ | + |
| thyme | + | ++ | +++++ | +++++ | − | ++++ | + | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazzaro, F.; Ombra, M.N.; Coppola, F.; De Giulio, B.; d’Acierno, A.; Coppola, R.; Fratianni, F. Antibacterial Activity and Prebiotic Properties of Six Types of Lamiaceae Honey. Antibiotics 2024, 13, 868. https://doi.org/10.3390/antibiotics13090868
Nazzaro F, Ombra MN, Coppola F, De Giulio B, d’Acierno A, Coppola R, Fratianni F. Antibacterial Activity and Prebiotic Properties of Six Types of Lamiaceae Honey. Antibiotics. 2024; 13(9):868. https://doi.org/10.3390/antibiotics13090868
Chicago/Turabian StyleNazzaro, Filomena, Maria Neve Ombra, Francesca Coppola, Beatrice De Giulio, Antonio d’Acierno, Raffaele Coppola, and Florinda Fratianni. 2024. "Antibacterial Activity and Prebiotic Properties of Six Types of Lamiaceae Honey" Antibiotics 13, no. 9: 868. https://doi.org/10.3390/antibiotics13090868









