Abstract
With nearly half of colorectal cancer (CRC) patients diagnosed at advanced stages where surgery alone is insufficient, chemotherapy remains a cornerstone for this cancer treatment. To prevent infections and improve outcomes, antibiotics are often co-administered. However, chemotherapeutic interactions with the gut microbiota cause significant non-selective toxicity, affecting not only tumor and normal epithelial cells but also the gut microbiota. This toxicity triggers the bacterial SOS response and loss of microbial diversity, leading to bacterial mutations and dysbiosis. Consequently, pathogenic overgrowth and systemic infections increase, necessitating broad-spectrum antibiotics intervention. This review underscores how prolonged antibiotic use during chemotherapy, combined with chemotherapy-induced bacterial mutations, creates selective pressures that drive de novo antimicrobial resistance (AMR), allowing resistant bacteria to dominate the gut. This compromises the treatment efficacy and elevates the mortality risk. Restoring gut microbial diversity may mitigate chemotherapy-induced toxicity and improve therapeutic outcomes, and emerging strategies, such as fecal microbiota transplantation (FMT), probiotics, and prebiotics, show considerable promise. Given the global threat posed by antibiotic resistance to cancer treatment, prioritizing antimicrobial stewardship is essential for optimizing antibiotic use and preventing resistance in CRC patients undergoing chemotherapy. Future research should aim to minimize chemotherapy’s impact on the gut microbiota and develop targeted interventions to restore microbial diversity affected during chemotherapy.
1. Introduction
Colorectal cancer (CRC) remains a significant global health burden, ranking as the third most commonly diagnosed cancer and the second leading cause of cancer-related deaths worldwide [1]. Recent estimates indicate that 1.9 million new CRC cases occurred globally in 2022, while projections suggest a further increase in CRC incidence to 2.5 million new cases annually by 2035 [2]. The pathogenesis of CRC is driven by a complex interplay of genetic and environmental factors, and Western lifestyles and physical inactivity, and dietary habits, especially among the younger population [3,4]. However, early detection and timely intervention are crucial for improving patient outcomes.
While surgical resection remains the primary curative treatment [5], chemotherapy is a widely employed CRC therapeutic regimen, especially in advanced stages of the disease [6]. During this treatment, anticancer drugs are administered to target and destroy cancer cells, inhibit their proliferation, and prevent tumor invasion, and metastasis [7], with the ultimate goal of eradicating the disease. However, the adverse side effects associated with chemotherapy have spurred interests in developing less toxic treatment alternatives [8,9], including modulating the gut microbiota.
The gut microbiota is a complex ecosystem of microorganisms residing in the human gastrointestinal tract with a crucial role of maintaining a proper digestion and host immunity [10,11]. Emerging evidence suggests a strong link between gut dysbiosis and the development and progression of CRC [12,13,14]. This growing evidence has opened up new therapeutic avenues such as fecal microbiota transplantation (FMT), probiotics, and prebiotics, which aim at restoring the altered gut microbiota to improve patient survival and the efficacy of CRC therapeutic regimens [15,16,17,18,19,20].
However, certain anticancer regimens may lead to the development of antimicrobial resistance (AMR) in the gut microbiota, increasing the risk of antibiotic-resistance-associated complications and CRC-related mortality [21,22,23]. Additionally, these regimens, particularly chemotherapy, not only disrupt gut microbiota homeostasis but also induce dysbiosis [24,25]. The induced dysbiosis creates a conducive environment for the proliferation of antibiotic-resistant bacteria [26,27]. Moreover, chemotherapy can activate the bacterial SOS response and accelerate bacterial mutations and horizontal gene transfer, thus driving the emergence of antibiotic-resistant mutants within the gut ecosystem [23,28,29]. Furthermore, chemotherapy compromises the integrity of the intestinal barrier, increasing the susceptibility to infections [30], and recurrent septic episodes [31]. This infection vulnerability often requires continued antimicrobial therapy, which may inadvertently contribute to the development of AMR pathogens [32,33]. These facts suggest that chemotherapy may increase the risk of superbug infections in CRC patients, potentially hindering their recovery and raising mortality rates. In this review, we explore how the prolonged use of antibiotics during chemotherapy, combined with chemotherapy-induced bacterial mutations, can intensify antibiotic resistance in CRC patients, ultimately contributing to poor treatment outcomes.
2. Chemotherapy for Colorectal Cancer Treatment
The five-year survival rate for patients diagnosed with early-stage CRC exceeds 60% [34]. However, over 50% of CRC patients are diagnosed at advanced stages [35], often with distant metastases, reducing survival rates to approximately 10% [36]. In such cases, surgical resection is rarely curative, necessitating chemotherapeutic intervention.
Chemotherapy is a cornerstone CRC treatment [37], used in adjuvant and palliative settings, especially in advanced or metastatic CRC (mCRC) [38,39]. Its application depends on the cancer’s stage, molecular characteristics, and the patient’s overall health [40]. This form of cancer treatment involves the systemic administration of anticancer agents that target to kill or inhibit rapidly dividing cancer cells.
Chemotherapy is categorized into two main approaches for CRC. Neoadjuvant chemotherapy, administered before surgery, is primarily used in locally advanced CRC to reduce tumor burden and improve resectability [41]. In contrast, adjuvant chemotherapy, given postoperatively, aims to eradicate micro-metastases and lower the risk of recurrence [38,42].
Therapeutic agents like fluoropyrimidines—including 5-fluorouracil (5-FU) and capecitabine—form the backbone of CRC chemotherapy [43]. These are often combined with oxaliplatin or irinotecan in regimens such as FOLFOX, CAPOX, and FOLFIRI to enhance efficacy and improve survival rates [44].
Despite their potent cytotoxic effects against cancer cells, these agents are associated with significant adverse effects that impair patients’ quality of life [9,45]. Hematological toxicities, such as neutropenia and anemia, gastrointestinal symptoms including diarrhea and mucositis, and neurotoxicity associated with oxaliplatin are often experienced by patients undergoing chemotherapy [46]. These often lead to dose adjustments and treatment delays, potentially compromising therapeutic efficacy [47,48].
Beyond these systemic effects, chemotherapy profoundly affects the gut microbiota [15,20,24]. The gut microbiota plays a crucial role in modulating the efficacy and toxicity of chemotherapeutic agents [20,49]. However, chemotherapy-induced mucositis disrupts the gut barrier and creates an inflammatory environment [50,51], altering the microbial composition and diversity [24]. This disruption contributes to secondary infections, thus complicating CRC treatment outcomes even further [52,53]. The interplay between the gut microbiota and chemotherapeutic drugs as illustrated in Figure 1 indicates how modulating the gut microbiota during chemotherapy could improve treatment outcomes.
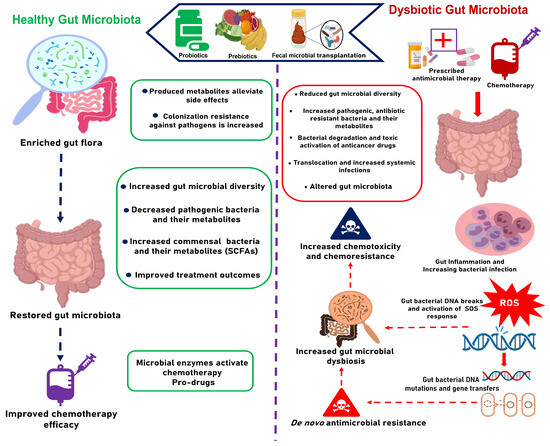
Figure 1.
The impact of gut microbiota to the efficacy and toxicity of CRC chemotherapy.
The increased vulnerability to infections implies prolonged antimicrobial therapy, heightening the risk of AMR and additional threats to patient survival [54,55,56]. Given the central role of chemotherapy in advanced CRC treatment, implementing gut-microbiota-targeted interventions is crucial for preventing dysbiosis, mitigating AMR, and improving treatment outcomes.
3. Gut Microbial Modulation of the Efficacy and Toxicity of Chemotherapy in Colorectal Cancer
Chemotherapy is a widely recommended treatment for advanced CRC; however, patient responses to this therapy exhibit considerable variability [57]. A significant factor contributing to this variability could be the composition of the patient gut microbiota [20,58,59]. A summary of the influence of gut bacteria in chemotherapy toxicity and efficacy is illustrated in Table 1. Specifically, recent studies indicate that the gut microbiota can modulate the efficacy and toxicity of chemotherapy through mechanisms outlined in the “TIMER” framework proposed by Alexander et al.: translocation, immunomodulation, metabolism, enzymatic degradation, and reduced diversity and ecological variation [60,61].

Table 1.
Summary of gut microbiota modulating the efficacy and toxicity of chemotherapeutic drugs.
Table 1.
Summary of gut microbiota modulating the efficacy and toxicity of chemotherapeutic drugs.
| Therapy | Model/Assay | Gut Microbiota Involved | Mechanism of Interaction | Reference |
|---|---|---|---|---|
| Irinotecan (SN38) | Mice | β-glucuronidase-producing gut bacteria | Chemical transformation of inactive SN38G into active SN38, inducing severe toxic effects | [62] |
| CB1954 (pro-drug of gemcitabine) | In vitro | Escherichia coli | Bacterial nitroreductase activity amplifies CB1954 activity | [63] |
| Gemcitabine | Mice | γ-proteobacteria, Mycoplasma hyorhinis, and Escherichia coli | Inactivation of gemcitabine to its inactive form 2′,2′-difluorodeoxyuridine by bacteria Cytidine deaminase | [64] |
| 5-FU | C. elegan | Escherichia coli | Interconversion of the 5-FU with vitamin B6 and B9 release and ribonucleotide metabolism | [65] |
| Mice and in vivo | Fusobacterium nucleatum | Causes chemoresistance by activating the autophagy pathway | [66] | |
| In vitro and mice | Gut microbiota | Causes gut dysbiosis and mucositis | [67,68] | |
| Mice | Bacteroides vulgatus | Bacteroides vulgatus-mediated nucleotide biosynthesis induces 5-fluorouracil resistance | [69] | |
| Methotrexate | In vitro | Escherichia coli | The drug selects for antibiotic resistance | [70] |
| Oxaliplatin | Mice and in vivo | Fusobacterium nucleatum | Causes chemoresistance by activating the autophagy pathway | [66] |
| Anti-PD-L1 | Mice | Commensal Bifidobacterium | Promotes the antitumor immunity and facilitates anti-PD-L1 efficacy | [71] |
| Etoposide | In vitro | Pseudomonas aeruginosa | Oxidative stress drives the emergence of antibiotic resistance to fluoroquinolones | [72] |
| Doxorubicin | Mice | Akkermansia muciniphila | Enhanced the responsiveness of doxorubicin in triple-negative breast cancer (mice model) | [73] |
In the context of the “TIMER” framework, one critical aspect in chemotherapy modulation is that chemotherapy-induced mucositis can compromise gut epithelial integrity, facilitating microbial translocation into the bloodstream or lymphatic system [74]. This translocation may trigger systemic inflammation, sepsis, and immune dysregulation, ultimately diminishing the therapeutic outcomes of chemotherapy [52,75].
Still in the same framework, certain gut microbial metabolites also play a role in influencing chemotherapy outcomes [76,77,78]. For instance, butyrate—a short-chain fatty acid (SCFA) produced via dietary fiber fermentation by gut microbes—has been demonstrated to enhance the anticancer effects of oxaliplatin by modulating CD8+ T-cell function within the tumor microenvironment (TME) through IL-12 signaling [79,80]. Additionally, commensal microbes can impact the anticancer response to oxaliplatin by altering the activity of myeloid-derived cells in the TME [20,81]. This underscores the potential of gut microbial metabolites to enhance chemotherapy efficacy through immunomodulation [82].
Besides the above mechanisms, gut microbial enzymatic functions have been shown to play a pivotal role in influencing the metabolism and toxicity of chemotherapeutic agents [49]. For example, Escherichia coli has been shown to activate tegafur, a prodrug of 5-FU, and CB1954 through nitroreductases [63]. Conversely, E. coli inhibits drugs like vidarabine, cladribine, doxorubicin, and others, while enhancing the anticancer activity of mercaptopurine [49,62,63]. This further underscores how the mechanisms of the “TIMER” framework modulate the efficacy of chemotherapy through gut microbiota.
A notable toxicity interaction of the gut microbiota with chemotherapeutic drugs involves irinotecan, where bacterial β-glucuronidases convert the inactive form (SN38G) into the active form (SN38), leading to dose-limiting side effects such as irinotecan-induced diarrhea [62,83,84]. Given that β-glucuronidases are prevalent in various gut microbiota [85,86], the co-administration of β-glucuronidase inhibitors during irinotecan therapy has been shown to mitigate diarrhea. This highlights the potential for the strategic modulation of gut microbiota to enhance chemotherapy efficacy while minimizing toxicity.
Certain gut bacteria have been implicated in promoting chemotherapy resistance, thereby undermining treatment outcomes [87]. For example, Fusobacterium nucleatum, which is enriched in CRC patients, has been associated with worse clinical outcomes, including chemoresistance to 5-FU and oxaliplatin through autophagy modulation [66,88]. Furthermore, F. nucleatum has been reported to upregulate BIRC3, which directly inhibits the caspase cascade, thus leading to 5-FU chemoresistance [89]. This evidence suggests that the targeted modulation of the gut microbiota against opportunistic microbes like F. nucleatum present promising strategies for reducing chemoresistance and improving therapeutic outcomes for patients with advanced CRC.
4. Chemotherapy-Induced Gut Dysbiosis in Colorectal Cancer
Recent studies highlight the crucial role of the gut microbiota in modulating the efficacy and toxicity of chemotherapeutic agents, making it a promising target for improving drug safety and efficacy through microbiota manipulation [49,90,91,92]. However, chemotherapy has been shown to induce significant perturbations in the gut microbiota, with pre- and post-treatment fecal analyses across various cancer cohorts as summarized in Table 2, which highlights its contribution to dysbiosis [24,25,93,94,95]. For instance, a recent study reported a significant reduction in gut microbiota in breast cancer patients undergoing chemotherapy [96]. Similarly, a study on lung cancer patients found that pemetrexed disrupted the gut microbial balance and compromised the colon barrier integrity [97]. In patients with non-Hodgkin’s lymphoma, chemotherapy was also associated with profound disruptions in the intestinal microbiome, affecting both its taxonomic composition and metabolic capacity [93]. These studies consistently indicate that chemotherapy depletes beneficial gut bacteria, while promoting the growth of opportunistic pathogens. This suggests that, while chemotherapy is the cornerstone of advanced CRC treatment, its side effects—including disrupting and depleting beneficial gut flora—can significantly impact patient recovery and quality of life. The depletion of such flora is particularly concerning as the gut microbiota modulates chemotherapy efficacy and mitigates chemotoxicity [49].
Adjuvant chemotherapy is commonly administered in advanced CRC patients to minimize recurrence [98,99]. However, its cytotoxic effects to normal cells and gut microbiota contribute significantly to side effects that impair one’s quality of life [100,101]. For example, chemotherapy-induced mucositis, driven by reactive oxygen species (ROS) formation [50] and the production of pro-inflammatory cytokines such as interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α) [102], leaves mucosal tissue vulnerable to infection and ulceration. It is also reported that most chemotherapeutic drugs harm not only normal epithelial cells but also commensal bacteria, highlighting their non-selective toxicity and contribution to dysbiosis [24].
The accumulating evidence links chemotherapy-induced mucositis as the cause of gut dysbiosis in patients undergoing chemotherapy [24,50,103,104]. Irinotecan, a key drug for metastatic CRC, exemplifies this by the reported damage caused to both epithelial cells and gut microbiota, leading to gastrointestinal toxicity and dysbiosis [105]. Similarly, 5-FU, a first-choice chemotherapeutic drug of CRC treatment, has been associated with mucositis and significant gut microbial alterations [104,106,107]. Preclinical studies have observed a shift from beneficial bacteria like Lactobacillus and Bifidobacterium to pathogenic genera such as Clostridium, Escherichia, and Enterococcus following a single dose of 5-FU [108]. These disruptions, as well as those summarized in Table 2, underscore the effect of chemotherapy on gut health and how this can be targeted to improve patient quality of life.

Table 2.
Summary of how chemotherapy drugs affect gut microbial community in different cancers.
Table 2.
Summary of how chemotherapy drugs affect gut microbial community in different cancers.
| Cancer Type | Model/Assay | Chemotherapy Regimen | Effects on Gut Microbiota | Reference |
|---|---|---|---|---|
| Lung cancer | Mice | Pemetrexed | Decrease in bacterial family of SCFA-producing taxa Ruminococcaeae. A significant increase in two opportunistic bacterial families, Enterobacteriaceae, and Enterococcaceae | [97] |
| Stage III CRC | Human | Capecitabine-Oxaliplatin (CapeOx) | Predominance of opportunistic Klebsiella pneumoniae (31%) in patients with chemotherapy-induced diarrhea | [109] |
| CRC | Human | 5-FU + oxaliplatin | Enrichment of Veillonella dispar, Bacteroides plebeius, and Prevotella copri in patients treated with this regimen | [110] |
| Breast, colorectal, esophageal, laryngeal, and melanoma | Human | Capecitabine, cisplatin/5-FU, FOLFOX4, FOLFOX6, FOLFIRI, 5-FU/folinic acid, paclitaxel, carboplatin, and gemcitabine | Reduced abundance of beneficial gut bacteria such as Lactobacillus spp., Bacteroides spp., Bifidobacterium spp., and Enterococcus spp., and increased abundance of opportunistic species such as Staphylococcus spp. and Escherichia coli were observed in patients undergoing chemotherapy | [111] |
| Ovarian cancer | Human | Paclitaxel, carboplatin, and cisplatin | Altered gut microbial composition in ovarian cancer patients, marked with increased abundance of Bacteroidetes and Firmicutes and a decreased abundance of Proteobacteria after chemotherapy | [112] |
| Breast cancer | Human | Taxane, cyclophosphamide, carboplatin, and doxorubicin | Altered gut microbiota which taxonomic shifts in Fusicatenibacter, Faecalibacterium, Erysipelotrichaceae UCG-003, Bacteroides, and Subdoligranulum, leading to cognitive decline during chemotherapy | [113] |
| CRC | Human | 5-FU | Decreased abundance of Deltaproteobacteria, Firmicutes, and Coriobacteria; with increased mRNA levels of inflammatory cytokines in the gut such as TNF-α, IL-6, IL-1β, and IL-10 and nitric oxide synthase | [25] |
| Pancreatic ductal adenocarcinoma (PDAC) | Mice | Gemcitabine | The drug decreased the abundance of Gram-positive Firmicutes from about 39 to 17%, as well as the Gram-negative Bacteroidetes from 38 to 17%. However, the abundance of inflammation-associated bacteria such as Akkermansia muciniphila increased from 5 to 33%. | [114] |
Recent studies have shown that the extent of chemotherapy-induced gut dysbiosis depends on the specific regimen administered. For example, the CapeOx regimen was associated with increased conditionally pathogenic bacteria, including Bilophila, Anaerostipes, Comamonas, Collinsella, Bacteroides, Eggerthella, and inflammation-related Weissella [109,115]. Another study which examined the effects of three regimens FOLFIRI, XELOX, and FOLFIRI combined with cetuximab showed varying impacts on microbial abundance. XELOX-treated patients exhibited an increase in the abundances of Humicola, Tremellomycetes, Veillonella, and Malassezia, alongside a decrease in the abundances of Clostridiales, Faecalibacterium, Phascolarctobacterium, Rhodotorula, and Humicola. Meanwhile, FOLFIRI altered the abundance of Magnusiomyces, Candida, Dipodascaceae, Tremellomycetes, and Saccharomycetales, whereas the abundances of Rhodotorula and Humicola were decreased [116]. In a combination therapy, the abundances of Dipodascaceae, Candida, Magnusiomyces, Tremellomycetes, Saccharomycetales, Lentinula, and Malassezia were increased in CRC patients treated with the FOLFIRI regimen plus cetuximab compared with those treated with the FOLFIRI regimen alone [116]. These findings emphasize that interventions targeting chemotherapy-induced gastrointestinal disorders or mucosal inflammation should consider regimen-specific microbial effects, as different regimens may exert distinct impacts on the gut microbiota.
5. Chemotherapy-Induced Microbial Mutations and the Emergence of De Novo AMR in Gut Microbiota
In addition to gut microbial perturbation, chemotherapeutic agents have been reported to induce mutations within the gut microbiota [117]. The mechanisms by which these agents operate often involve damaging bacterial DNA and inhibiting DNA replication, thereby exerting selective pressure that influences the evolution of gut bacteria [118]. This selective pressure can lead to increased genotypic diversity, proliferation, and adaptations among the bacterial populations selected for, within the gut ecosystem. Damaging bacterial DNA activates the SOS response, a cellular mechanism that drives de novo mutations [119]. First described by Radman in 1975, the SOS response is triggered by the accumulation of single-stranded DNA breaks, leading to the formation of a nucleoprotein complex with the ubiquitous protein RecA. This complex cleaves the LexA repressor, thereby removing the inhibition of the SOS response [120]. Under the conditions created by chemotherapeutic regimens, DNA repair during the SOS response relies on low-fidelity DNA polymerases, which consequently heightens mutation rates [121].
Most chemotherapeutic agents (including alkylating agents and platinum-based compounds) as summarized in Table 3 strongly induce the SOS response in bacteria [122]. This response is also activated by nucleotide analogs, nucleotide synthesis inhibitors, and topoisomerase inhibitors [123]. The exposure of the gut microbiota to these agents through chemotherapy is likely to enhance bacterial mutagenesis through the activation of the SOS response [21]. A recent study investigating the induction of the SOS response in commensal E. coli with 39 chemotherapeutic drugs at therapeutic concentrations demonstrated that ten of these drugs activated the SOS response. Notably, eight of these accelerated the mutations in commensal E. coli through SOS activation [28]. This shows that chemotherapeutic agents could expedite the evolution of the gut microbiota and facilitate the emergence of resistant mutants within commensal bacteria.
Mutations arising from the activation of the SOS response may significantly contribute to the rapid emergence of antibiotic resistance among cancer patients [124,125]. This has been reported by numerous studies which indicated the emergence of antibiotic-resistant pathogenic bacteria in patients, attributed to SOS activation by chemotherapy drugs [21,22,23,28]. When gut bacteria acquire antibiotic resistance, they gain the ability to survive the exposure to antibiotic therapies that would otherwise eliminate them or inhibit their growth [126], which threatens patient survival.

Table 3.
A summary of mechanisms underlying chemotherapy-induced antimicrobial resistance across different drugs and bacteria.
Table 3.
A summary of mechanisms underlying chemotherapy-induced antimicrobial resistance across different drugs and bacteria.
| Chemotherapy Drug | Model/Assay | Mechanism of Antibiotic Resistance Emergence | Targeted Bacteria | Targeted Antibiotics | Reference |
|---|---|---|---|---|---|
| Cisplatin and oxaliplatin | In vitro | Induction of SOS response | Escherichia coli MG1655 | Rifampicin, ciprofloxacin | [127] |
| 39 chemotherapeutic drugs | In vitro | SOS-response induction | Escherichia coli MG1655, Enterobacter cloacae ATCC 1304, Pseudomonas aeruginosa ATCC 27853, and Staphylococcus aureus ATCC 25923 | Ciprofloxacin, cefotaxime, imipenem | [28] |
| Methotrexate | In vitro | Selection for acquired resistance and co-selection for genetically linked resistance | Escherichia coli, and Klebsiella pneumoniae | Trimethoprim | [70] |
| Mercaptopurine, cytarabine, azacitidine, dacarbazine, daunorubicin, mitoxantrone, and cyclophosphamide | In vitro | Genotoxic effect stress activated the SOS response, thus increasing bacterial mutation | Klebsiella pneumoniae carbapenemase (KPC)-producing Enterobacteriaceae | Ceftazidime-avibactam | [128] |
| Etoposide | In vitro | Oxidative stress | Pseudomonas aeruginosa | Ciprofloxacin | [72] |
| Paclitaxel and its derivative docetaxel | In vitro | Upregulation of rpoS expression, activated SOS response, conjugative transfer of resistance genes | Escherichia coli | Rifampicin and ampicillin | [129] |
A recent study involving seven clinical isolates of Enterobacteriaceae producing KPC-type carbapenemases revealed that mutations induced by anticancer therapies in experimental bacteria increased the resistance against ceftazidime-avibactam and rifampicin up to 104-fold compared to no resistance in the control experiment [128]. This phenomenon highlights an additional layer of complexity and threat that chemotherapy drugs create on the gut microbiota.
Besides the activation of the SOS response, recent studies have implicated chemotherapeutic drugs in inducing antibiotic resistance in tumor-associated bacteria through oxidative stress [22,128]. For example, a recent study stated that oxidative stress induced by Etoposide (ETO), an FDA-approved chemotherapy drug, drives the emergence of Pseudomonas aeruginosa resistance to fluoroquinolones [72]. However, another study indicated that a widely used chemotherapeutic drug methotrexate induces the selection of antibiotic resistance in E. coli [70], which implies that chemotherapy may promote the spread of antibiotic resistance genes. In addition, paclitaxel and its derivative docetaxel have been reported to promote the transfer of antibiotic resistance through a series of cellular responses such as an enhanced cell membrane permeability, activated SOS response, increased plasmid replication, pilus formation, and the upregulated expression of rpoS gene [129]. These studies indicate that chemotherapy can also induce selective pressure against the gut microbiota, encouraging them to acquire and transfer antibiotic resistance. This highlights the need for novel anti-cancer regimens with minimal effects on the gut microbiota.
Horizontal gene transfer (HGT) events are crucial for the rapid acquisition of complex antibiotic resistance mechanisms within bacterial ecosystems under selective pressure [129,130,131]. HGT can occur through bacteriophage-mediated transduction, transformation with extracellular DNA, or plasmid exchange via bacterial conjugation [132]. The rate of HGT—particularly through prophage induction—increases substantially during the SOS response, suggesting that a heightened rate of SOS activation due to chemotherapy may facilitate a rapid spread of antibiotic resistance in a patient under chemotherapy, posing additional threats to the patient health [133]. The combined effects of the SOS response on commensal microbiota and increased HGT rates resulting from chemotherapy can contribute to elevated rates of plasmid exchange within the gut microbiota. This further exacerbates bacterial adaptation and the dissemination of acquired mutations, including those conferring antibiotic resistance [133]. Therefore, the outlined risk of acquiring antibiotic resistance during chemotherapy calls for future research to focus on developing safer chemotherapeutic regimens and strategies to protect the gut microbiota against them.
6. Effects of Antibiotic–Chemotherapy Combinations on Persistence of AMR in Gut Microbiota
Bacterial infections are prevalent among CRC patients undergoing chemotherapy [134,135]. This is primarily due to mucositis, which creates open sores that facilitate bacterial colonization, and could also be due to the direct effects of chemotherapy that induce dysbiosis in the gut microbiota [50,103,136]. Consequently, these patients rely on effective antibiotics for both the prevention and treatment of infections [137]. Mucositis further heightens the risk of bacterial translocation into the bloodstream, necessitating additional antibiotic therapy [138,139]. This cycle can lead to increased selection pressure for antibiotic-resistant bacteria, resulting in their persistence within the gut microbiota. The predominance of resistant pathogens poses a significant risk to CRC patient survival, as bacterial infections represent one of the most frequent complications in cancer care [140,141,142]. The failure of antibiotic treatment could lead to a higher incidence of systemic infections and the associated mortality [142]. Moreover, cancer patients are particularly vulnerable to hospital-acquired infections; yet, hospitals are major reservoirs of antibiotic-resistant bacteria [143,144,145]. This vulnerability exacerbates the antibiotic resistance and increases the mortality associated with antibiotic failure. This explains why over 40% of oncologists in the United Kingdom express concern that rising antibiotic resistance threatens the effectiveness of cancer treatments [22].
Similar to chemotherapeutic agents, most antibiotics used to treat bacterial infections have been shown to activate the SOS response [124,125]. For instance, quinolones such as ciprofloxacin inhibit bacterial topoisomerases II and IV, leading to double-stranded DNA breaks that trigger the SOS response [146,147]. This suggests that combining antimicrobial prophylaxis with chemotherapy may inadvertently heighten the risk of de novo antibiotic-resistance mutations in gut bacteria. This reverses the intended mitigation of bacterial infections to potentially increasing mortality in CRC patients due to antibiotic failure—even though antibiotic-resistant infections are rarely recorded as direct causes of death on death certificates [22]. This aligns with recent comparative studies which indicate that cancer patients undergoing chemotherapy with antimicrobial prophylaxis have lower overall survival (OS) than those without prophylaxis [148,149,150,151]. For instance, a study on the impact of antibiotic treatment during platinum chemotherapy for epithelial ovarian cancer found that antibiotics reduced OS in the treatment group compared to the control group (45.6 vs. 62.4 months). Notably, using antibiotics that target Gram-positive bacteria further worsened OS, with a survival difference of approximately 17 months (35.0 vs. 62.4 months) [148]. Similarly, a study on non-small-cell lung cancer (NSCLC) patients revealed that antibiotics treatment one month prior to chemotherapy significantly decreased OS compared to those who did not receive antibiotics (13.8 vs. 17.6 months, p < 0.001) [151]. These findings indicate that antibiotic use before or during chemotherapy is consistently associated with poor treatment outcomes in cancer patients.
While modern cancer treatment approaches have significantly improved overall survival, they continue to render patients vulnerable to bacterial infections [142]. This implies that the emergence of antibiotic resistance could thus lead to unfavorable outcomes for patients who depend on antibiotics for infection prevention and treatment. A study by Bodro et al. [152] reported the increased persistence of bacteremia and higher early case-fatality rates among cancer patients infected with antibiotic-resistant ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, P. aeruginosa, and Enterobacter spp.) [152,153]. Additionally, another study found that 88% of deaths from hospital-acquired infections in an oncology intensive care unit were attributed to multidrug-resistant pathogens, underscoring the devastating impact of antibiotic resistance in cancer care [154].
In CRC care, chemotherapy is typically administered in cycles lasting two to three weeks, for over a period of up to six months [38,155,156]. Due to the high likelihood of mucositis and associated bacterial infections during treatment, there is a high expectation of antimicrobial therapy during each chemotherapy cycle. This prolonged antibiotic use inevitably contributes to the emergence and selective pressure for antibiotic resistance at the end of the treatment [137,157,158]. Therefore, the concurrent use of chemotherapy and antibiotics may significantly contribute to prolonged or even persistent AMR within the gut microbiota of patients.
Additionally, the combined effects of chemotherapy and antibiotics create a multifaceted model that can lead to worse cancer treatment outcomes [21,159]. Firstly, the combination disrupts the commensal gut microbiota, resulting in dysbiosis and facilitating pathogen proliferation [21]. Secondly, it accelerates the activation of the bacterial SOS response, promoting de novo mutations conferring antibiotic resistance [21]. Additionally, this therapy exerts strong selective pressure favoring resistant bacteria within the gut microbiota. Furthermore, it increases the extent of bacterial translocation and systemic infections due to mucositis-related open sores and compromised intestinal barriers [21]. Therefore, while combining chemotherapy and antibiotics may offer therapeutic benefits in cancer treatment, it also has the potential to produce detrimental effects that may outweigh these advantages.
7. Strategies to Restore Chemotherapy-Induced Dysbiosis and Mitigate Antibiotic Resistance in CRC Patients
Recent studies have underscored the critical role of the gut microbiota in modulating the efficacy and toxicity of chemotherapy agents, resulting in variability in host responses to treatment [49,59,63,78,160]. However, chemotherapy itself acts as a significant stressor on the gut microbiota, often driving it into an unstable and transient state [24,93]. Notably, chemotherapy is administered in cycles, with each cycle exacerbating gut dysbiosis and mucositis. Moreover, mucositis encourages bacterial translocation, requiring the use of broad-spectrum antibiotics to treat and prevent systemic infections [52,161]. This antibiotic intervention creates selective pressure that drives microbial mutations, diminishes colonization resistance, and promotes the proliferation of resistant pathogens. Consequently, this vicious cycle heightens the risk of infections and accelerates the development of antibiotic resistance, which poses a significant threat to patient survival. Given the heightened vulnerability to infections among such patients, antibiotic resistance could promote CRC-related mortality. This highlights the urgent need for strategies aimed at preventing chemotherapy-induced dysbiosis and the proliferation of antibiotic resistance in CRC patients.
Targeting the gut microbiota has emerged as a promising strategy for managing CRC, aiming not only to mitigate the adverse effects of chemotherapy on the gut microbiota but also to enhance treatment efficacy and improve patients’ quality of life [15,18,162]. Unlike other side effects, the impacts of chemotherapy on the gut microbiota can be ameliorated through dysbiosis restoration [49,162]. Several strategies have been proposed for ameliorating dysbiosis and alleviating associated complications in chemotherapy patients, including fecal microbiota transplantation (FMT), probiotics, and phage therapy [163,164].
Recent studies have demonstrated significant restoration of commensal microbiota and a reduction in antibiotic resistance genes in recurrent Clostridium difficile infection patients undergoing FMT [165,166,167]. Although not yet widely available for all diseases, clinical cases indicate that FMT can restore microbial diversity and improve the levels of metabolites such as SCFAs to levels comparable to those of healthy donors [168,169]. In acute myeloid leukemia patients, autologous FMT was shown to restore dysbiosis by increasing proportions of beneficial taxa such as Ruminococcaceae, Clostridiales, and Lachnospiraceae, while decreasing pro-inflammatory taxa from the Enterococcaceae and Enterobacteriaceae families that predominated during chemotherapy [170]. These findings suggest that FMT may be effective beyond its current FDA-approved application, potentially reversing chemotherapy- and antibiotic-induced gut dysbiosis in CRC patients [169].
Although FMT offers a rapid restoration of gut microbial diversity, probiotic administration ensures a more targeted approach with limited chances of pathogen colonization from donors [171,172,173]. The effectiveness of probiotics can be optimized based on knowledge of key species linked to antibiotic resistance, adverse effects, and specific gut microbiota recovery goals [174,175]. This explains why beneficial outcomes of probiotic use are influenced by factors such as strain selection, dosage, duration of treatment, and host physiology [176]. For instance, a recent study involving nasopharyngeal cancer patients indicated that a probiotics cocktail comprising Lactobacillus plantarum, Bifidobacterium animalis, Lactobacillus rhamnosus, and Lactobacillus acidophilus could reduce the severity of chemotherapy-induced mucositis in 21 days by regulating gut dysbiosis and enhancing immune responses [177]. In CRC cases, yeast-based probiotics, particularly Saccharomyces, have been reported as adjunctive therapies for CRC [178,179,180]. Furthermore, another study involving CRC patients revealed that the administration of two daily tablets totaling 7 × 109 CFUs Lactobacillus acidophilus NCFM and 1.4 × 1010 CFUs Bifidobacterium lactis Bl-04 improved the gut microbiota by increasing the abundance of butyrate-producing bacteria such as Faecalibacterium and Clostridiales spp., while decreasing levels of CRC-associated genera like Fusobacterium and Peptostreptococcus [181]. However, not all probiotic strains are equally effective in restoring chemotherapy-induced dysbiosis; thus, screening for potent strains is essential for developing effective probiotic-based interventions [175,182,183].
In addition to probiotics, prebiotic dietary supplements can serve as substrates to promote the growth of low-abundance commensal bacteria and enhance SCFA production—including acetate, propionate, and butyrate—impacted by chemotherapy treatment [184,185]. A recent study indicated that SCFAs can effectively suppress inflammatory responses induced by 5-FU and maintain the integrity of intestinal mucosal epithelium [186,187]. This could alleviate mucositis and associated effects, thus improving chemotherapy side effects.
Research indicates that dietary habits are crucial in modulating the gut microbial community structure [11,188]. For example, a Western dietary style increases the risk for CRC-associated gut microbiota while CRC patients are recommended to eat a plant-based diet with appropriate dietary fiber intake [189,190,191]. These fibers undergo microbial degradation in the large intestine, increasing essential gut metabolites such as SCFAs [192]. Research also indicates that dietary fiber intake leads to increased abundances of beneficial gut bacteria such as Bacteroidetes and lactic acid bacteria, including species such as E. rectale, Ruminococcus, and Roseburia [193]. Although such interventions may take long to significantly alter the gut microbiota, they remain a viable long-term strategy for ameliorating chemotherapy-induced gut dysbiosis.
Recent microbiome modulation strategies have been proposed, including phage therapy, SCFA supplementation, and bacterial consortium transplantation, with a shared goal to replace opportunistic gut microbes and restore eubiosis [194,195,196]. However, comprehensive studies on their safety, efficacy, and application remain limited. For instance, despite decades of phage therapy use in Eastern Europe, key data on phage amplification, dosing, and timing are still needed to address safety concerns [197]. Therefore, further research with larger study populations is still needed to establish the effectiveness, safety, and optimal treatment duration of these approaches before routine application.
In response to the global public health challenge posed by AMR [198], antimicrobial stewardship is strongly recommended in order to optimize antibiotic use and mitigate the risk of antibiotic resistance among chemotherapy-treated patients, which can either be chemotherapy-induced or arise from prolonged antibiotic use [199,200]. Through these deliberate stewardship efforts, physicians, hospitals, nursing homes, palliative care facilities, and other healthcare providers ensure they prescribe the appropriate antibiotics at the right dose, at the right time, and for the right duration to achieve the best clinical outcomes [201]. Cancer patients stand to benefit significantly from antibiotic stewardship [202], as prior antibiotic exposure is a known risk factor for developing antibiotic-resistant infections [203,204]. A recent study indicates that interventions by antimicrobial stewardship teams (ASTs) have improved clinical and microbiological outcomes, particularly concerning resistant Gram-negative bacteria [205]. These improvements enhance antibiotic susceptibility rates and overall patient outcomes, which is particularly relevant for CRC patients undergoing chemotherapy, given that infections are a major contributor to cancer-related mortality in these patients [206]. Therefore, CRC societies and infectious disease centers should collaborate to guide data collection on variables pertinent to antibiotic resistance. Such collaborations can develop robust antibiotic stewardship and surveillance policies for optimized antibiotic use across all infections [201,207]. For CRC patients, the accurate reporting of deaths attributable to antibiotic-resistant infections should be emphasized in such policies because it could provide a clear understanding of the magnitude of antibiotic resistance [208,209], enabling timely interventions to restrict the spread of resistant infections within oncology hospitals.
8. Conclusions
Although chemotherapy remains a cornerstone in the treatment of advanced CRC, its interactions with the gut microbiota highlight a significant aspect of its non-selective toxicity, which extends beyond tumor cells to induce gut dysbiosis. This non-selective toxicity encompasses a range of side effects, including damage to the colorectal epithelium and the disruption of commensal bacteria, resulting in mucositis and the activation of the bacterial SOS response. The compromised gut epithelium, combined with chemotherapy-induced dysbiosis, elevates the risk of pathogenic bacterial overgrowth and bacterial translocation through open sores, leading to severe systemic infections that often require broad-spectrum antibiotics for prevention and treatment. The concurrent use of antimicrobial therapy throughout chemotherapy cycles, along with chemotherapy-induced mutations, creates selective pressure that favors the dominance of antibiotic-resistant bacteria in the gut. This compromises the efficacy of chemotherapy and increases the risk of CRC mortality. Given the critical role of the gut microbiota in influencing both the toxicity and efficacy of chemotherapy, restoring the gut microbial balance is essential. Emerging approaches, such as FMT, probiotics, and prebiotics, present promising strategies for restoring the gut microbiota and mitigating the chemotherapy-induced antibiotic resistance. However, it is important to note that FMT so far has been approved by the FDA for the treatment of recurrent Clostridium difficile infection, implying that clinical studies in other conditions, including CRC, are still needed in order to establish efficacy and safety. Furthermore, not all probiotics are effective in alleviating chemotherapy-induced dysbiosis, underscoring the necessity for screening potent probiotic strains. Therefore, antimicrobial stewardship is recommended in order to optimize antibiotic use and mitigate the emergence and spread of antibiotic resistance among chemotherapy-treated CRC patients. Future research should prioritize reducing the impact of chemotherapy on the gut microbiota and optimize drug–probiotic combinations to improve gut microbiota restoration and chemotherapy outcomes.
Author Contributions
Conceptualization, M.J.K., C.-C.W., C.-Y.F., I.-C.L., T.-K.H., and B.-M.H.; resources, B.-M.H.; writing—original draft preparation, M.J.K.; writing—review and editing, C.-C.W., C.-Y.F., I.-C.L., T.-K.H., S.-W.H., Y.-C.C., and B.-M.H.; supervision, C.-C.W., C.-Y.F., I.-C.L., T.-K.H., and B.-M.H.; funding acquisition, B.-M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Cheng Hsin General Hospital, Dalin Tzu Chi Hospital, Asia University Hospital, and Ditmanson Medical Foundation Chiayi Christian Hospital–National Chung Cheng University Joint Research Program.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ou, D.; Xie, A.; Chen, D.; Li, X. Global burden and cross-country health inequalities of early-onset colorectal cancer and its risk factors from 1990 to 2021 and its projection until 2036. BMC Public Health 2024, 24, 3124. [Google Scholar] [CrossRef] [PubMed]
- Bener, A.; Öztürk, A.E.; Dasdelen, M.F.; Barisik, C.C.; Dasdelen, Z.B.; Agan, A.F.; De La Rosette, J.; Day, A.S. Colorectal cancer and associated genetic, lifestyle, cigarette, nargileh-hookah use and alcohol consumption risk factors: A comprehensive case-control study. Oncol. Rev. 2024, 18, 1449709. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A review of colorectal cancer in terms of epidemiology, risk factors, development, symptoms and diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef]
- Guraya, S.Y. Pattern, stage, and time of recurrent colorectal cancer after curative surgery. Clin. Color. Cancer 2019, 18, e223–e228. [Google Scholar] [CrossRef]
- Biller, L.H.; Schrag, D. Diagnosis and treatment of metastatic colorectal cancer: A review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef]
- Mukherjee, O.; Rakshit, S.; Shanmugam, G.; Sarkar, K. Role of chemotherapeutic drugs in immunomodulation of cancer. Curr. Res. Immunol. 2023, 4, 100068. [Google Scholar] [CrossRef]
- Juthani, R.; Punatar, S.; Mittra, I. New light on chemotherapy toxicity and its prevention. BJC Rep. 2024, 2, 41. [Google Scholar] [CrossRef]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human gut microbiota in health and disease: Unveiling the relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef]
- Rinninella, E.; Tohumcu, E.; Raoul, P.; Fiorani, M.; Cintoni, M.; Mele, M.C.; Cammarota, G.; Gasbarrini, A.; Ianiro, G. The role of diet in shaping human gut microbiota. Best. Pract. Res. Clin. Gastroenterol. 2023, 62, 101828. [Google Scholar] [CrossRef] [PubMed]
- Artemev, A.; Naik, S.; Pougno, A.; Honnavar, P.; Shanbhag, N.M. The Association of Microbiome Dysbiosis With Colorectal Cancer. Cureus 2022, 14, e22156. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Du, L.; Shi, D.; Kong, C.; Liu, J.; Liu, G.; Li, X.; Ma, Y. Dysbiosis of human gut microbiome in young-onset colorectal cancer. Nat. Commun. 2021, 12, 6757. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Ye, J.; Zhao, B.; Sun, J.; Cao, P.; Yang, Y. The role of intestinal microbiota in colorectal cancer. Front. Pharmacol. 2021, 12, 674807. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, P.; Deng, K.; Zhou, Y.; Hu, K. Targeting the gut microbiota: A new strategy for colorectal cancer treatment. J. Transl. Med. 2024, 22, 915. [Google Scholar] [CrossRef]
- Zhao, J.; Liao, Y.; Wei, C.; Ma, Y.; Wang, F.; Chen, Y.; Zhao, B.; Ji, H.; Wang, D.; Tang, D. Potential Ability of Probiotics in the Prevention and Treatment of Colorectal Cancer. Clin. Med. Insights Oncol. 2023, 17, 11795549231188225. [Google Scholar] [CrossRef]
- John Kenneth, M.; Tsai, H.C.; Fang, C.Y.; Hussain, B.; Chiu, Y.C.; Hsu, B.M. Diet-mediated gut microbial community modulation and signature metabolites as potential biomarkers for early diagnosis, prognosis, prevention and stage-specific treatment of colorectal cancer. J. Adv. Res. 2023, 52, 45–57. [Google Scholar] [CrossRef]
- Fong, W.; Li, Q.; Yu, J. Gut microbiota modulation: A novel strategy for prevention and treatment of colorectal cancer. Oncogene 2020, 39, 4925–4943. [Google Scholar] [CrossRef]
- Ma, W.; Mao, Q.; Xia, W.; Dong, G.; Yu, C.; Jiang, F. Gut Microbiota Shapes the Efficiency of Cancer Therapy. Front. Microbiol. 2019, 10, 1050. [Google Scholar] [CrossRef]
- Zhao, L.-Y.; Mei, J.-X.; Yu, G.; Lei, L.; Zhang, W.-H.; Liu, K.; Chen, X.-L.; Kołat, D.; Yang, K.; Hu, J.-K. Role of the gut microbiota in anticancer therapy: From molecular mechanisms to clinical applications. Signal Transduct. Target. Ther. 2023, 8, 201. [Google Scholar] [CrossRef]
- Papanicolas, L.E.; Gordon, D.L.; Wesselingh, S.L.; Rogers, G.B. Not just antibiotics: Is cancer chemotherapy driving antimicrobial resistance? Trends Microbiol. 2018, 26, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Nanayakkara, A.K.; Boucher, H.W.; Fowler, V.G., Jr.; Jezek, A.; Outterson, K.; Greenberg, D.E. Antibiotic resistance in the patient with cancer: Escalating challenges and paths forward. CA A Cancer J. Clin. 2021, 71, 488–504. [Google Scholar] [CrossRef] [PubMed]
- Chifiriuc, M.C.; Filip, R.; Constantin, M.; Pircalabioru, G.G.; Bleotu, C.; Burlibasa, L.; Ionica, E.; Corcionivoschi, N.; Mihaescu, G. Common themes in antimicrobial and anticancer drug resistance. Front. Microbiol. 2022, 13, 960693. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wen, X.-S.; Xian, C.J. Chemotherapy-Induced Intestinal Microbiota Dysbiosis Impairs Mucosal Homeostasis by Modulating Toll-like Receptor Signaling Pathways. Int. J. Mol. Sci. 2021, 22, 9474. [Google Scholar] [CrossRef]
- Yixia, Y.; Sripetchwandee, J.; Chattipakorn, N.; Chattipakorn, S.C. The alterations of microbiota and pathological conditions in the gut of patients with colorectal cancer undergoing chemotherapy. Anaerobe 2021, 68, 102361. [Google Scholar] [CrossRef]
- Langdon, A.; Schwartz, D.J.; Bulow, C.; Sun, X.; Hink, T.; Reske, K.A.; Jones, C.; Burnham, C.-A.D.; Dubberke, E.R.; Dantas, G. Microbiota restoration reduces antibiotic-resistant bacteria gut colonization in patients with recurrent Clostridioides difficile infection from the open-label PUNCH CD study. Genome Med. 2021, 13, 28. [Google Scholar] [CrossRef]
- Panwar, R.B.; Sequeira, R.P.; Clarke, T.B. Microbiota-mediated protection against antibiotic-resistant pathogens. Genes. Immun. 2021, 22, 255–267. [Google Scholar] [CrossRef]
- Meunier, A.; Nerich, V.; Fagnoni-Legat, C.; Richard, M.; Mazel, D.; Adotevi, O.; Bertrand, X.; Hocquet, D. Enhanced emergence of antibiotic-resistant pathogenic bacteria after in vitro induction with cancer chemotherapy drugs. J. Antimicrob. Chemother. 2019, 74, 1572–1577. [Google Scholar] [CrossRef]
- Liu, H.; Liu, B.; Zheng, F.; Chen, X.; Ye, L.; He, Y. Distribution of pathogenic bacteria in lower respiratory tract infection in lung cancer patients after chemotherapy and analysis of integron resistance genes in respiratory tract isolates of uninfected patients. J. Thorac. Dis. 2020, 12, 4216–4223. [Google Scholar] [CrossRef]
- Haussner, F.; Chakraborty, S.; Halbgebauer, R.; Huber-Lang, M. Challenge to the intestinal mucosa during sepsis. Front. Immunol. 2019, 10, 891. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Yu, Y.; Liang, J. Risk factors for sepsis in patients with colorectal cancer complicated with gastrointestinal perforation and its impact on prognosis. J. Gastrointest. Oncol. 2023, 14, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Busch, L.M.; Kadri, S.S. Antimicrobial treatment duration in sepsis and serious infections. J. Infect. Dis. 2020, 222, S142–S155. [Google Scholar] [CrossRef]
- Niederman, M.S.; Baron, R.M.; Bouadma, L.; Calandra, T.; Daneman, N.; DeWaele, J.; Kollef, M.H.; Lipman, J.; Nair, G.B. Initial antimicrobial management of sepsis. Crit. Care 2021, 25, 307. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.W.; Sundaram, V.; Chew, T.A.; Ladabaum, U. Advanced-Stage Colorectal Cancer in Persons Younger Than 50 Years Not Associated With Longer Duration of Symptoms or Time to Diagnosis. Clin. Gastroenterol. Hepatol. 2017, 15, 728–737.e3. [Google Scholar] [CrossRef]
- Filip, S.; Vymetalkova, V.; Petera, J.; Vodickova, L.; Kubecek, O.; John, S.; Cecka, F.; Krupova, M.; Manethova, M.; Cervena, K.; et al. Distant Metastasis in Colorectal Cancer Patients-Do We Have New Predicting Clinicopathological and Molecular Biomarkers? A Comprehensive Review. Int. J. Mol. Sci. 2020, 21, 5255. [Google Scholar] [CrossRef]
- Kumar, A.; Gautam, V.; Sandhu, A.; Rawat, K.; Sharma, A.; Saha, L. Current and emerging therapeutic approaches for colorectal cancer: A comprehensive review. World J. Gastrointest. Surg. 2023, 15, 495–519. [Google Scholar] [CrossRef]
- Taieb, J.; Gallois, C. Adjuvant chemotherapy for stage III colon cancer. Cancers 2020, 12, 2679. [Google Scholar] [CrossRef]
- Shinji, S.; Yamada, T.; Matsuda, A.; Sonoda, H.; Ohta, R.; Iwai, T.; Takeda, K.; Yonaga, K.; Masuda, Y.; Yoshida, H. Recent advances in the treatment of colorectal cancer: A review. J. Nippon. Med. Sch. 2022, 89, 246–254. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes. Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Smith, H.G.; Nilsson, P.J.; Shogan, B.D.; Harji, D.; Gambacorta, M.A.; Romano, A.; Brandl, A.; Qvortrup, C. Neoadjuvant treatment of colorectal cancer: Comprehensive review. BJS Open 2024, 8, zrae038. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, R.S.; Smith, J.J. Adjuvant Chemotherapy for Colon Cancer. Dis. Colon. Rectum 2019, 62, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Takeshima, N.; Hayasaka, Y.; Notsu, A.; Yamazaki, M.; Kawabata, T.; Yamazaki, K.; Mori, K.; Yasui, H. Comparison of irinotecan and oxaliplatin as the first-line therapies for metastatic colorectal cancer: A meta-analysis. BMC Cancer 2021, 21, 116. [Google Scholar] [CrossRef]
- Negarandeh, R.; Salehifar, E.; Saghafi, F.; Jalali, H.; Janbabaei, G.; Abdhaghighi, M.J.; Nosrati, A. Evaluation of adverse effects of chemotherapy regimens of 5-fluoropyrimidines derivatives and their association with DPYD polymorphisms in colorectal cancer patients. BMC Cancer 2020, 20, 560. [Google Scholar] [CrossRef]
- Khan, M.; Alharbi, S.; Aljuhani, S.; Tunkar, M.; Morya, A.; Alnatsheh, A.; Alshamrani, M.; Felemban, R. The Incidence of Hematological Toxicities in Colorectal Cancer Patients Treated With Fluoropyrimidine-Based Regimens at Princess Noorah Oncology Center. Cureus 2023, 15, e44267. [Google Scholar] [CrossRef]
- Zainal Abidin, M.N.; Omar, M.S.; Islahudin, F.; Mohamed Shah, N. The survival impact of palliative chemotherapy dose modifications on metastatic colon cancer. BMC Cancer 2022, 22, 731. [Google Scholar] [CrossRef]
- Kogan, L.G.; Davis, S.L.; Brooks, G.A. Treatment delays during FOLFOX chemotherapy in patients with colorectal cancer: A multicenter retrospective analysis. J. Gastrointest. Oncol. 2019, 10, 841–846. [Google Scholar] [CrossRef]
- Chrysostomou, D.; Roberts, L.A.; Marchesi, J.R.; Kinross, J.M. Gut microbiota modulation of efficacy and toxicity of cancer chemotherapy and immunotherapy. Gastroenterology 2023, 164, 198–213. [Google Scholar] [CrossRef]
- Sougiannis, A.T.; VanderVeen, B.N.; Davis, J.M.; Fan, D.; Murphy, E.A. Understanding chemotherapy-induced intestinal mucositis and strategies to improve gut resilience. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G712–G719. [Google Scholar] [CrossRef]
- Dahlgren, D.; Sjöblom, M.; Hellström, P.M.; Lennernäs, H. Chemotherapeutics-induced intestinal mucositis: Pathophysiology and potential treatment strategies. Front. Pharmacol. 2021, 12, 681417. [Google Scholar] [CrossRef] [PubMed]
- Kouzu, K.; Tsujimoto, H.; Kishi, Y.; Ueno, H.; Shinomiya, N. Bacterial translocation in gastrointestinal cancers and cancer treatment. Biomedicines 2022, 10, 380. [Google Scholar] [CrossRef] [PubMed]
- Ogizbayeva, A.; Turgunov, Y. Bacterial translocation in colorectal cancer patients. J. Clin. Med. Kazakhstan 2021, 18, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.S.M.; Mohammed, Z.; Häggström, C.; Myte, R.; Lindquist, E.; Gylfe, Å.; Van Guelpen, B.; Harlid, S. Antibiotics Use and Subsequent Risk of Colorectal Cancer: A Swedish Nationwide Population-Based Study. J. Natl. Cancer Inst. 2022, 114, 38–46. [Google Scholar] [CrossRef]
- Simin, J.; Fornes, R.; Liu, Q.; Olsen, R.S.; Callens, S.; Engstrand, L.; Brusselaers, N. Antibiotic use and risk of colorectal cancer: A systematic review and dose–response meta-analysis. Br. J. Cancer 2020, 123, 1825–1832. [Google Scholar] [CrossRef]
- Imai, H.; Saijo, K.; Komine, K.; Yoshida, Y.; Sasaki, K.; Suzuki, A.; Ouchi, K.; Takahashi, M.; Takahashi, S.; Shirota, H.; et al. Antibiotics Improve the Treatment Efficacy of Oxaliplatin-Based but Not Irinotecan-Based Therapy in Advanced Colorectal Cancer Patients. J. Oncol. 2020, 2020, 1701326. [Google Scholar] [CrossRef]
- Yang, R.; Niepel, M.; Mitchison, T.K.; Sorger, P.K. Dissecting variability in responses to cancer chemotherapy through systems pharmacology. Clin. Pharmacol. Ther. 2010, 88, 34–38. [Google Scholar] [CrossRef]
- Cheng, W.Y.; Wu, C.-Y.; Yu, J. The role of gut microbiota in cancer treatment: Friend or foe? Gut 2020, 69, 1867–1876. [Google Scholar] [CrossRef]
- Madkour, M.A.; Altaf, R.A.; Sayed, Z.S.; Yasen, N.S.; Elbary, H.A.; Elsayed, R.A.; Mohamed, E.N.; Toema, M.; Wadan, A.-H.S.; Nafady, M.H.; et al. The Role of Gut Microbiota in Modulating Cancer Therapy Efficacy. Adv. Gut Microbiome Res. 2024, 2024, 9919868. [Google Scholar] [CrossRef]
- Alexander, J.L.; Wilson, I.D.; Teare, J.; Marchesi, J.R.; Nicholson, J.K.; Kinross, J.M. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 356–365. [Google Scholar] [CrossRef]
- Ting, N.L.; Lau, H.C.; Yu, J. Cancer pharmacomicrobiomics: Targeting microbiota to optimise cancer therapy outcomes. Gut 2022, 71, 1412–1425. [Google Scholar] [CrossRef] [PubMed]
- Wallace, B.D.; Wang, H.; Lane, K.T.; Scott, J.E.; Orans, J.; Koo, J.S.; Venkatesh, M.; Jobin, C.; Yeh, L.-A.; Mani, S.; et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science 2010, 330, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Lehouritis, P.; Cummins, J.; Stanton, M.; Murphy, C.T.; McCarthy, F.O.; Reid, G.; Urbaniak, C.; Byrne, W.L.; Tangney, M. Local bacteria affect the efficacy of chemotherapeutic drugs. Sci. Rep. 2015, 5, 14554. [Google Scholar] [CrossRef] [PubMed]
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 357, 1156–1160. [Google Scholar] [CrossRef]
- Scott, T.A.; Quintaneiro, L.M.; Norvaisas, P.; Lui, P.P.; Wilson, M.P.; Leung, K.-Y.; Herrera-Dominguez, L.; Sudiwala, S.; Pessia, A.; Clayton, P.T.; et al. Host-microbe co-metabolism dictates cancer drug efficacy in C. elegans. Cell 2017, 169, 442–456.e18. [Google Scholar] [CrossRef]
- Yu, T.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell 2017, 170, 548–563.e16. [Google Scholar] [CrossRef]
- Lin, C.; Cai, X.; Zhang, J.; Wang, W.; Sheng, Q.; Hua, H.; Zhou, X. Role of gut microbiota in the development and treatment of colorectal cancer. Digestion 2019, 100, 72–78. [Google Scholar] [CrossRef]
- Pereira, V.B.; Melo, A.T.; Assis-Júnior, E.M.; Wong, D.V.; Brito, G.A.; Almeida, P.R.; Ribeiro, R.A.; Lima-Júnior, R.C. A new animal model of intestinal mucositis induced by the combination of irinotecan and 5-fluorouracil in mice. Cancer Chemother. Pharmacol. 2016, 77, 323–332. [Google Scholar] [CrossRef]
- Teng, H.; Wang, Y.; Sui, X.; Fan, J.; Li, S.; Lei, X.; Shi, C.; Sun, W.; Song, M.; Wang, H.; et al. Gut microbiota-mediated nucleotide synthesis attenuates the response to neoadjuvant chemoradiotherapy in rectal cancer. Cancer Cell 2023, 41, 124–138.e6. [Google Scholar] [CrossRef]
- Guðmundsdóttir, J.S.; Fredheim, E.G.; Koumans, C.I.; Hegstad, J.; Tang, P.-C.; Andersson, D.I.; Samuelsen, Ø.; Johnsen, P.J. The chemotherapeutic drug methotrexate selects for antibiotic resistance. EBioMedicine 2021, 74, 103742. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chan, S.Y.; Deng, Y.; Khoo, B.L.; Chua, S.L. Oxidative stress induced by Etoposide anti-cancer chemotherapy drives the emergence of tumor-associated bacteria resistance to fluoroquinolones. J. Adv. Res. 2024, 55, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Bawaneh, A.; Wilson, A.S.; Levi, N.; Howard-McNatt, M.M.; Chiba, A.; Soto-Pantoja, D.R.; Cook, K.L. Intestinal microbiota influence doxorubicin responsiveness in triple-negative breast cancer. Cancers 2022, 14, 4849. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hwang, A.Y.M.; Jia, Y.; Kim, B.; Iskandar, M.; Mohammed, A.I.; Cirillo, N. Experimental Chemotherapy-Induced Mucositis: A Scoping Review Guiding the Design of Suitable Preclinical Models. Int. J. Mol. Sci. 2022, 23, 15434. [Google Scholar] [CrossRef]
- Potruch, A.; Schwartz, A.; Ilan, Y. The role of bacterial translocation in sepsis: A new target for therapy. Ther. Adv. Gastroenterol. 2022, 15, 17562848221094214. [Google Scholar] [CrossRef]
- Li, S.; Zhu, S.; Yu, J. The role of gut microbiota and metabolites in cancer chemotherapy. J. Adv. Res. 2024, 64, 223–235. [Google Scholar] [CrossRef]
- Song, P.; Peng, Z.; Guo, X. Gut microbial metabolites in cancer therapy. Trends Endocrinol. Metab. 2024, 36, 55–69. [Google Scholar] [CrossRef]
- Kwon, B. A metabolite of the gut microbiota: A facilitator of chemotherapy efficacy in cancer. Signal Transduct. Target. Ther. 2023, 8, 238. [Google Scholar] [CrossRef]
- He, Y.; Fu, L.; Li, Y.; Wang, W.; Gong, M.; Zhang, J.; Dong, X.; Huang, J.; Wang, Q.; Mackay, C.R.; et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8+ T cell immunity. Cell Metab. 2021, 33, 988–1000.e7. [Google Scholar] [CrossRef]
- Danne, C.; Sokol, H. Butyrate, a new microbiota-dependent player in CD8+ T cells immunity and cancer therapy? Cell Rep. Med. 2021, 2, 100328. [Google Scholar] [CrossRef]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013, 342, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Inamura, K. Gut microbiota contributes towards immunomodulation against cancer: New frontiers in precision cancer therapeutics. Semin. Cancer Biol. 2021, 70, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.-W.; Tseng, C.-H.; Tzeng, C.-C.; Leu, Y.-L.; Cheng, T.-C.; Wang, J.-Y.; Chang, J.-M.; Lu, Y.-C.; Cheng, C.-M.; Chen, I.-J.; et al. Pharmacological inhibition of bacterial β-glucuronidase prevents irinotecan-induced diarrhea without impairing its antitumor efficacy in vivo. Pharmacol. Res. 2019, 139, 41–49. [Google Scholar] [CrossRef]
- Okunaka, M.; Kano, D.; Matsui, R.; Kawasaki, T.; Uesawa, Y. Evaluation of the Expression Profile of Irinotecan-Induced Diarrhea in Patients with Colorectal Cancer. Pharmaceuticals 2021, 14, 377. [Google Scholar] [CrossRef] [PubMed]
- Candeliere, F.; Raimondi, S.; Ranieri, R.; Musmeci, E.; Zambon, A.; Amaretti, A.; Rossi, M. β-Glucuronidase pattern predicted from gut metagenomes indicates potentially diversified pharmacomicrobiomics. Front. Microbiol. 2022, 13, 826994. [Google Scholar] [CrossRef]
- Simpson, J.B.; Walker, M.E.; Sekela, J.J.; Ivey, S.M.; Jariwala, P.B.; Storch, C.M.; Kowalewski, M.E.; Graboski, A.L.; Lietzan, A.D.; Walton, W.G.; et al. Gut microbial β-glucuronidases influence endobiotic homeostasis and are modulated by diverse therapeutics. Cell Host Microbe 2024, 32, 925–944.e10. [Google Scholar] [CrossRef]
- Wong, C.C.; Fong, W.; Yu, J. Gut microbes promote chemoradiotherapy resistance via metabolic cross-feeding. Cancer Cell 2023, 41, 12–14. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, L.; Leng, X.X.; Xie, Y.L.; Kang, Z.R.; Zhao, L.C.; Song, L.H.; Zhou, C.B.; Fang, J.Y. Fusobacterium nucleatum induces chemoresistance in colorectal cancer by inhibiting pyroptosis via the Hippo pathway. Gut Microbes 2024, 16, 2333790. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Y.; Weng, W.; Guo, B.; Cai, G.; Ma, Y.; Cai, S. Fusobacterium nucleatum promotes chemoresistance to 5-fluorouracil by upregulation of BIRC3 expression in colorectal cancer. J. Exp. Clin. Cancer Res. 2019, 38, 14. [Google Scholar] [CrossRef]
- Gori, S.; Inno, A.; Belluomini, L.; Bocus, P.; Bisoffi, Z.; Russo, A.; Arcaro, G. Gut microbiota and cancer: How gut microbiota modulates activity, efficacy and toxicity of antitumoral therapy. Crit. Rev. Oncol. Hematol. 2019, 143, 139–147. [Google Scholar] [CrossRef]
- Cazzaniga, M.; Zonzini, G.B.; Di Pierro, F.; Palazzi, C.M.; Cardinali, M.; Bertuccioli, A. Influence of the microbiota on the effectiveness and toxicity of oncological therapies, with a focus on chemotherapy. Pathol. Oncol. Res. 2023, 29, 1611300. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Du, H.; Chen, Y.; Ma, C.; Zhang, Q.; Li, H.; Xie, Z.; Hong, Y. Targeting the gut microbiota to alleviate chemotherapy-induced toxicity in cancer. Crit. Rev. Microbiol. 2024, 50, 564–580. [Google Scholar] [CrossRef] [PubMed]
- Montassier, E.; Gastinne, T.; Vangay, P.; Al-Ghalith, G.A.; Bruley des Varannes, S.; Massart, S.; Moreau, P.; Potel, G.; de La Cochetière, M.F.; Batard, E.; et al. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment. Pharmacol. Ther. 2015, 42, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Li, S.; Chen, W.; Han, Y.; Yao, Y.; Yang, L.; Li, Q.; Xiao, Q.; Wei, J.; Liu, Z.; et al. Postoperative probiotics administration attenuates gastrointestinal complications and gut microbiota dysbiosis caused by chemotherapy in colorectal cancer patients. Nutrients 2023, 15, 356. [Google Scholar] [CrossRef]
- Deleemans, J.M.; Chleilat, F.; Reimer, R.A.; Henning, J.-W.; Baydoun, M.; Piedalue, K.-A.; McLennan, A.; Carlson, L.E. The chemo-gut study: Investigating the long-term effects of chemotherapy on gut microbiota, metabolic, immune, psychological and cognitive parameters in young adult Cancer survivors; study protocol. BMC Cancer 2019, 19, 1243. [Google Scholar] [CrossRef]
- Wu, A.H.; Vigen, C.; Tseng, C.; Garcia, A.A.; Spicer, D. Effect of Chemotherapy on the Gut Microbiome of Breast Cancer Patients During the First Year of Treatment. Breast Cancer Targets Ther. 2022, 14, 433–451. [Google Scholar] [CrossRef]
- Pensec, C.; Gillaizeau, F.; Guenot, D.; Bessard, A.; Carton, T.; Leuillet, S.; Campone, M.; Neunlist, M.; Blottière, H.M.; Le Vacon, F.; et al. Impact of pemetrexed chemotherapy on the gut microbiota and intestinal inflammation of patient-lung-derived tumor xenograft (PDX) mouse models. Sci. Rep. 2020, 10, 9094. [Google Scholar] [CrossRef]
- Piercey, O.; Wong, H.-L.; Leung, C.; To, Y.H.; Heong, V.; Lee, M.; Tie, J.; Steel, M.; Yeung, J.M.; McCormick, J.; et al. Adjuvant Chemotherapy for Older Patients With Stage III Colorectal Cancer: A Real-World Analysis of Treatment Recommendations, Treatment Administered and Impact on Cancer Recurrence. Clin. Color. Cancer 2024, 23, 95–103.e3. [Google Scholar] [CrossRef]
- Matsuda, A.; Yamada, T.; Takahashi, G.; Matsumoto, S.; Yokoyama, Y.; Sonoda, H.; Uehara, K.; Shinji, S.; Sekiguchi, K.; Kanaka, S.; et al. Significance of Adjuvant Chemotherapy for Obstructive Colorectal Cancer After Stent Placement: A Multicenter Retrospective Study. Anticancer. Res. 2024, 44, 2737–2745. [Google Scholar] [CrossRef]
- Baltussen, J.C.; de Glas, N.A.; van Holstein, Y.; van der Elst, M.; Trompet, S.; den Boogaard, A.U.; van der Plas-Krijgsman, W.; Labots, G.; Holterhues, C.; van der Bol, J.M.; et al. Chemotherapy-related toxic effects and Quality of Life and Physical Functioning in older patients. JAMA Netw. Open 2023, 6, e2339116. [Google Scholar] [CrossRef]
- Han, C.J.; Ning, X.; Burd, C.E.; Spakowicz, D.J.; Tounkara, F.; Kalady, M.F.; Noonan, A.M.; McCabe, S.; Von Ah, D. Chemotoxicity and associated risk factors in colorectal cancer: A systematic review and meta-analysis. Cancers 2024, 16, 2597. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Wardill, H.R.; Bowen, J. Role of toll-like receptor 4 (TLR4)-mediated interleukin-6 (IL-6) production in chemotherapy-induced mucositis. Cancer Chemother. Pharmacol. 2018, 82, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.-Y.; Sobue, T.; Choquette, L.; Dupuy, A.K.; Thompson, A.; Burleson, J.A.; Salner, A.L.; Schauer, P.K.; Joshi, P.; Fox, E.; et al. Chemotherapy-induced oral mucositis is associated with detrimental bacterial dysbiosis. Microbiome 2019, 7, 66. [Google Scholar] [CrossRef]
- Li, H.-L.; Lu, L.; Wang, X.-S.; Qin, L.-Y.; Wang, P.; Qiu, S.-P.; Wu, H.; Huang, F.; Zhang, B.-B.; Shi, H.-L.; et al. Alteration of gut microbiota and inflammatory cytokine/chemokine profiles in 5-fluorouracil induced intestinal mucositis. Front. Cell. Infect. Microbiol. 2017, 7, 455. [Google Scholar] [CrossRef] [PubMed]
- Mahdy, M.S.; Azmy, A.F.; Dishisha, T.; Mohamed, W.R.; Ahmed, K.A.; Hassan, A.; Aidy, S.E.; El-Gendy, A.O. Irinotecan-gut microbiota interactions and the capability of probiotics to mitigate Irinotecan-associated toxicity. BMC Microbiol. 2023, 23, 53. [Google Scholar] [CrossRef]
- Sougiannis, A.T.; VanderVeen, B.N.; Enos, R.T.; Velazquez, K.T.; Bader, J.E.; Carson, M.; Chatzistamou, I.; Walla, M.; Pena, M.M.; Kubinak, J.L.; et al. Impact of 5 fluorouracil chemotherapy on gut inflammation, functional parameters, and gut microbiota. Brain Behav. Immun. 2019, 80, 44–55. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, H.; Zhang, R.; Liu, Y.; Kong, N.; Guo, Y.; Xu, M. 5-Fluorouracil induced dysregulation of the microbiome-gut-brain axis manifesting as depressive like behaviors in rats. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165884. [Google Scholar] [CrossRef]
- Hamouda, N.; Sano, T.; Oikawa, Y.; Ozaki, T.; Shimakawa, M.; Matsumoto, K.; Amagase, K.; Higuchi, K.; Kato, S. Apoptosis, dysbiosis and expression of inflammatory cytokines are sequential events in the development of 5-fluorouracil-induced intestinal mucositis in mice. Basic. Clin. Pharmacol. Toxicol. 2017, 121, 159–168. [Google Scholar] [CrossRef]
- Fei, Z.; Lijuan, Y.; Xi, Y.; Wei, W.; Jing, Z.; Miao, D.; Shuwen, H. Gut microbiome associated with chemotherapy-induced diarrhea from the CapeOX regimen as adjuvant chemotherapy in resected stage III colorectal cancer. Gut Pathog. 2019, 11, 18. [Google Scholar] [CrossRef]
- Deng, X.; Li, Z.; Li, G.; Li, B.; Jin, X.; Lyu, G. Comparison of microbiota in patients treated by surgery or chemotherapy by 16S rRNA sequencing reveals potential biomarkers for colorectal cancer therapy. Front. Microbiol. 2018, 9, 1607. [Google Scholar] [CrossRef]
- Stringer, A.M.; Al-Dasooqi, N.; Bowen, J.M.; Tan, T.H.; Radzuan, M.; Logan, R.M.; Mayo, B.; Keefe, D.M.; Gibson, R.J. Biomarkers of chemotherapy-induced diarrhoea: A clinical study of intestinal microbiome alterations, inflammation and circulating matrix metalloproteinases. Support. Care Cancer 2013, 21, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Zhang, X.; Fan, Y.; Chen, L.; Ma, X.; Yu, H.; Li, J.; Guan, X.; Zhao, P.; Yang, J. Changes of intestinal microbiota in ovarian cancer patients treated with surgery and chemotherapy. Cancer Manag. Res. 2020, 12, 8125–8135. [Google Scholar] [CrossRef] [PubMed]
- Otto-Dobos, L.; Grant, C.; Lahoud, A.; Wilcox, O.; Strehle, L.; Loman, B.; Yiadom, S.A.; Seng, M.; Halloy, N.; Russart, K.; et al. Chemotherapy-induced gut microbiome disruption, inflammation, and cognitive decline in female patients with breast cancer. Brain Behav. Immun. 2024, 120, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, C.; Adamberg, K.; Jaagura, M.; Copetti, M.; Fontana, A.; Adamberg, S.; Kolk, K.; Vilu, R.; Andriulli, A.; Pazienza, V. Influence of gemcitabine chemotherapy on the microbiota of pancreatic cancer xenografted mice. Cancer Chemother. Pharmacol. 2018, 81, 773–782. [Google Scholar] [CrossRef]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; He, J.; Li, H.; You, J.; Qin, H. Alterations in intestinal microbiota of colorectal cancer patients receiving radical surgery combined with adjuvant CapeOx therapy. Sci. China Life Sci. 2019, 62, 1178–1193. [Google Scholar] [CrossRef]
- Shuwen, H.; Xi, Y.; Yuefen, P.; Jiamin, X.; Quan, Q.; Haihong, L.; Yizhen, J.; Wei, W. Effects of postoperative adjuvant chemotherapy and palliative chemotherapy on the gut microbiome in colorectal cancer. Microb. Pathog. 2020, 149, 104343. [Google Scholar] [CrossRef]
- Wan, L.; Li, H.; Sun, G.; Zhang, L.; Xu, H.; Su, F.; He, S.; Xiao, F. Mutational Pattern Induced by 5-Fluorouracil and Oxaliplatin in the Gut Microbiome. Front. Microbiol. 2022, 13, 841458. [Google Scholar] [CrossRef]
- Bernacchia, L.; Gupta, A.; Paris, A.; Moores, A.A.; Kad, N.M. Developing novel antimicrobials by combining cancer chemotherapeutics with bacterial DNA repair inhibitors. PLoS Pathog. 2023, 19, e1011875. [Google Scholar] [CrossRef]
- Lima-Noronha, M.A.; Fonseca, D.L.; Oliveira, R.S.; Freitas, R.R.; Park, J.H.; Galhardo, R.S. Sending out an SOS-the bacterial DNA damage response. Genet. Mol. Biol. 2022, 45, e20220107. [Google Scholar] [CrossRef]
- Fitzgerald, D.M.; Rosenberg, S.M. Biology before the SOS Response-DNA Damage Mechanisms at Chromosome Fragile Sites. Cells 2021, 10, 2275. [Google Scholar] [CrossRef]
- Jaszczur, M.; Bertram, J.G.; Robinson, A.; van Oijen, A.M.; Woodgate, R.; Cox, M.M.; Goodman, M.F. Mutations for Worse or Better: Low-Fidelity DNA Synthesis by SOS DNA Polymerase V Is a Tightly Regulated Double-Edged Sword. Biochemistry 2016, 55, 2309–2318. [Google Scholar] [CrossRef] [PubMed]
- Rycenga, H.B.; Long, D.T. The evolving role of DNA inter-strand crosslinks in chemotherapy. Curr. Opin. Pharmacol. 2018, 41, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Sass, T.H.; Lovett, S.T. The DNA damage response of Escherichia coli, revisited: Differential gene expression after replication inhibition. Proc. Natl. Acad. Sci. USA 2024, 121, e2407832121. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.K.; Alvarado, C.L.; Sutton, M.D. Role of the SOS Response in the Generation of Antibiotic Resistance In Vivo. Antimicrob. Agents Chemother. 2021, 65, e0001321. [Google Scholar] [CrossRef]
- Yakimov, A.; Bakhlanova, I.; Baitin, D. Targeting evolution of antibiotic resistance by SOS response inhibition. Comput. Struct. Biotechnol. J. 2021, 19, 777–783. [Google Scholar] [CrossRef]
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; Sulaiman, A.M.; Chee, J.Y.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial resistance: Prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur. J. Pharm. Sci. 2022, 170, 106103. [Google Scholar] [CrossRef]
- Chistyakov, V.; Prazdnova, E.; Mazanko, M.; Churilov, M.; Chmyhalo, V. Increase in bacterial resistance to antibiotics after cancer therapy with platinum-based drugs. Mol. Biol. 2018, 52, 232–236. [Google Scholar] [CrossRef]
- Hobson, C.A.; Bonacorsi, S.; Hocquet, D.; Baruchel, A.; Fahd, M.; Storme, T.; Tang, R.; Doit, C.; Tenaillon, O.; Birgy, A. Impact of anticancer chemotherapy on the extension of beta-lactamase spectrum: An example with KPC-type carbapenemase activity towards ceftazidime-avibactam. Sci. Rep. 2020, 10, 589. [Google Scholar] [CrossRef]
- Yang, B.; Wang, Z.; Jia, Y.; Fang, D.; Li, R.; Liu, Y. Paclitaxel and its derivative facilitate the transmission of plasmid-mediated antibiotic resistance genes through conjugative transfer. Sci. Total Environ. 2022, 810, 152245. [Google Scholar] [CrossRef]
- Sun, D.; Jeannot, K.; Xiao, Y.; Knapp, C.W. Horizontal gene transfer mediated bacterial antibiotic resistance. Front. Microbiol. 2019, 10, 1933. [Google Scholar] [CrossRef]
- Tao, S.; Chen, H.; Li, N.; Wang, T.; Liang, W. The Spread of Antibiotic Resistance Genes In Vivo Model. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 3348695. [Google Scholar] [CrossRef] [PubMed]
- Blakely, G.W. Mechanisms of horizontal gene transfer and DNA recombination. In Molecular Medical Microbiology; Elsevier: Amsterdam, The Netherlands, 2024; pp. 309–324. [Google Scholar]
- Wendling, C.C.; Refardt, D.; Hall, A.R. Fitness benefits to bacteria of carrying prophages and prophage-encoded antibiotic-resistance genes peak in different environments. Evolution 2021, 75, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, K.; Sampath, V.; Umar, S. Bacterial Infections and Cancer: Exploring This Association And Its Implications for Cancer Patients. Int. J. Mol. Sci. 2023, 24, 3110. [Google Scholar] [CrossRef] [PubMed]
- Attiê, R.; Chinen, L.T.; Yoshioka, E.M.; Silva, M.C.; de Lima, V.C. Acute bacterial infection negatively impacts cancer specific survival of colorectal cancer patients. World J. Gastroenterol. 2014, 20, 13930–13935. [Google Scholar] [CrossRef]
- Blijlevens, N.M.; Reijnders, B.; Molendijk, E. Gastrointestinal mucositis: A sign of a (systemic) inflammatory response. Curr. Opin. Support. Palliat. Care 2024, 18, 78–85. [Google Scholar] [CrossRef]
- Gao, Y.; Shang, Q.; Li, W.; Guo, W.; Stojadinovic, A.; Mannion, C.; Man, Y.G.; Chen, T. Antibiotics for cancer treatment: A double-edged sword. J. Cancer 2020, 11, 5135–5149. [Google Scholar] [CrossRef]
- Martins, J.O.; Borges, M.M.; Malta, C.E.; Carlos, A.C.; Crispim, A.A.; Moura, J.F.; Fernandes-Lima, I.J.; Silva, P.G. Risk factors for oral mucositis during chemotherapy treatment for solid tumors: A retrospective STROBE-guided study. Med. Oral Patol. Oral Cir. Bucal 2022, 27, e319–e329. [Google Scholar] [CrossRef]
- Blijlevens, N.M.A.; de Mooij, C.E.M. Mucositis and Infection in Hematology Patients. Int. J. Mol. Sci. 2023, 24, 9592. [Google Scholar] [CrossRef]
- Rolston, K.V. Infections in cancer patients with solid tumors: A review. Infect. Dis. Ther. 2017, 6, 69–83. [Google Scholar] [CrossRef]
- Islas-Muñoz, B.; Volkow-Fernández, P.; Ibanes-Gutiérrez, C.; Villamar-Ramírez, A.; Vilar-Compte, D.; Cornejo-Juárez, P. Bloodstream infections in cancer patients. Risk factors associated with mortality. Int. J. Infect. Dis. 2018, 71, 59–64. [Google Scholar]
- Bhat, S.; Muthunatarajan, S.; Mulki, S.S.; Archana Bhat, K.; Kotian, K.H. Bacterial infection among cancer patients: Analysis of isolates and antibiotic sensitivity pattern. Int. J. Microbiol. 2021, 2021, 8883700. [Google Scholar] [CrossRef] [PubMed]
- MacPhail, A.; Dendle, C.; Slavin, M.; McQuilten, Z. Hospital-acquired bloodstream infections in cancer patients: Current knowledge and future directions. J. Hosp. Infect. 2024, 148, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Cornejo-Juárez, P.; Vilar-Compte, D.; García-Horton, A.; López-Velázquez, M.; Ñamendys-Silva, S.; Volkow-Fernández, P. Hospital-acquired infections at an oncological intensive care cancer unit: Differences between solid and hematological cancer patients. BMC Infect. Dis. 2016, 16, 274. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.; Guddati, A.K. Infections in Hospitalized Cancer Patients. World J. Oncol. 2021, 12, 195–205. [Google Scholar] [CrossRef]
- Hooper, D.C.; Jacoby, G.A. Topoisomerase Inhibitors: Fluoroquinolone Mechanisms of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025320. [Google Scholar] [CrossRef]
- Shariati, A.; Arshadi, M.; Khosrojerdi, M.A.; Abedinzadeh, M.; Ganjalishahi, M.; Maleki, A.; Heidary, M.; Khoshnood, S. The resistance mechanisms of bacteria against ciprofloxacin and new approaches for enhancing the efficacy of this antibiotic. Front. Public Health 2022, 10, 1025633. [Google Scholar] [CrossRef]
- Chambers, L.M.; Kuznicki, M.; Yao, M.; Chichura, A.; Gruner, M.; Reizes, O.; Debernardo, R.; Rose, P.G.; Michener, C.; Vargas, R. Impact of antibiotic treatment during platinum chemotherapy on survival and recurrence in women with advanced epithelial ovarian cancer. Gynecol. Oncol. 2020, 159, 699–705. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, G.; Wong, W.C.; Hu, D.H.; Zhu, J.W.; Li, R.; Zhou, H. The impact of antibiotic use on clinical features and survival outcomes of cancer patients treated with immune checkpoint inhibitors. Front. Immunol. 2022, 13, 968729. [Google Scholar] [CrossRef]
- Morrell, S.; Kohonen-Corish, M.R.; Ward, R.L.; Sorrell, T.C.; Roder, D.; Currow, D.C. Antibiotic exposure within six months before systemic therapy was associated with lower cancer survival. J. Clin. Epidemiol. 2022, 147, 122–131. [Google Scholar] [CrossRef]
- Tian, X.; Mei, T.; Yu, M.; Li, Y.; Ao, R.; Gong, Y. The impact of antibiotic selection and interval time among advanced non-small cell lung cancer patients receiving prior antibacterial treatment and first-line chemotherapy. Cancer Med. 2022, 11, 4849–4864. [Google Scholar] [CrossRef]
- Bodro, M.; Gudiol, C.; Garcia-Vidal, C.; Tubau, F.; Contra, A.; Boix, L.; Domingo-Domenech, E.; Calvo, M.; Carratalà, J. Epidemiology, antibiotic therapy and outcomes of bacteremia caused by drug-resistant ESKAPE pathogens in cancer patients. Support. Care Cancer 2014, 22, 603–610. [Google Scholar] [CrossRef] [PubMed]
- El-Mahallawy, H.A.; Hassan, S.S.; El-Wakil, M.; Moneer, M.M. Bacteremia due to ESKAPE pathogens: An emerging problem in cancer patients. J. Egypt. Natl. Cancer Inst. 2016, 28, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Cornejo-Juárez, P.; Vilar-Compte, D.; Pérez-Jiménez, C.; Ñamendys-Silva, S.; Sandoval-Hernández, S.; Volkow-Fernández, P. The impact of hospital-acquired infections with multidrug-resistant bacteria in an oncology intensive care unit. Int. J. Infect. Dis. 2015, 31, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Damato, A.; Ghidini, M.; Dottorini, L.; Tomasello, G.; Iaculli, A.; Ghidini, A.; Luciani, A.; Petrelli, F. Chemotherapy Duration for Various Indications in Colorectal Cancer: A Review. Curr. Oncol. Rep. 2023, 25, 341–352. [Google Scholar] [CrossRef]
- Boublikova, L.; Novakova, A.; Simsa, J.; Lohynska, R. Total neoadjuvant therapy in rectal cancer: The evidence and expectations. Crit. Rev. Oncol./Hematol. 2023, 192, 104196. [Google Scholar] [CrossRef]
- Mithuna, R.; Tharanyalakshmi, R.; Jain, I.; Singhal, S.; Sikarwar, D.; Das, S.; Ranjitha, J.; Ghosh, D.; Rahman, M.M.; Das, B. Emergence of antibiotic resistance due to the excessive use of antibiotics in medicines and feed additives: A global scenario with emphasis on the Indian perspective. Emerg. Contam. 2024, 10, 100389. [Google Scholar] [CrossRef]
- Elkrief, A.; Derosa, L.; Kroemer, G.; Zitvogel, L.; Routy, B. The negative impact of antibiotics on outcomes in cancer patients treated with immunotherapy: A new independent prognostic factor? Ann. Oncol. 2019, 30, 1572–1579. [Google Scholar] [CrossRef]
- Amanati, A.; Sajedianfard, S.; Khajeh, S.; Ghasempour, S.; Mehrangiz, S.; Nematolahi, S.; Shahhosein, Z. Bloodstream infections in adult patients with malignancy, epidemiology, microbiology, and risk factors associated with mortality and multi-drug resistance. BMC Infect. Dis. 2021, 21, 636. [Google Scholar] [CrossRef]
- Wilson, I.D.; Nicholson, J.K. Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl. Res. 2017, 179, 204–222. [Google Scholar] [CrossRef]
- Dahlgren, D.; Lennernäs, H. Review on the effect of chemotherapy on the intestinal barrier: Epithelial permeability, mucus and bacterial translocation. Biomed. Pharmacother. 2023, 162, 114644. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H. Gut microbiota modulation: A tool for the management of colorectal cancer. J. Transl. Med. 2022, 20, 178. [Google Scholar] [CrossRef] [PubMed]
- Dixit, K.; Chaudhari, D.; Dhotre, D.; Shouche, Y.; Saroj, S. Restoration of dysbiotic human gut microbiome for homeostasis. Life Sci. 2021, 278, 119622. [Google Scholar] [CrossRef] [PubMed]
- Roggiani, S.; Mengoli, M.; Conti, G.; Fabbrini, M.; Brigidi, P.; Barone, M.; D’Amico, F.; Turroni, S. Gut microbiota resilience and recovery after anticancer chemotherapy. Microbiome Res. Rep. 2023, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Millan, B.; Park, H.; Hotte, N.; Mathieu, O.; Burguiere, P.; Tompkins, T.A.; Kao, D.; Madsen, K.L. Fecal Microbial Transplants Reduce Antibiotic-resistant Genes in Patients With Recurrent Clostridium difficile Infection. Clin. Infect. Dis. 2016, 62, 1479–1486. [Google Scholar] [CrossRef]
- Leung, V.; Vincent, C.; Edens, T.J.; Miller, M.; Manges, A.R. Antimicrobial resistance gene acquisition and depletion following fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin. Infect. Dis. 2018, 66, 456–457. [Google Scholar] [CrossRef]
- Hyun, J.; Lee, S.K.; Cheon, J.H.; Yong, D.E.; Koh, H.; Kang, Y.K.; Kim, M.H.; Sohn, Y.; Cho, Y.; Baek, Y.J.; et al. Faecal microbiota transplantation reduces amounts of antibiotic resistance genes in patients with multidrug-resistant organisms. Antimicrob. Resist. Infect. Control 2022, 11, 20. [Google Scholar] [CrossRef]
- Jess, A.T.; Eskander, G.H.; Vu, M.H.; Michail, S. Short-chain fatty acid levels after fecal microbiota transplantation in a pediatric cohort with recurrent Clostridioides difficile infection. Metabolites 2023, 13, 1039. [Google Scholar] [CrossRef]
- Lou, X.; Xue, J.; Shao, R.; Yang, Y.; Ning, D.; Mo, C.; Wang, F.; Chen, G. Fecal microbiota transplantation and short-chain fatty acids reduce sepsis mortality by remodeling antibiotic-induced gut microbiota disturbances. Front. Immunol. 2022, 13, 1063543. [Google Scholar] [CrossRef]
- Malard, F.; Vekhoff, A.; Lapusan, S.; Isnard, F.; D’Incan-Corda, E.; Rey, J.; Saillard, C.; Thomas, X.; Ducastelle-Lepretre, S.; Paubelle, E.; et al. Gut microbiota diversity after autologous fecal microbiota transfer in acute myeloid leukemia patients. Nat. Commun. 2021, 12, 3084. [Google Scholar] [CrossRef]
- Zhou, J.; Li, M.; Chen, Q.; Li, X.; Chen, L.; Dong, Z.; Zhu, W.; Yang, Y.; Liu, Z.; Chen, Q. Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nat. Commun. 2022, 13, 3432. [Google Scholar] [CrossRef]
- Van Zyl, W.F.; Deane, S.M.; Dicks, L.M. Molecular insights into probiotic mechanisms of action employed against intestinal pathogenic bacteria. Gut Microbes 2020, 12, 1831339. [Google Scholar] [CrossRef] [PubMed]
- Wieërs, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; Philippart de Foy, J.-M.; Dequenne, I.; de Timary, P.; Cani, P.D. How probiotics affect the microbiota. Front. Cell. Infect. Microbiol. 2020, 9, 454. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Niu, Z.; Zou, M.; Liu, S.; Wang, M.; Gu, X.; Lu, H.; Tian, H.; Jha, R. Probiotics, prebiotics, and synbiotics regulate the intestinal microbiota differentially and restore the relative abundance of specific gut microorganisms. J. Dairy. Sci. 2020, 103, 5816–5829. [Google Scholar] [CrossRef] [PubMed]
- Sniffen, J.C.; McFarland, L.V.; Evans, C.T.; Goldstein, E.J. Choosing an appropriate probiotic product for your patient: An evidence-based practical guide. PLoS ONE 2018, 13, e0209205. [Google Scholar] [CrossRef]
- Ma, T.; Shen, X.; Shi, X.; Sakandar, H.A.; Quan, K.; Li, Y.; Jin, H.; Kwok, L.-Y.; Zhang, H.; Sun, Z. Targeting gut microbiota and metabolism as the major probiotic mechanism-An evidence-based review. Trends Food Sci. Technol. 2023, 138, 178–198. [Google Scholar] [CrossRef]
- Xia, C.; Jiang, C.; Li, W.; Wei, J.; Hong, H.; Li, J.; Feng, L.; Wei, H.; Xin, H.; Chen, T. A phase II randomized clinical trial and mechanistic studies using improved probiotics to prevent oral mucositis induced by concurrent radiotherapy and chemotherapy in nasopharyngeal carcinoma. Front. Immunol. 2021, 12, 618150. [Google Scholar] [CrossRef]
- Sambrani, R.; Abdolalizadeh, J.; Kohan, L.; Jafari, B. Recent advances in the application of probiotic yeasts, particularly Saccharomyces, as an adjuvant therapy in the management of cancer with focus on colorectal cancer. Mol. Biol. Rep. 2021, 48, 951–960. [Google Scholar] [CrossRef]
- Vivarelli, S.; Falzone, L.; Basile, M.S.; Nicolosi, D.; Genovese, C.; Libra, M.; Salmeri, M. Benefits of using probiotics as adjuvants in anticancer therapy. World Acad. Sci. J. 2019, 1, 125–135. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kumar, D.; Guha, D. Insights of probiotics as an alternative medicine for cancer therapy, mechanism, and applications. Med. Microecol. 2024, 22, 100111. [Google Scholar] [CrossRef]
- Hibberd, A.A.; Lyra, A.; Ouwehand, A.C.; Rolny, P.; Lindegren, H.; Cedgård, L.; Wettergren, Y. Intestinal microbiota is altered in patients with colon cancer and modified by probiotic intervention. BMJ Open Gastroenterol. 2017, 4, e000145. [Google Scholar] [CrossRef]
- McFarland, L.V.; Evans, C.T.; Goldstein, E.J.C. Strain-Specificity and Disease-Specificity of Probiotic Efficacy: A Systematic Review and Meta-Analysis. Front. Med. 2018, 5, 124. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, M.; Radziwill-Bienkowska, J.M.; Marć, M.A.; Jastrząb, R.; Mytych, J.; Siedlecki, P.; Szczepankowska, A.K. Screening for probiotic properties and potential immunogenic effects of lactobacilli strains isolated from various food products. Front. Microbiol. 2024, 15, 1430582. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Jung, S.-C.; Kwak, K.; Kim, J.-S. The Role of Prebiotics in Modulating Gut Microbiota: Implications for Human Health. Int. J. Mol. Sci. 2024, 25, 4834. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Zhu, R.; Zhu, X.; Yan, W.; Li, Y.; Zhai, Y.; Wu, T.; Huang, X.; Yin, Q.; Li, Y. Combining gut microbiota modulation and chemotherapy by capecitabine-loaded prebiotic nanoparticle improves colorectal cancer therapy. Nat. Commun. 2023, 14, 4746. [Google Scholar] [CrossRef]
- Yue, X.; Wen, S.; Long-Kun, D.; Man, Y.; Chang, S.; Min, Z.; Shuang-Yu, L.; Xin, Q.; Jie, M.; Liang, W. Three important short-chain fatty acids (SCFAs) attenuate the inflammatory response induced by 5-FU and maintain the integrity of intestinal mucosal tight junction. BMC Immunol. 2022, 23, 19. [Google Scholar] [CrossRef]
- Zhang, Y.; Xi, Y.; Yang, C.; Gong, W.; Wang, C.; Wu, L.; Wang, D. Short-Chain Fatty Acids Attenuate 5-Fluorouracil-Induced THP-1 Cell Inflammation through Inhibiting NF-κB/NLRP3 Signaling via Glycerolphospholipid and Sphingolipid Metabolism. Molecules 2023, 28, 494. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Newsome, R.; Yang, Y.; Jobin, C. Western diet influences on microbiome and carcinogenesis. Semin. Immunol. 2023, 67, 101756. [Google Scholar] [CrossRef]
- Mehta, R.S.; Song, M.; Nishihara, R.; Drew, D.A.; Wu, K.; Qian, Z.R.; Fung, T.T.; Hamada, T.; Masugi, Y.; da Silva, A.; et al. Dietary Patterns and Risk of Colorectal Cancer: Analysis by Tumor Location and Molecular Subtypes. Gastroenterology 2017, 152, 1944–1953.e1. [Google Scholar] [CrossRef]
- Zheng, Y.; Meng, L.; Liu, H.; Sun, L.; Nie, Y.; Wu, Q.; Fan, D.; Li, M. Let food be thy medicine: The role of diet in colorectal cancer: A narrative review. J. Gastrointest. Oncol. 2022, 13, 2020. [Google Scholar] [CrossRef]
- Pu, G.; Hou, L.; Du, T.; Zhou, W.; Liu, C.; Niu, P.; Wu, C.; Bao, W.; Huang, R.; Li, P. Increased Proportion of Fiber-Degrading Microbes and Enhanced Cecum Development Jointly Promote Host To Digest Appropriate High-Fiber Diets. mSystems 2023, 8, e0093722. [Google Scholar] [CrossRef] [PubMed]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Totino, V.; Cacciotti, F.; Iebba, V.; Neroni, B.; Bonfiglio, G.; Trancassini, M.; Passariello, C.; Pantanella, F.; Schippa, S. Rebuilding the Gut Microbiota Ecosystem. Int. J. Environ. Res. Public Health 2018, 15, 1679. [Google Scholar] [CrossRef]
- Lee, J.G.; Lee, J.; Lee, A.-r.; Jo, S.V.; Park, C.H.; Han, D.S.; Eun, C.S. Impact of short-chain fatty acid supplementation on gut inflammation and microbiota composition in a murine colitis model. J. Nutr. Biochem. 2022, 101, 108926. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, B.; Domingo-Calap, P. Phage Therapy in Gastrointestinal Diseases. Microorganisms 2020, 8, 1420. [Google Scholar] [CrossRef]
- Nilsson, A.S. Pharmacological limitations of phage therapy. Upsala J. Med. Sci. 2019, 124, 218–227. [Google Scholar] [CrossRef]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Aguilar, G.R.; Mestrovic, T.; Smith, G.; Han, C. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Barlam, T.F.; Cosgrove, S.E.; Abbo, L.M.; MacDougall, C.; Schuetz, A.N.; Septimus, E.J.; Srinivasan, A.; Dellit, T.H.; Falck-Ytter, Y.T.; Fishman, N.O.; et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 2016, 62, e51–e77. [Google Scholar] [CrossRef]
- Durand, C.; Risso, K.; Loschi, M.; Retur, N.; Emery, A.; Courjon, J.; Cluzeau, T.; Carles, M. Efficacy of an antimicrobial stewardship intervention for early adaptation of antibiotic therapy in high-risk neutropenic patients. Antimicrob. Resist. Infect. Control 2024, 13, 5. [Google Scholar] [CrossRef]
- Majumder, M.A.A.; Rahman, S.; Cohall, D.; Bharatha, A.; Singh, K.; Haque, M.; Gittens-St Hilaire, M. Antimicrobial stewardship: Fighting antimicrobial resistance and protecting global public health. Infect. Drug Resist. 2020, 13, 4713–4738. [Google Scholar] [CrossRef]
- Bansal, N.; Goyal, P.; Basu, D.; Batra, U.; Sachdeva, N.; Joga, S.; Jain, A.; Doval, D. Impact of improving infection control and antibiotic stewardship practices on nosocomial infections and antimicrobial resistance in an oncology centre from India. Indian J. Med. Microbiol. 2023, 45, 100383. [Google Scholar] [CrossRef] [PubMed]
- Boursi, B.; Mamtani, R.; Haynes, K.; Yang, Y.-X. Recurrent antibiotic exposure may promote cancer formation–another step in understanding the role of the human microbiota? Eur. J. Cancer 2015, 51, 2655–2664. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Ghidini, M.; Ghidini, A.; Perego, G.; Cabiddu, M.; Khakoo, S.; Oggionni, E.; Abeni, C.; Hahne, J.C.; Tomasello, G.; et al. Use of Antibiotics and Risk of Cancer: A Systematic Review and Meta-Analysis of Observational Studies. Cancers 2019, 11, 1174. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Imoto, W.; Yamairi, K.; Shibata, W.; Namikawa, H.; Yoshii, N.; Fujimoto, H.; Nakaie, K.; Okada, Y.; Fujita, A.; et al. The intervention by an antimicrobial stewardship team can improve clinical and microbiological outcomes of resistant gram-negative bacteria. J. Infect. Chemother. 2019, 25, 1001–1006. [Google Scholar] [CrossRef]
- Elhadi, M.; Khaled, A.; Msherghi, A. Infectious diseases as a cause of death among cancer patients: A trend analysis and population-based study of outcome in the United States based on the Surveillance, Epidemiology, and End Results database. Infect. Agent. Cancer 2021, 16, 72. [Google Scholar] [CrossRef]
- Ashiru-Oredope, D.; Langford, B.J.; Bonaconsa, C.; Nampoothiri, V.; Charani, E.; Goff, D.A. Global collaborations in antimicrobial stewardship: All hands on deck. Antimicrob. Steward. Healthc. Epidemiol. 2023, 3, e66. [Google Scholar] [CrossRef]
- O’neill, J. Antimicrobial resistance: Tackling a crisis for the health and wealth of nations. Rev. Antimicrob. Resist. 2014. Available online: https://www.who.int/news/item/29-04-2019-new-report-calls-for-urgent-action-to-avert-antimicrobial-resistance-crisis (accessed on 21 February 2025).
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).