Lysyl-Phosphatidylglycerol: A Lipid Involved in the Resistance of Staphylococcus aureus to Antimicrobial Peptide Activity
Abstract
1. Introduction
2. Staphylococcus aureus
3. Membrane Composition
4. Aminoacyl Lipids: From Discovery to Function
5. Adaptive Membrane Lipid Modifications in Bacteria: Role of Lysyl-PG and Related Lipoamino Acids
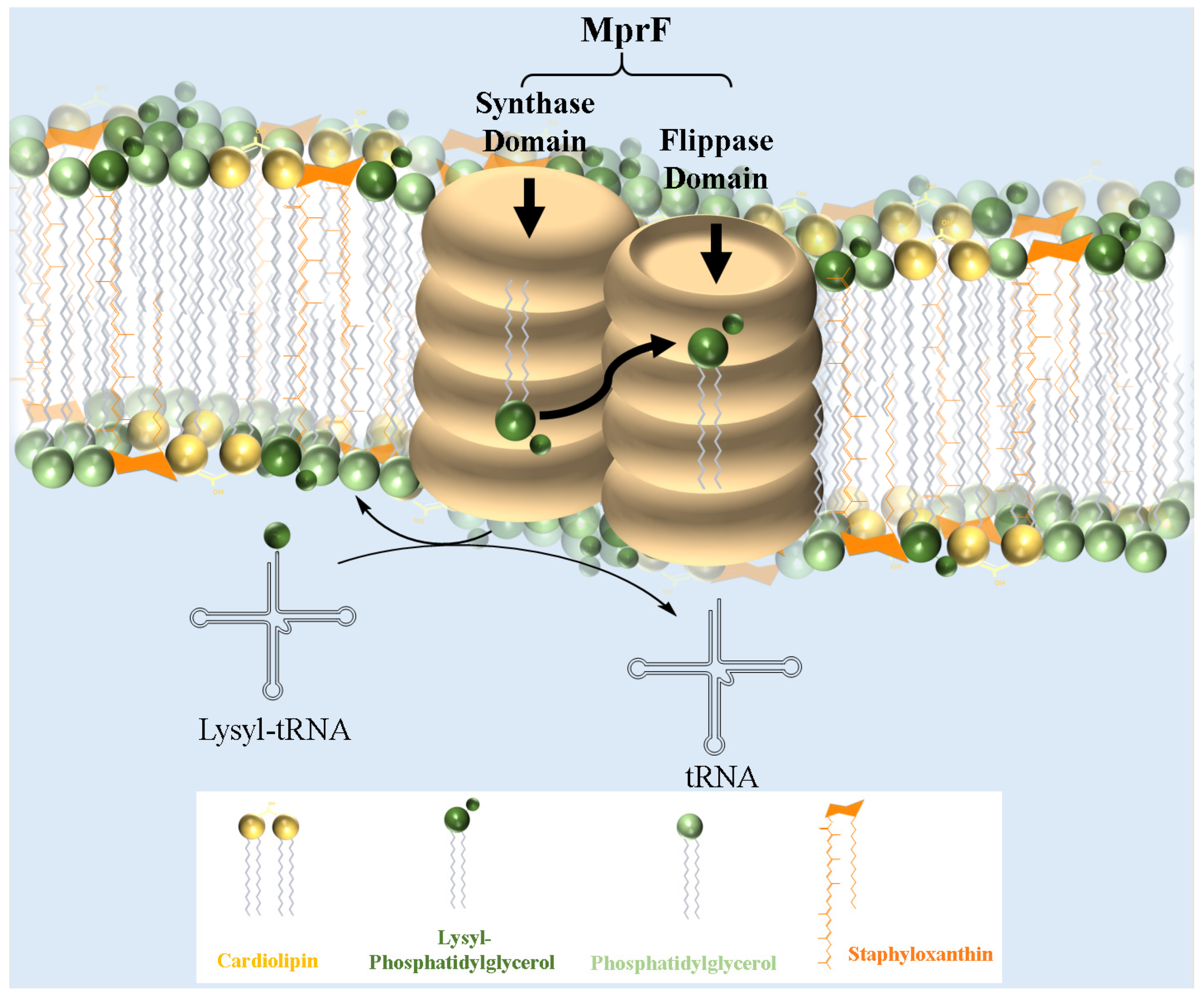
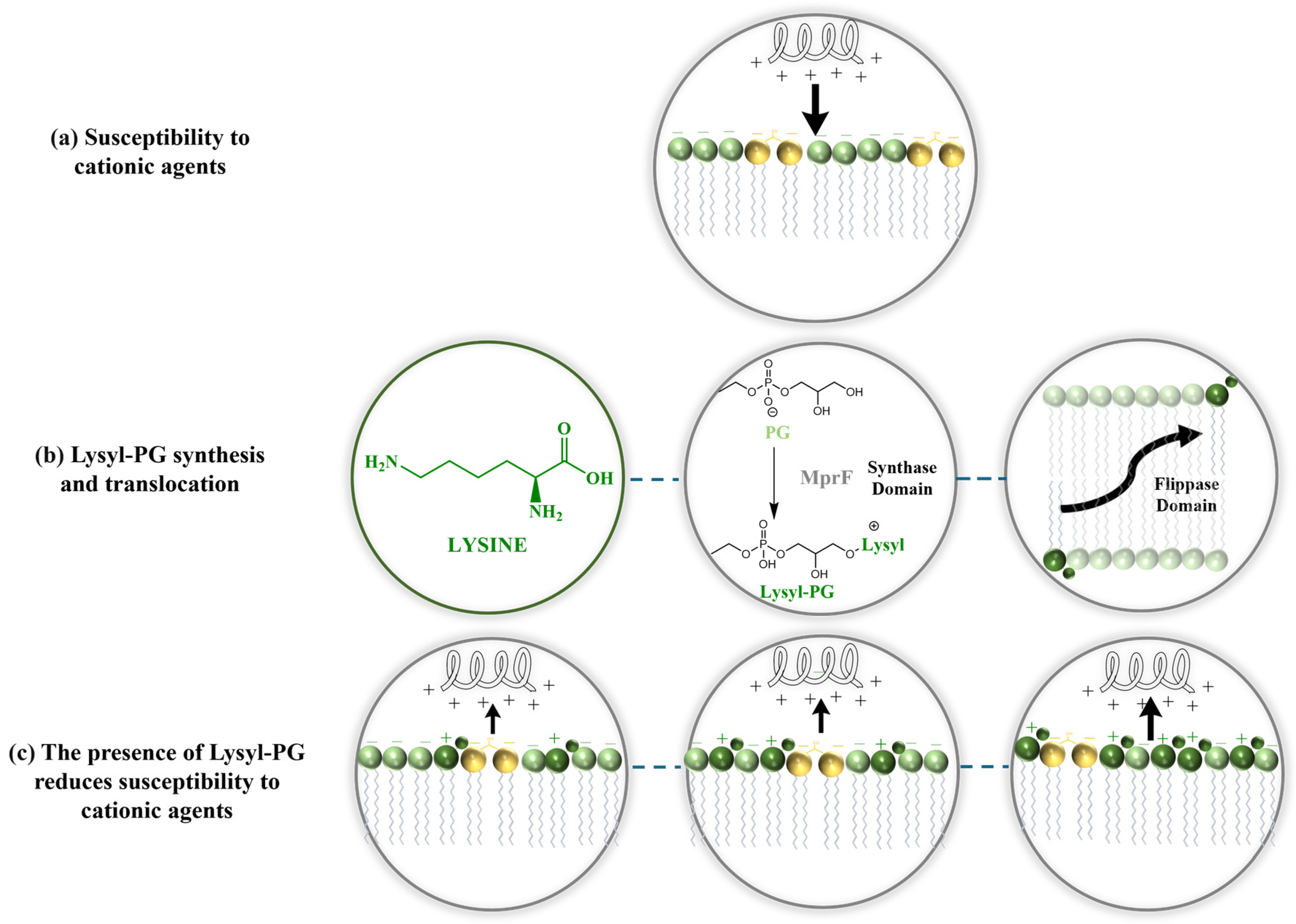
6. Mechanisms of Lysyl-PG Biosynthesis and Translocation: The Role of MprF
7. Role of Lysyl-PG in Modulating S. aureus Resistance to Antimicrobial Agents
8. Environmental Regulation of Lysyl-PG Synthesis
9. The Role of CL and STX in S. aureus Membrane Adaptation and Resistance
10. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D TLC | Two-Dimensional Thin-Layer Chromatography |
| 31P NMR | Phosphorus-31 Nuclear Magnetic Resonance |
| aaPGSs | aminoacylphosphatidylglycerol synthases |
| cAMPs | Cationic Antimicrobial Peptides |
| CL | Cardiolipin |
| d62DP3adLPG | d62 1,2 O dipalmitoyl 3 aza dehydroxy lysyl phosphatidylglycerol, trifluoroacetate salt |
| d62DPPG | d62 1,2 O dipalmitoyl sn glycero 3 phospho (1′ rac glycerol), triethyl ammonium salt |
| DMPG | 1,2-Dimyristoyl-sn-glycero-3-phospho-rac-(1-glycerol) sodium salt |
| DMPC | 1,2-Dimyristoyl-sn-glycero-3-phosphocholine |
| DOPG | 1,2-Dioleoyl-sn-glycero-3-phosphoglycerol |
| DP3adLPG | Dipalmitoyl-3-aza-dehydroxy-lysylphosphatidylglycerol |
| DPPC | 1,2-Dipalmitoyl-rac-glycero-3-phosphocholine |
| DPPG | 1,2-Dihexadecanoyl-sn-glycero-3-phospho-(1′-rac-glycerol) ammonium salt |
| GL | Glycolipid |
| GLASS | Global Antimicrobial Resistance and Use Surveillance System |
| HILIC | Hydrophilic Interaction Liquid Chromatography |
| HPLC | High-Performance Liquid Chromatography |
| HPTLC | High-Performance Thin-Layer Chromatography |
| LC/ESI–MS/MS | Liquid Chromatography–Electrospray Ionization Tandem Mass Spectrometry |
| LC/MS | Liquid Chromatography–Mass Spectrometry |
| LC-MS/MS | Liquid Chromatography–Tandem Mass Spectrometry |
| LUVs | Large Unilamellar Vesicles |
| lysyl-PG | lysyl-phosphatidylglycerol |
| lysyl-DMPG | 1,2-Dimyristoyl-sn-glycero-3-[phospho-1-(3-lysyl(1-glycerol))] |
| lysyl-DOPG | 1,2-Dioleoyl-sn-glycero-3-[phospho-rac-(3-lysyl(1-glycerol))] |
| MprF | Multiple peptide resistance factor |
| MALDI-MS | Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry |
| MS | Mass Spectrometry |
| NPLC-ESI/MS | Normal Phase Liquid Chromatography Coupled with Electrospray Ionization Mass Spectrometry |
| PE | Phosphatidylethanolamine |
| PG | Phosphatidylglycerol |
| POPC | 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine |
| POPG | 1-Palmitoyl-2-oleoyl-sn-glycero-3-(phospho-rac-(1-glycerol)) |
| S. aureus | Staphylococcus aureus |
| STX | Staphyloxanthin |
| Tandem MS | MS/MS |
| TLC | Thin-Layer Chromatography |
| TMCL | 1,1′,2,2′-Tetramyristoyl cardiolipin |
References
- Vestergaard, M.; Frees, D.; Ingmer, H. Antibiotic resistance and the MRSA problem. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022; Progress in the Development of National Surveillance Systems Reporting Data to GLASS-AMR; WHO: Geneva, Switzerland, 2022; pp. 10–41. [Google Scholar]
- Hasanpour, A.H.; Sepidarkish, M.; Mollalo, A.; Ardekani, A.; Almukhtar, M.; Mechaal, A.; Hosseini, S.R.; Bayani, M.; Javanian, M.; Rostami, A. The global prevalence of methicillin-resistant Staphylococcus aureus colonization in residents of elderly care centers: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2023, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.N.; Liu, G.Y.; Yeaman, M.R.; Nast, C.C.; Proctor, R.A.; McKinnell, J.; Bayer, A.S. Carotenoid-related alteration of cell membrane fluidity impacts Staphylococcus aureus susceptibility to host defense peptides. Antimicrob. Agents Chemother. 2011, 55, 526–531. [Google Scholar] [CrossRef]
- Bisignano, C.; Ginestra, G.; Smeriglio, A.; La Camera, E.; Crisafi, G.; Franchina, F.A.; Tranchida, P.Q.; Alibrandi, A.; Trombetta, D.; Mondello, L. Study of the lipid profile of ATCC and clinical strains of Staphylococcus aureus in relation to their antibiotic resistance. Molecules 2019, 24, 1276. [Google Scholar] [CrossRef]
- Haest, C.; De Gier, J.; Den Kamp, J.O.; Bartels, P.; Van Deenen, L. Changes in permeability of Staphylococcus aureus and derived liposomes with varying lipid composition. Biochim. Biophys. Acta (BBA)-Biomembr. 1972, 255, 720–733. [Google Scholar] [CrossRef]
- Sievers, S.; Ernst, C.M.; Geiger, T.; Hecker, M.; Wolz, C.; Becher, D.; Peschel, A. Changing the phospholipid composition of Staphylococcus aureus causes distinct changes in membrane proteome and membrane-sensory regulators. Proteomics 2010, 10, 1685–1693. [Google Scholar] [CrossRef]
- Zheng, X.; Marsman, G.; Lacey, K.; Chapman, J.; Goosmann, C.; Ueberheide, B.; Torres, V. The cell envelope of Staphylococcus aureus selectively controls the sorting of virulence factors. Nat. Commun. 2021, 12, 6193. [Google Scholar]
- Cheung, G.Y.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar]
- Pietrocola, G.; Nobile, G.; Rindi, S.; Speziale, P. Staphylococcus aureus manipulates innate immunity through own and host-expressed proteases. Front. Cell. Infect. Microbiol. 2017, 7, 166. [Google Scholar] [CrossRef]
- Alkuraythi, D. Virulence Factors and Pathogenicity of Staphylococcus aureus. In Advances and Perspectives of Infections Caused by Staphylococcus aureus; IntechOpen: London, UK, 2024. [Google Scholar]
- Bear, A.; Locke, T.; Rowland-Jones, S.; Pecetta, S.; Bagnoli, F.; Darton, T.C. The immune evasion roles of Staphylococcus aureus protein A and impact on vaccine development. Front. Cell. Infect. Microbiol. 2023, 13, 1242702. [Google Scholar] [CrossRef]
- Arunachalam, K.; Pandurangan, P.; Shi, C.; Lagoa, R. Regulation of Staphylococcus aureus virulence and application of nanotherapeutics to eradicate s. aureus infection. Pharmaceutics 2023, 15, 310. [Google Scholar] [CrossRef] [PubMed]
- Peschel, A.; Jack, R.W.; Otto, M.; Collins, L.V.; Staubitz, P.; Nicholson, G.; Kalbacher, H.; Nieuwenhuizen, W.F.; Jung, G.; Tarkowski, A. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J. Exp. Med. 2001, 193, 1067–1076. [Google Scholar]
- Rehal, R.; Gaffney, P.R.; Hubbard, A.T.; Barker, R.D.; Harvey, R.D. The pH-dependence of lipid-mediated antimicrobial peptide resistance in a model staphylococcal plasma membrane: A two-for-one mechanism of epithelial defense circumvention. Eur. J. Pharm. Sci. 2019, 128, 43–53. [Google Scholar]
- Nikolic, P.; Mudgil, P. The cell wall, cell membrane and virulence factors of Staphylococcus aureus and their role in antibiotic resistance. Microorganisms 2023, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Dennison, S.R.; Morton, L.H.; Harris, F.; Phoenix, D.A. Low pH enhances the action of maximin H5 against Staphylococcus aureus and helps mediate lysylated phosphatidylglycerol-induced resistance. Biochemistry 2016, 55, 3735–3751. [Google Scholar] [PubMed]
- Kilelee, E.; Pokorny, A.; Yeaman, M.R.; Bayer, A.S. Lysyl-phosphatidylglycerol attenuates membrane perturbation rather than surface association of the cationic antimicrobial peptide 6W-RP-1 in a model membrane system: Implications for daptomycin resistance. Antimicrob. Agents Chemother. 2010, 54, 4476–4479. [Google Scholar]
- Ernst, C.M.; Staubitz, P.; Mishra, N.N.; Yang, S.-J.; Hornig, G.; Kalbacher, H.; Bayer, A.S.; Kraus, D.; Peschel, A. The bacterial defensin resistance protein MprF consists of separable domains for lipid lysinylation and antimicrobial peptide repulsion. PLoS Pathog. 2009, 5, e1000660. [Google Scholar] [CrossRef]
- Zhang, J.; Suo, Y.; Zhang, D.; Jin, F.; Zhao, H.; Shi, C. Genetic and virulent difference between pigmented and non-pigmented Staphylococcus aureus. Front. Microbiol. 2018, 9, 598. [Google Scholar]
- Riedel, S.; Hobden, J.A.; Miller, S.; Morse, S.A.; Mietzner, T.A.; Detrick, B.; Mitchell, T.G.; Sakanari, J.A.; Hotez, P.; Mejia, R. The Staphylococci. In Jawetz, Melnick & Adelberg’s Medical Microbiology, 28e; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar]
- Missiakas, D.; Winstel, V. Selective host cell death by Staphylococcus aureus: A strategy for bacterial persistence. Front. Immunol. 2021, 11, 621733. [Google Scholar] [CrossRef] [PubMed]
- Loeb, L. The influence of certain bacteria on the coagulation of the blood. J. Med. Res. 1903, 10, 407. [Google Scholar]
- Crosby, H.A.; Kwiecinski, J.; Horswill, A.R. Staphylococcus aureus aggregation and coagulation mechanisms, and their function in host–pathogen interactions. Adv. Appl. Microbiol. 2016, 96, 1–41. [Google Scholar] [PubMed]
- Williams, R. Healthy carriage of Staphylococcus aureus: Its prevalence and importance. Bacteriol. Rev. 1963, 27, 56–71. [Google Scholar] [CrossRef]
- Kluytmans, J.; Van Belkum, A.; Verbrugh, H. Nasal carriage of Staphylococcus aureus: Epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 1997, 10, 505–520. [Google Scholar] [CrossRef]
- Monecke, S.; Luedicke, C.; Slickers, P.; Ehricht, R. Molecular epidemiology of Staphylococcus aureus in asymptomatic carriers. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1159–1165. [Google Scholar] [CrossRef]
- Hanselman, B.A.; Kruth, S.A.; Rousseau, J.; Weese, J.S. Coagulase positive staphylococcal colonization of humans and their household pets. Can. Vet. J. 2009, 50, 954. [Google Scholar] [PubMed]
- Strominger, J.L.; Park, J.T.; Thompson, R.E. Composition of the cell wall of Staphylococcus aureus: Its relation to the mechanism of action of penicillin. J. Biol. Chem. 1959, 234, 3263–3268. [Google Scholar] [CrossRef]
- Sutton, J.A.; Carnell, O.T.; Lafage, L.; Gray, J.; Biboy, J.; Gibson, J.F.; Pollitt, E.J.; Tazoll, S.C.; Turnbull, W.; Hajdamowicz, N.H. Staphylococcus aureus cell wall structure and dynamics during host-pathogen interaction. PLoS Pathog. 2021, 17, e1009468. [Google Scholar] [CrossRef]
- Wang, M.; Buist, G.; van Dijl, J.M. Staphylococcus aureus cell wall maintenance–the multifaceted roles of peptidoglycan hydrolases in bacterial growth, fitness, and virulence. FEMS Microbiol. Rev. 2022, 46, fuac025. [Google Scholar] [CrossRef]
- Peschel, A.; Vuong, C.; Otto, M.; Götz, F. The d-Alanine Residues ofStaphylococcus aureus Teichoic Acids Alter the Susceptibility to Vancomycin and the Activity of Autolytic Enzymes. Antimicrob. Agents Chemother. 2000, 44, 2845–2847. [Google Scholar] [CrossRef] [PubMed]
- Weidenmaier, C.; Peschel, A.; Kempf, V.A.; Lucindo, N.; Yeaman, M.R.; Bayer, A.S. DltABCD-and MprF-mediated cell envelope modifications of Staphylococcus aureus confer resistance to platelet microbicidal proteins and contribute to virulence in a rabbit endocarditis model. Infect. Immun. 2005, 73, 8033–8038. [Google Scholar] [PubMed]
- Cooper, G.M. The Cell, 2nd ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2000. [Google Scholar]
- Rehal, R.P.; Marbach, H.; Hubbard, A.T.; Sacranie, A.A.; Sebastiani, F.; Fragneto, G.; Harvey, R.D. The influence of mild acidity on lysyl-phosphatidylglycerol biosynthesis and lipid membrane physico-chemical properties in methicillin-resistant Staphylococcus aureus. Chem. Phys. Lipids 2017, 206, 60–70. [Google Scholar] [PubMed]
- Hernandez-Villa, L.; Manrique-Moreno, M.; Leidy, C.; Jemiola-Rzeminska, M.; Ortiz, C.; Strzalka, K. Biophysical evaluation of cardiolipin content as a regulator of the membrane lytic effect of antimicrobial peptides. Biophys. Chem. 2018, 238, 8–15. [Google Scholar]
- Epand, R.M.; Hui, S.-W. Effect of electrostatic repulsion on the morphology and thermotropic transitions of anionic phospholipids. FEBS Lett. 1986, 209, 257–260. [Google Scholar]
- Prossnigg, F.; Hickel, A.; Pabst, G.; Lohner, K. Packing behaviour of two predominant anionic phospholipids of bacterial cytoplasmic membranes. Biophys. Chem. 2010, 150, 129–135. [Google Scholar]
- Nishi, H.; Komatsuzawa, H.; Fujiwara, T.; McCallum, N.; Sugai, M. Reduced content of lysyl-phosphatidylglycerol in the cytoplasmic membrane affects susceptibility to moenomycin, as well as vancomycin, gentamicin, and antimicrobial peptides, in Staphylococcus aureus. Antimicrob. Agents Chemother. 2004, 48, 4800–4807. [Google Scholar]
- Koprivnjak, T.; Zhang, D.; Ernst, C.; Peschel, A.; Nauseef, W.; Weiss, J. Characterization of Staphylococcus aureus cardiolipin synthases 1 and 2 and their contribution to accumulation of cardiolipin in stationary phase and within phagocytes. J. Bacteriol. 2011, 193, 4134–4142. [Google Scholar]
- Ohniwa, R.L.; Kitabayashi, K.; Morikawa, K. Alternative cardiolipin synthase Cls1 compensates for stalled Cls2 function in Staphylococcus aureus under conditions of acute acid stress. Fems Microbiol. Lett. 2013, 338, 141–146. [Google Scholar]
- Short, S.A.; White, D.C. Metabolism of phosphatidylglycerol, lysylphosphatidylglycerol, and cardiolipin of Staphylococcus aureus. J. Bacteriol. 1971, 108, 219–226. [Google Scholar]
- Yeo, W.-S.; Dyzenhaus, S.; Torres, V.J.; Brinsmade, S.R.; Bae, T. Regulation of Bacterial Two-Component Systems by Cardiolipin. Infect. Immun. 2023, 91, e00046-23. [Google Scholar] [CrossRef] [PubMed]
- Clauditz, A.; Resch, A.; Wieland, K.-P.; Peschel, A.; Götz, F. Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect. Immun. 2006, 74, 4950–4953. [Google Scholar] [CrossRef]
- López, G.-D.; Suesca, E.; Álvarez-Rivera, G.; Rosato, A.E.; Ibáñez, E.; Cifuentes, A.; Leidy, C.; Carazzone, C. Carotenogenesis of Staphylococcus aureus: New insights and impact on membrane biophysical properties. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2021, 1866, 158941. [Google Scholar]
- Múnera-Jaramillo, J.; López, G.-D.; Suesca, E.; Carazzone, C.; Leidy, C.; Manrique-Moreno, M. The role of staphyloxanthin in the regulation of membrane biophysical properties in Staphylococcus aureus. Biochim. Biophys. Acta (BBA)-Biomembr. 2024, 1866, 184288. [Google Scholar]
- Seel, W.; Baust, D.; Sons, D.; Albers, M.; Etzbach, L.; Fuss, J.; Lipski, A. Carotenoids are used as regulators for membrane fluidity by Staphylococcus xylosus. Sci. Rep. 2020, 10, 330. [Google Scholar]
- Campbell, A.E.; McCready-Vangi, A.R.; Uberoi, A.; Murga-Garrido, S.M.; Lovins, V.M.; White, E.K.; Pan, J.T.-C.; Knight, S.A.; Morgenstern, A.R.; Bianco, C. Variable staphyloxanthin production by Staphylococcus aureus drives strain-dependent effects on diabetic wound-healing outcomes. Cell Rep. 2023, 42, 113281. [Google Scholar]
- Gaby, W.; Naughten, R.; Logan, C. The role of phospholipides in the metabolism of amino acids by the mold Penicillium chrysogenum. Arch. Biochem. Biophys. 1959, 82, 34–41. [Google Scholar]
- Gaby, W.; Wolin, H.; Zajac, I. The role of phospholipides in the uptake of amino acids by Ehrlich ascites carcinoma cells. Cancer Res. 1960, 20, 1508–1513. [Google Scholar]
- Hunter, G.; Goodsall, R. Lipo-amino acid complexes from Bacillus megaterium and their possible role in protein synthesis. Biochem. J. 1961, 78, 564. [Google Scholar] [CrossRef]
- Macfarlane, M. Lipid components of Staphylococcus aureus and Salmonella typhimurium. Biochem. J. 1962, 82, 40p. [Google Scholar]
- Macfarlane, M.G. Characterization of lipoamino-acids as O-amino-acid esters of phosphatidyl-glycerol. Nature 1962, 196, 136–138. [Google Scholar] [CrossRef]
- Houtsmuller, U.; Van Deenen, L. Identification of a bacterial phospholipid as an O-ornithine ester of phosphatidyl glycerol. Biochim. Biophys. Acta 1963, 70, 211–213. [Google Scholar] [CrossRef][Green Version]
- Houtsmuller, U.; Van Deenen, L. On the accumulation of amino acid derivates of phosphatidylglycerol in bacteria. Biochim. Biophys. Acta (BBA)-Spec. Sect. Lipids Relat. Subj. 1964, 84, 96–98. [Google Scholar] [CrossRef][Green Version]
- Nesbitt, J.A.; Lennarz, W.J. Participation of Aminoacyl Transfer Ribonucleic Acid in Aminoacyl Phosphatidylglycerol Synthesis. J. Biol. Chem. 1968, 243, 3088–3095. [Google Scholar] [CrossRef] [PubMed]
- Gould, R.M.; Lennarz, W. Metabolism of phosphatidylglycerol and lysyl phosphatidylglycerol in Staphylococcus aureus. J. Bacteriol. 1970, 104, 1135–1144. [Google Scholar] [CrossRef]
- Houtsmuller, U.; Van Deenen, L. On the amino acid esters of phosphatidyl glycerol from bacteria. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1965, 106, 564–576. [Google Scholar] [CrossRef]
- Lennarz, W.; Nesbitt, J., 3rd; Reiss, J. The participation of sRNA in the enzymatic synthesis of OL-lysyl phosphatidylgylcerol in Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 1966, 55, 934–941. [Google Scholar] [CrossRef]
- Gould, R.M.; Lennarz, W. Biosynthesis of aminoacyl derivatives of phosphatidylglycerol. Biochem. Biophys. Res. Commun. 1967, 26, 510–515. [Google Scholar] [CrossRef]
- Nahaie, M.; Goodfellow, M.; Minnikin, D.; Hajek, V. Polar lipid and isoprenoid quinone composition in the classification of Staphylococcus. Microbiology 1984, 130, 2427–2437. [Google Scholar] [CrossRef]
- Roy, H. Tuning the properties of the bacterial membrane with aminoacylated phosphatidylglycerol. IUBMB Life 2009, 61, 940–953. [Google Scholar] [CrossRef]
- Roy, H.; Dare, K.; Ibba, M. Adaptation of the bacterial membrane to changing environments using aminoacylated phospholipids. Mol. Microbiol. 2009, 71, 547–550. [Google Scholar] [CrossRef]
- Dare, K.; Shepherd, J.; Roy, H.; Seveau, S.; Ibba, M. LysPGS formation in Listeria monocytogenes has broad roles in maintaining membrane integrity beyond antimicrobial peptide resistance. Virulence 2014, 5, 534–546. [Google Scholar]
- Slavetinsky, C.; Kuhn, S.; Peschel, A. Bacterial aminoacyl phospholipids–Biosynthesis and role in basic cellular processes and pathogenicity. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2017, 1862, 1310–1318. [Google Scholar] [CrossRef]
- Sohlenkamp, C.; Geiger, O. Bacterial membrane lipids: Diversity in structures and pathways. FEMS Microbiol. Rev. 2016, 40, 133–159. [Google Scholar] [PubMed]
- Fischer, W.; Leopold, K. Polar lipids of four Listeria species containing L-lysylcardiolipin, a novel lipid structure, and other unique phospholipids. Int. J. Syst. Evol. Microbiol. 1999, 49, 653–662. [Google Scholar]
- Den Kamp, J.O.; Houtsmuller, U.; Van Dehnen, L. On the phospholipids of Bacillus megaterium. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1965, 106, 438–441. [Google Scholar]
- Kocun, F. Amino acid containing phospholipids as major components of the phospholipids of Streptococcus faecalis 10C1. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1970, 202, 277–282. [Google Scholar]
- Kenward, M.; Brown, M.; Fryer, J. The influence of calcium or manganese on the resistance to EDTA, polymyxin B or cold shock, and the composition of Pseudomonas aeruginosa grown in glucose-or magnesium-depleted batch cultures. J. Appl. Bacteriol. 1979, 47, 489–503. [Google Scholar]
- Maloney, E.; Stankowska, D.; Zhang, J.; Fol, M.; Cheng, Q.-J.; Lun, S.; Bishai, W.R.; Rajagopalan, M.; Chatterjee, D.; Madiraju, M.V. The two-domain LysX protein of Mycobacterium tuberculosis is required for production of lysinylated phosphatidylglycerol and resistance to cationic antimicrobial peptides. PLoS Pathog. 2009, 5, e1000534. [Google Scholar]
- Mishra, N.N.; Yang, S.-J.; Sawa, A.; Rubio, A.; Nast, C.C.; Yeaman, M.R.; Bayer, A.S. Analysis of cell membrane characteristics of in vitro-selected daptomycin-resistant strains of methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2009, 53, 2312–2318. [Google Scholar] [CrossRef]
- Jones, T.; Yeaman, M.R.; Sakoulas, G.; Yang, S.-J.; Proctor, R.A.; Sahl, H.-G.; Schrenzel, J.; Xiong, Y.Q.; Bayer, A.S. Failures in clinical treatment of Staphylococcus aureus infection with daptomycin are associated with alterations in surface charge, membrane phospholipid asymmetry, and drug binding. Antimicrob. Agents Chemother. 2008, 52, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Roy, H.; Ibba, M. RNA-dependent lipid remodeling by bacterial multiple peptide resistance factors. Proc. Natl. Acad. Sci. USA 2008, 105, 4667–4672. [Google Scholar] [CrossRef]
- Roy, H.; Ibba, M. Broad range amino acid specificity of RNA-dependent lipid remodeling by multiple peptide resistance factors. J. Biol. Chem. 2009, 284, 29677–29683. [Google Scholar] [CrossRef] [PubMed]
- Řezanka, T.; Kambourova, M.; Derekova, A.; Kolouchová, I.; Sigler, K. LC–ESI–MS/MS identification of polar lipids of two thermophilic Anoxybacillus bacteria containing a unique lipid pattern. Lipids 2012, 47, 729–739. [Google Scholar] [CrossRef]
- Groenewold, M.K.; Hebecker, S.; Fritz, C.; Czolkoss, S.; Wiesselmann, M.; Heinz, D.W.; Jahn, D.; Narberhaus, F.; Aktas, M.; Moser, J. Virulence of Agrobacterium tumefaciens requires lipid homeostasis mediated by the lysyl-phosphatidylglycerol hydrolase AcvB. Mol. Microbiol. 2019, 111, 269–286. [Google Scholar] [CrossRef]
- Samant, S.; Hsu, F.-F.; Neyfakh, A.A.; Lee, H. The Bacillus anthracis protein MprF is required for synthesis of lysylphosphatidylglycerols and for resistance to cationic antimicrobial peptides. J. Bacteriol. 2009, 191, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Arendt, W.; Hebecker, S.; Jäger, S.; Nimtz, M.; Moser, J. Resistance phenotypes mediated by aminoacyl-phosphatidylglycerol synthases. J. Bacteriol. 2012, 194, 1401–1416. [Google Scholar] [CrossRef]
- Den Kamp, J.O.; Van Iterson, W.; Van Deenen, L. Studies on the phospholipids and morphology of protoplasts of Bacillus megaterium. Biochim. Biophys. Acta (BBA)-Biomembr. 1967, 135, 862–884. [Google Scholar] [CrossRef]
- Sumpavapol, P.; Tongyonk, L.; Tanasupawat, S.; Chokesajjawatee, N.; Luxananil, P.; Visessanguan, W. Bacillus siamensis sp. nov. isolated from salted crab (poo-khem) in Thailand. Int. J. Syst. Evol. Microbiol. 2010, 60, 2364–2370. [Google Scholar] [CrossRef]
- Den Kamp, J.O.; Redai, I.; Van Deenen, L. Phospholipid composition of Bacillus subtilis. J. Bacteriol. 1969, 99, 298–303. [Google Scholar] [CrossRef]
- Lopez, C.S.; Alice, A.F.; Heras, H.; Rivas, E.A.; Sanchez-Rivas, C. Role of anionic phospholipids in the adaptation of Bacillus subtilis to high salinity. Microbiology 2006, 152, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Atila, M.; Luo, Y. Profiling and tandem mass spectrometry analysis of aminoacylated phospholipids in Bacillus subtilis. F1000Research 2016, 5, 121. [Google Scholar] [CrossRef]
- Atila, M.; Katselis, G.; Chumala, P.; Luo, Y. Characterization of N-succinylation of L-lysylphosphatidylglycerol in Bacillus subtilis using tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 2016, 27, 1606–1613. [Google Scholar] [PubMed]
- Mahabadi, M. Effect of the Deuteration Conditions on the Lipid Profile of Escherichia coli and Bacillus subtilis. Master’s Thesis, Université du Québec à Montréal, Montreal, QC, Canada, 2020. [Google Scholar]
- Jones, D.E.; Smith, J.D. Phospholipids of the differentiating bacterium Caulobacter crescentus. Can. J. Biochem. 1979, 57, 424–428. [Google Scholar] [PubMed]
- Johnston, N.C.; Baker, J.K.; Goldfine, H. Phospholipids of Clostridium perfringens: A reexamination. FEMS Microbiol. Lett. 2004, 233, 65–68. [Google Scholar]
- Yoon, J.-H.; Jung, Y.-T. Cohnella boryungensis sp. nov. isolated from soil. Antonie Van Leeuwenhoek 2012, 101, 769–775. [Google Scholar]
- Mota, J.D.S.; Den Kamp, J.O.; Verheij, H.; Van Deenen, L. Phospholipids of Streptococcus faecalis. J. Bacteriol. 1970, 104, 611–619. [Google Scholar]
- Rashid, R.; Cazenave-Gassiot, A.; Gao, I.H.; Nair, Z.J.; Kumar, J.K.; Gao, L.; Kline, K.A.; Wenk, M.R. Comprehensive analysis of phospholipids and glycolipids in the opportunistic pathogen Enterococcus faecalis. PLoS ONE 2017, 12, e0175886. [Google Scholar]
- Tague, E.D.; Woodall, B.M.; Harp, J.R.; Farmer, A.T.; Fozo, E.M.; Campagna, S.R. Expanding lipidomics coverage: Effective ultra performance liquid chromatography-high resolution mass spectrometer methods for detection and quantitation of cardiolipin, phosphatidylglycerol, and lysyl-phosphatidylglycerol. Metabolomics 2019, 15, 1–10. [Google Scholar] [CrossRef]
- Exterkate, F.; Otten, B.; Wassenberg, H.; Veerkamp, J. Comparison of the phospholipid composition of Bifidobacterium and Lactobacillus strains. J. Bacteriol. 1971, 106, 824–829. [Google Scholar] [CrossRef]
- Thedieck, K.; Hain, T.; Mohamed, W.; Tindall, B.J.; Nimtz, M.; Chakraborty, T.; Wehland, J.; Jänsch, L. The MprF protein is required for lysinylation of phospholipids in listerial membranes and confers resistance to cationic antimicrobial peptides (CAMPs) on Listeria monocytogenes. Mol. Microbiol. 2006, 62, 1325–1339. [Google Scholar] [CrossRef]
- Koyama, T.; Yoshida, I.; Ogura, K. Undecaprenyl diphosphate synthase from Micrococcus luteus BP 26: Essential factors for the enzymatic activity. J. Biochem. 1988, 103, 867–871. [Google Scholar] [CrossRef]
- Sohlenkamp, C.; Galindo-Lagunas, K.A.; Guan, Z.; Vinuesa, P.; Robinson, S.; Thomas-Oates, J.; Raetz, C.R.; Geiger, O. The lipid lysyl-phosphatidylglycerol is present in membranes of Rhizobium tropici CIAT899 and confers increased resistance to polymyxin B under acidic growth conditions. Mol. Plant-Microbe Interact. 2007, 20, 1421–1430. [Google Scholar] [CrossRef]
- Hayami, M.; Okabe, A.; Kariyama, R.; Abe, M.; Kanemasa, Y. Lipid composition of Staphylococcus aureus and its derived L-forms. Microbiol. Immunol. 1979, 23, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Javed, M.A.; Deneer, H. Comparative study on nutrient depletion-induced lipidome adaptations in Staphylococcus haemolyticus and Staphylococcus epidermidis. Sci. Rep. 2018, 8, 2356. [Google Scholar]
- Joyce, L.R.; Guan, Z.; Palmer, K.L. Streptococcus pneumoniae, S. pyogenes and S. agalactiae membrane phospholipid remodelling in response to human serum. Microbiology 2021, 167, 001048. [Google Scholar]
- Fischer, W.; Arneth-Seifert, D. d-Alanylcardiolipin, a Major Component of the Unique Lipid Pattern of Vagococcus fluvialis. J. Bacteriol. 1998, 180, 2950–2957. [Google Scholar]
- Ernst, C.M.; Slavetinsky, C.J.; Kuhn, S.; Hauser, J.N.; Nega, M.; Mishra, N.N.; Gekeler, C.; Bayer, A.S.; Peschel, A. Gain-of-function mutations in the phospholipid flippase MprF confer specific daptomycin resistance. mBio 2018, 9. [Google Scholar] [CrossRef]
- Slavetinsky, C.J.; Peschel, A.; Ernst, C.M. Alanyl-phosphatidylglycerol and lysyl-phosphatidylglycerol are translocated by the same MprF flippases and have similar capacities to protect against the antibiotic daptomycin in Staphylococcus aureus. Antimicrob. Agents Chemother. 2012, 56, 3492–3497. [Google Scholar] [CrossRef]
- Koprivnjak, T.; Peschel, A.; Gelb, M.H.; Liang, N.S.; Weiss, J.P. Role of charge properties of bacterial envelope in bactericidal action of human group IIA phospholipase A2against Staphylococcus aureus. J. Biol. Chem. 2002, 277, 47636–47644. [Google Scholar] [CrossRef]
- Andrä, J.; Goldmann, T.; Ernst, C.M.; Peschel, A.; Gutsmann, T. Multiple peptide resistance factor (MprF)-mediated resistance of Staphylococcus aureus against antimicrobial peptides coincides with a modulated peptide interaction with artificial membranes comprising lysyl-phosphatidylglycerol. J. Biol. Chem. 2011, 286, 18692–18700. [Google Scholar] [CrossRef] [PubMed]
- Khatib, T.O.; Stevenson, H.; Yeaman, M.R.; Bayer, A.S.; Pokorny, A. Binding of daptomycin to anionic lipid vesicles is reduced in the presence of lysyl-phosphatidylglycerol. Antimicrob. Agents Chemother. 2016, 60, 5051–5053. [Google Scholar] [CrossRef]
- Rehal, R.; Barker, R.D.; Lu, Z.; Bui, T.T.; Demé, B.; Hause, G.; Wölk, C.; Harvey, R.D. Lipid domain formation and non-lamellar structures associated with varied lysylphosphatidylglycerol analogue content in a model Staphylococcal plasma membrane. Biochim. Biophys. Acta (BBA)-Biomembr. 2021, 1863, 183571. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.; Taylor, S.D. The impact of lysyl-phosphatidylglycerol on the interaction of daptomycin with model membranes. Org. Biomol. Chem. 2022, 20, 9319–9329. [Google Scholar] [CrossRef]
- Koch, H.U.; Haas, R.; Fischer, W. The role of lipoteichoic acid biosynthesis in membrane lipid metabolism of growing Staphylococcus aureus. Eur. J. Biochem. 1984, 138, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.B.; Yao, J.; Jackson, P.; Frank, M.; Rock, C.O. Phosphatidylglycerol homeostasis in glycerol-phosphate auxotrophs of Staphylococcus aureus. BMC Microbiol. 2013, 13, 1–9. [Google Scholar] [CrossRef]
- Bimpeh, K.; Hines, K.M. A rapid single-phase extraction for polar staphylococcal lipids. Anal. Bioanal. Chem. 2023, 415, 4591–4602. [Google Scholar] [CrossRef]
- Zamudio-Chávez, L.; Suesca, E.; López, G.-D.; Carazzone, C.; Manrique-Moreno, M.; Leidy, C. Staphylococcus aureus Modulates Carotenoid and Phospholipid Content in Response to Oxygen-Restricted Growth Conditions, Triggering Changes in Membrane Biophysical Properties. Int. J. Mol. Sci. 2023, 24, 14906. [Google Scholar] [CrossRef]
- Rocha-Roa, C.; Orjuela, J.D.; Leidy, C.; Cossio, P.; Aponte-Santamaria, C. Cardiolipin prevents pore formation in phosphatidylglycerol bacterial membrane models. FEBS Lett. 2021, 595, 2701–2714. [Google Scholar] [CrossRef]
- Wilson, B.A.; Ramanathan, A.; Lopez, C.F. Cardiolipin-dependent properties of model mitochondrial membranes from molecular simulations. Biophys. J. 2019, 117, 429–444. [Google Scholar] [CrossRef]
- Elmesseri, R.A.; Saleh, S.E.; Elsherif, H.M.; Yahia, I.S.; Aboshanab, K.M. Staphyloxanthin as a potential novel target for deciphering promising anti-Staphylococcus aureus agents. Antibiotics 2022, 11, 298. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, D.R.; Cabrera, J.E.; Delgado, J.A.M.; Miscione, G.P.; Leidy, C.; Aponte-Santamaria, C.A. Variations in the Biophysical Properties of DMPG and DPPG Membranes with Staphyloxanthin: A Molecular Dynamics Simulation Study. Biophys. J. 2021, 120, 43a. [Google Scholar]
- Perry, W.J.; Grunenwald, C.M.; Van de Plas, R.; Witten, J.C.; Martin, D.R.; Apte, S.S.; Cassat, J.E.; Pettersson, G.B.; Caprioli, R.M.; Skaar, E.P. Visualizing Staphylococcus aureus pathogenic membrane modification within the host infection environment by multimodal imaging mass spectrometry. Cell Chem. Biol. 2022, 29, 1209–1217.e4. [Google Scholar] [CrossRef] [PubMed]

| Bacteria or Species | Type | Strain | Concentration (Lysyl-PG) | Technique | Ref. |
|---|---|---|---|---|---|
| Agrobacterium tumefaciens | Gram-negative | C58 | ~1% | HPTLC and 2DTLC | [79] |
| Anoxibacillus rupiensis | Gram-positive | Is not named | 2.8–23.2% (pH 6.5) | HILIC and LC–ESI–MS/MS | [78] |
| Bacillus Anthracis | Gram-positive | Sterne 34F2 | + | 2D-TLC profiles of 32P-labeled phospholipids and MS | [80] |
| Bacillus licheniformis | Gram-positive | ATCC 14580 | 3% | 2DTLC | [81] |
| Bacillus megaterium | Gram-positive | MK 10D MK 10D | 8–14% 15–16% | Phospholipids were analyzed from extracted 32P-labeled lipids located in the chromatogram by autoradiography. | [70] [82] |
| Bacillus siamensis | Gram-positive | PD-A10T | + | TLC | [83] |
| Bacillus subtilis | Gram-positive | Marburg | 22% (pH: 7.0) 42% (pH: 5.0) | Phospholipids were analyzed from extracted 32P-labeled lipids located in the chromatogram by autoradiography. | [84] |
| SDB110, YB886 and mutants | 8–31% | HPTLC | [85] | ||
| 168 and BKE08425 | + | TLC and MS/MS | [86] | ||
| 168 | + | MS/MS | [87] | ||
| 168 | 8.5% | 31P NMR | [88] | ||
| Bacillus thuringiensis | Gram-positive | ATCC 10792 | 10% | 2DTLC | [81] |
| Caulobacter acrescentes | Gram-negative | CB13 | 10.4–11.4% | Phospholipids were analyzed from extracted 32P-labeled lipids located in the chromatogram by autoradiography. | [89] |
| Clostridium perfringens | Gram-positive | Is not named ATCC 3624 | + + | TLC and infrared spectra TLC and MS | [55] [90] |
| Cohnella boryungensis | Gram-positive | BR-29T | + | 2DTLC | [91] |
| Enterococcus faecalis | Gram-positive | 10CI | 20exp–35%stat | Incorporation of L-[14C] lysine into lipids and chromatography on Silicic acid impregnated paper | [71] |
| ATCC 9790 | + | Autoradiogram of 32P-labeled phospholipids | [92] | ||
| OG1RF, Dap21 and Dap22 | + | LC-MS/MS | [93] | ||
| OG1RF | 0.05–2.97 μM | HILIC and high-resolution MS | [94] | ||
| Lactobacillus species | Gram-positive | L. casei L.arabinosus 9K L.plantarum 9P L.acidophilus 9MB L. lactis 9T L.bulgaricus 9LB L. fermenti 9H | 27–30% 14% 23% 3% 23% 32% 14% | 2DTLC Autoradiogram of 32P-labeled phospholipids. The data are expressed as a percentage of total lipid phosphorus radioactivity. | [95] |
| Listeria innocua | Gram-positive | NCTC 11288T | 8.6%stat–12.3%exp | 2DTLC. Data are the percentage of total lipid phosphorus | [69] |
| Listeria monocytogenes | Gram-positive | NCTC 7973 | 5.4%exp | 2DTLC. Data are the percentage of total lipid phosphorus. | [69] |
| EGD-e | + | 2DTLC, ESI-MS, and GC-MS | [96] | ||
| EGD-e | 0–54% (30 °C) 1–73% (37 °C) | 2DTLC of 32P-phospholipids | [66] | ||
| Listeria seeligeri | Gram-positive | SLCC 3954T | 2.9%exp | 2DTLC. Data are the percentage of total lipid phosphorus | [69] |
| Listeria welshimeri | Gram-positive | SLCC 5334T | 10.8%exp | 2DTLC. Data are the percentage of total lipid phosphorus | [69] |
| Mammaliicoccus sciuri | Gram-positive | SCH89 SCH91 (DMS20352, DMS20345) | + | 2DTLC and MS | [63] |
| Micrococcus luteus | Gram-positive | B-P26 | + | TLC | [97] |
| Mycobacterium tuberculosis | Gram-positive | Rv-03 Rv-80lys Rv-81ami Rv-82med | + | 14C lysine-labeled lipid and TLC plates were either visualized by autoradiography, MALDI-MS, and 31P-NMR | [73] |
| Pseudomonas aeruginosa | Gram-negative | NCTC 6750 | + | Spectrophotometric techniques | [72] |
| Rhizobium tropici | Gram-negative | CIAT899 | 0.6–1.2% | 14C lysine-labeled lipid and TLC | [98] |
| Staphylococcus aureus | Gram-positive | Is not named COL, HN001 and HN002 | 1–70% 0.2–3.4% | Is not named Radiolabeling. Data expressed as percentages of total radioactivity | [57] [41] |
| L-form S. aureus | Gram-positive | Newman and Tazaki | 6.1–17.3% | 2DTLC on silica gel G plates, 32P-labeled or detected by rhodamine G6 | [99] |
| Staphylococcus capitis | Gram-positive | NCTC 11045 | + | 2DTLC and MS | [63] |
| Staphylococcus cohnii | Gram-positive | NCTC 11041 | + | 2DTLC and MS | [63] |
| Staphylococcus epidermidis | Gram-positive | NCTC 11047 Skin isolated | + +exp | 2DTLC and MS TLC and MS/MS | [63] [100] |
| Staphylococcus haemolyticus | Gram-positive | Skin isolated NCTC 11042 | + +exp | 2DTLC and MS TLC and MS/MS | [63] [100] |
| Staphylococcus hominis | Gram-positive | NCTC 11320 | + | 2DTLC and MS | [63] |
| Staphylococcus hyicus | Gram-positive | NCTC 10530 | + | 2DTLC and MS | [63] |
| Staphylococcus intermedius | Gram-positive | NCTC 11048 | + | 2DTLC and MS | [63] |
| Staphylococcus saprophyticus | Gram-positive | SCH94 and SCH95 | + | 2DTLC and MS | [63] |
| Staphylococcus simulans | Gram-positive | NCTC 11046 | + | 2DTLC and MS | [63] |
| Staphylococcus warneri | Gram-positive | NCTC 11044 | + | 2DTLC and MS | [63] |
| Staphylococcus xylosus | Gram-positive | NCTC 11043 | + | 2DTLC and MS | [63] |
| Streptococcus agalactiae | Gram-positive | COH1A909 | + | NPLC-ESI/MS | [101] |
| Streptococcus thermophilus | Gram-positive | ATCC 19258 | 10% | 2DTLC | [81] |
| Vagococcus fluvialis | Gram-positive | NCDO 2497 | 10.1%stat–21.2%exp | HPLC and TLC. Abundance (mol%) at phase | [102] |
| Lipid Model | Strain | Composition | Antimicrobial Agent | Sequence | Result | Charge | Ref. |
|---|---|---|---|---|---|---|---|
| Liposomes | Wild-type: Newman and Sa113 | Bacterial extracts from wild-type strains have lysyl-PG concentrations up to 38% | Defensin HNP-1 * | ACYCRIPACIAGERRYGTCIYQGRLWAFCC (Cys2-Cys30,Cys4-Cys19,Cys9-Cys29) | The mutant strains were significantly more susceptible to a broad range of antimicrobial agents, while the neutral gramicidin D exhibited equal activity against both wild-type and mutant strains. Enhanced binding of mutant cells to a tachyplesin 1 and gallidermin further demonstrated that lysyl-PG reduced the attraction and binding of antimicrobial agents. | +3 | [15] |
| Protegrins 3 | RGGGLCYCRRRFCVCVGR-NH2 (Cys6-Cys15,Cys8-Cys13) | +6 | |||||
| Protegrins 5 | RGGRLCYCRPRFCVCVGR-NH2 (Cys6-Cys15,Cys8-Cys13) | +5 | |||||
| Tachyplesin 1 | KWCFRVCYRGICYRRCR-NH2 (Cys3-Cys16,Cys7-Cys12) | +6 | |||||
| Gallidermin | 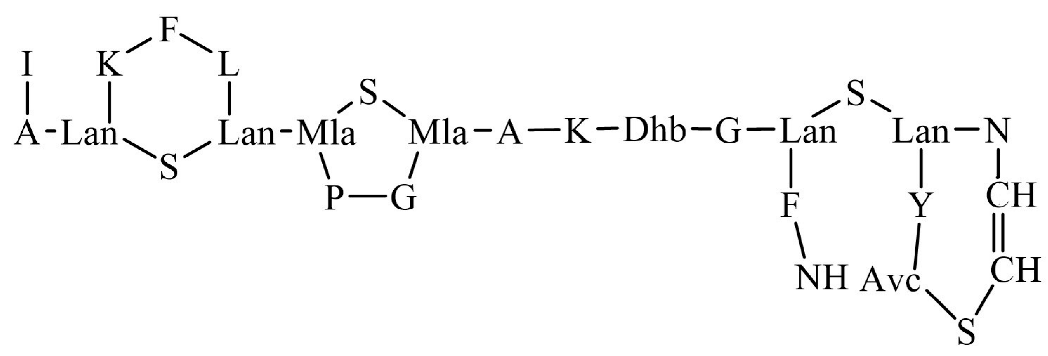 | - | |||||
| Mutants: pBR473 and pRBmprF | Mutant strains have 0% lysyl-PG | Nisin | 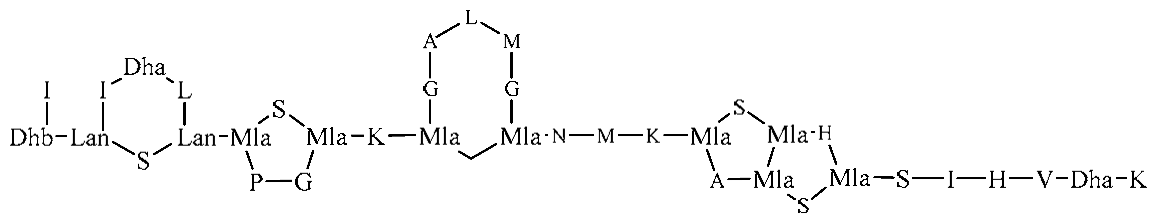 | - | |||
| Magainin II | GIGKFLHAAKKFAKAFVAEIMNS-NH2 | +4 | |||||
| Melittin | GIGAVLKVLTTGLPALISWIKRKRQQ-NH2 | +5 | |||||
| Gramicidin S |  | - | |||||
| Gramicidin D | HCO-VGADLADVWDLWDLW-NHCH2CH2OH | 0 | |||||
| Vesicles | Wild-type: SA113 mutants mprF− dltA− | Bacterial extracts from wild-type and dltA strains contain lysyl-PG, while the mprF− mutant does not produce lysyl-PG | Human Group IIA Phospholipase A2 | Mutations affecting the charge properties of the bacterial envelope had a significant impact on PLA2 activity. For instance, dltA− deficient mutants, unable to modify teichoic acids with D-alanine, exhibited a sensitivity to PLA2 that was 30 to 100 times greater than that of the parental strain. In contrast, mprF-deficient bacteria, which lacked lysyl-phosphatidylglycerol synthesis, displayed only a modest increase in susceptibility to PLA2, with a fold change of no more than three (≤3-fold) compared to the wild-type S. aureus strain. These findings highlight the distinct roles of dltA− and mprF− in modulating bacterial envelope properties and their differential effects on resistance to PLA2. | Ranging from +12 to +17 | [105] | |
| Is not named | Wild-type: COL Mutants: HN001 HN002 | Bacterial extract lysyl-PG:PG:GL:CL 3.4:90:5.6:1 0.2:89.7:7.3:2.8 1.3:89.0:7.0:2.7 | Moenomycin |  | The mutants showed slightly decreased susceptibility to moenomycin and vancomycin, they were more vulnerable to positively charged antimicrobial agents (e.g., α-defensins and CAP18) due to the increased negative charge of their membranes. | −1 | [41] |
| Vancomycin |  | +1 | |||||
| hBD3 | IINTLQKYYCRVRGGRCAVLSCLPKEEQIGKCSTRGRKCCRRKK | +11 | |||||
| CAP18 (Precursor of LL-37) | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPR | +7 | |||||
| Synthetic LUVs | - | POPG:POPC:Lysyl-DOPG 70:30:0 50:30:20 40:30:30 30:30:40 | 6W-RP-1 | ALYKKWKKKLLKSLKRLG | The presence of lysyl-PG significantly inhibited dye leakage induced by the antimicrobial peptide 6W-RP-1. Additionally, the authors suggested that the cationic peptide preferentially interacted with anionic PG, leading to the formation of PG-enriched lipid domains and the exclusion of cationic lysyl-PG. | +8 | [19] |
| LUVs and planar bilayers | Clinical isolate wild-type: Sa113 (ATCC 35556) Mutants: SA113-derived (ΔmprF), and ΔmprF-derived (ΔmprFpRBsyn) | PE:DOPG:Lysyl-DOPG 50:0:50 0:100:0 0:50:50 50:25:25 | NK-2 | KILRGVCKKIMRTFLRRISKDILTGKK-NH2 | The presence of lysyl-PG in the membrane significantly reduced the effectiveness of the cAMPs. The mutant strains lacking lysyl-PG were more susceptible to the tested peptides compared to the wild-type strain. NK-2 increased the acyl chain order in lysyl-PG-containing membranes, indicating a rigidifying effect, while it had a fluidizing effect in PG-only membranes. | +10 | [106] |
| C7A | KILRGVAKKIMRTFLRRISKDILTGKK-NH2 | +10 | |||||
| C7S/NK27 | KILRGVSKKIMRTFLRRISKDILTGKK-NH2 | +10 | |||||
| NK23a | KISKKIMRTFLRRISKDILTGKK-NH2 | +9 | |||||
| NK23b | KILRGVSKKIMRRISKDILTGKK-NH2 | +9 | |||||
| NK23c | KILRGVSKKIMRTFLRRILTGKK-NH2 | +10 | |||||
| NK11 | KISKRILTGKK-NH2 | +6 | |||||
| Melittin | GIGAVLKVLTTGLPALISWIKRKRQQ-NH2 | +5 | |||||
| Ar-1 | RWCVYAYVRVRGVLVRYRRCW-OH | +6 | |||||
| C/S-Ar-1, | RWSVYAYVRVRGVLVRYRRSW-OH | +6 | |||||
| R/K-Ar-1 | KWCVYAYVKVKGVLVKYKKCW-OH | +6 | |||||
| Synthetic LUVs | - | POPC:POPG:lysyl-PG 70:30:0 70:10:20 | Daptomycin |  | The presence of lysyl-PG in lipid vesicles moderately reduced the binding affinity of daptomycin. The reduction in daptomycin binding affinity was linked to a decrease in the electrostatic contribution to the Gibbs free energy of binding, caused by the cationic nature of lysyl-PG. This indicated that the interaction between daptomycin and the lipid bilayer was affected by the overall charge distribution in the membrane. | −2 | [107] |
| Lipid extract monolayer | 476 | PG:lysyl-PG:CL 43:51:6 | Magainin 2 F5W | GIGKWLHSAKKFGKAFVGEIMNS | Lysyl-PG biosynthesis in S. aureus increased significantly under mildly acidic conditions. This modification helped to neutralize the membrane’s surface charge and increased membrane rigidity, enhancing stability under stress. The ionization of the headgroup amine of lysyl-PG at acidic pH reduced interactions between antimicrobial peptides and the membrane, providing protection against lysis. | +3 | [37] |
| 62:33:4 | |||||||
| MRSA G32 | 40:52:8 | ||||||
| 67:28:5 | |||||||
| MRSA G33 | 66:28:6 | ||||||
| 58:34:9 | |||||||
| MRSA H64 | 50:44:6 | ||||||
| 64:33:4 | |||||||
| MRSA H66 | 40:55:5 | ||||||
| 61:34:5 | |||||||
| Synthetic Monolayer and vesicles | - | Molar ratios: DPPG:DP3adLPG:TMCL 67:28:5 41:51:8 d62DPPG/d62DP3adLPG 70:30 45:55 | Magainin 2 F5W | GIGKWLHSAKKFGKAFVGEIMNS | The presence of lysyl-PG in the lipid bilayer altered the membrane’s charge characteristics, making it predominantly zwitterionic at neutral pH and cationic in mildly acidic conditions, which reduced the peptide’s ability to adopt its active α-helical conformation necessary for membrane disruption. | +3 | [16] |
| Synthetic Liposomes | - | DPPG:DP3adLPG 70:30 60:40 55:45 40:60 30:70 DPPC:DPPG 75:25 | Magainin 2 F5W | GIGKWLHSAKKFGKAFVGEIMNS | Magainin 2 F5W showed a higher binding affinity and α-helical content in DPPG-rich environments, indicating that anionic lipids enhanced its interaction. The formation of distinct lamellar and non-lamellar phases in these mixtures further altered membrane properties, influencing the peptide’s mechanism. | +3 | [108] |
| Synthetic LUVs | - | DMPC:Lysyl-DMPG 75:25 DMPC:DMPG 75:25 DMPG:DMPC:Lysyl-DMPC 12.5:0:87.5 12.5:6.25:81.25 12.5:12.5:75 12.5:25:62.5 12.5:18.75: 68.75 0:25:75 25:25:50 25: 0:75 | Daptomycin | 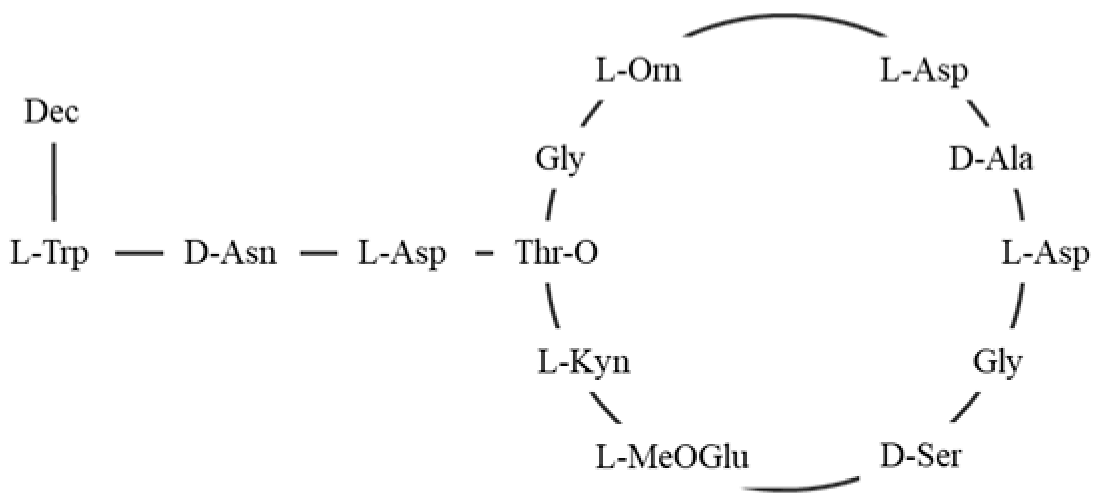 | The findings suggested that lysyl-PG could affect the oligomerization of daptomycin, potentially altering its antibacterial activity, indicating that while lysyl-PG could mask the binding sites for daptomycin, the overall impact on membrane fluidity and the structural conformation of daptomycin remained marginal. | −2 | [109] |
| Strain | Conditions | Lipid Composition | Technique | Results and Notes | Ref. | ||
|---|---|---|---|---|---|---|---|
| PG | Lysyl-PG | CL | |||||
| Is not named | I: pH 4.8 F: pH 7.2 Glucose + 16 h, 37 °C | 50% | 38% | 12% | The percentage of phospholipids was measured by analysis of phospholipid composition in bacterial cultures and TLC. Data are expressed as a percentage of total lipids. The percentages of phospholipids were extracted using PlotDigitizer, as the scientific article does not directly provide the values. | The amount of lysyl-PG increased significantly when glucose was present, while the relative amount of PG decreased. | [57] |
| I: pH 7.2 F: pH 7.2 Glucose + 16 h 37 °C The pH was adjusted to 7.2 and incubated again for 3 h | 79% | 13% | 9% | ||||
| I: pH 7.2 F: pH 7.4 Glucose – 16 h, 37 °C | 93% | 4% | 3% | ||||
| I: pH 7.2 F: pH 4.8 Glucose − Incubated for more 3 h | 35% | 58% | 6% | ||||
| pH range: 7.2–6.2 Glucose + 12 h | 63% | 22% | 17% | ||||
| pH range: 5.2–4.8 Glucose - | 21% | 70% | 11% | ||||
| PS 187 (NCTC 9754) | pH: 4.7 A | 30.5% | 67.5% | - | Radiolabeled lipids and chromatography. Cells harvested at pH 4.7 (A) and 7.2 (B) were extracted according to the procedure of Bligh and Dyer. Data expressed as a percentage of total lipids and the values are the means of six experiments and are expressed as mg lipid per g of lyophilized cells. | The phospholipid composition varied significantly with changes in the pH of the culture medium. Specifically, CL was observed to accumulate at low pH values, while the percentages of PG and lysyl-PG changed ostensibly with pH adjustments. | [60] |
| pH: 6.3 A | 44.0% | 54.0% | - | ||||
| pH: 7.0 A | 72.5% | 25.5% | - | ||||
| pH: 4.7 B | 89.0% | 9.0% | - | ||||
| pH: 6.3 B | 90.0% | 8.0% | - | ||||
| pH: 7.0 B | 94.0% | 4.0% | - | ||||
| pH: 7.2 | 19.3 mg | 3.8 mg | 0.5 mg | ||||
| pH: 4.8 | 3.2 mg | 5.8 mg | 3.0 mg | ||||
| Newman | L-forms were grown in brain heart infusion broth (Difco) supplemented with 5% NaCl and 10% horse serum on a reciprocal shaker at 37 °C. | 43.1 | 30.1 | 22.5 | Two-dimensional chromatography on silica gel G plates. Data expressed as percentages of phosphorus in total phospholipids. | The analysis revealed that lysyl-PG was identified as the primary aminoacyl-PG in S. aureus, with lysine being the only amino acid detected in this lipid fraction. The L-forms showed a decrease in lysyl-PG and PG content, and a significant increase in CL content, which exceeded 50% of the total phospholipid phosphorus, compared to less than 25% in the parental strains. | [99] |
| Newman Lf | 26.1 | 17.3 | 53.9 | ||||
| Tazaki | 43.1 | 35.1 | 17.9 | ||||
| Tazaki Lf | 11.8 | 6.1 | 78.6 | ||||
| DSM 346 | This involved the labeling of logarithmically growing bacteria with 44 µM [14C] acetate. | 50.4% | 9.9% | 1.1% | Data expressed as %mol. The mole percentage of these lipids was determined under different labeling conditions, revealing their relative abundance in the bacterial membrane. | The lipid composition in S. aureus showed that PG, lysyl-PG, and bisphosphatidylglycerol (CL) were among the key components. The type of radioactive labeling did not affect the quantification of lysyl-PG. | [110] |
| This involved the labeling of the bacteria with 5 mM [2–3H] glycerol. | 54.5% | 10.5% | 0.7% | ||||
| COL (wild-type) | S. aureus strains were grown in trypticase soy broth. | 90% | 3.4% | 1.0% | Radiolabeling with [2–3H] glycerol. Data expressed as percentages of total radioactivity. | The fmtC gene played a crucial role in the synthesis of lysyl-PG. When this gene is disrupted, as observed in mutant HN001, the production of lysyl-PG is significantly reduced. Similarly, the lysC gene is involved in the biosynthesis of lysine, which serves as a precursor for lysyl-PG. A mutation in this gene, as seen in mutant HN002, also leads to decreased levels of lysine available for lysyl-PG synthesis, further contributing to the reduction in lysyl-PG in the membrane. | [41] |
| HN001 mutant (fmtC) | 89.7% | 0.2% | 2.8 | ||||
| HN002 mutant (lysC:) | 89.0% | 1.3% | 2.7 | ||||
| PDJ28 (ΔgpsA) | With glycerol | 55.0 | 23.2 | <1 | TLC, lipid mass spectrometry, and radiolabeling. Data expressed as percentages of total 14C-label. | Removal of glycerol from the growth medium led to the rapid cessation of phospholipid synthesis. | [111] |
| Without glycerol | 28.4 | 18.4 | 12.5 | ||||
| 476 | pH: 5.5 | 43% | 51% | 6% | 31P NMR. Data expressed as a percentage of total phospholipid content. | Mildly acidic conditions significantly stimulate the biosynthesis of lysyl-PG in S. aureus. This study found that under these conditions, the proportion of lysyl-PG in the total phospholipid content increased markedly, with some strains showing levels as high as 50% of total phospholipids. | [37] |
| pH: 7.4 | 62% | 33% | 4% | ||||
| MRSA G32 | pH: 5.5 | 40% | 52% | 8% | |||
| pH: 7.4 | 67% | 28% | 5% | ||||
| MRSA G33 | pH: 5.5 | 66% | 28% | 6% | |||
| pH: 7.4 | 58% | 34% | 9% | ||||
| MRSA H64 | pH: 5.5 | 50% | 44% | 6% | |||
| pH: 7.4 | 64% | 33% | 4% | ||||
| MRSA H66 | pH: 5.5 | 40% | 55% | 5% | |||
| pH: 7.4 | 61% | 34% | 5% | ||||
| JE2 (NR-4653) and JE2-Dap2 | Bligh and Dyer | 13.07 µM | 0.10 µM | - | HILIC and MS. Data expressed in µM. | This study found that the addition of 0.5% (v/v) acetic acid during the extraction process led to a twofold increase in the total yields of lysyl-PG across all species analyzed. | [112] |
| Methanol/acetonitrile/water, method developed for the recovery of lipids from bacteria | 25.48 µM | 0.18 µM | - | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vásquez, A.; Leidy, C.; Manrique-Moreno, M. Lysyl-Phosphatidylglycerol: A Lipid Involved in the Resistance of Staphylococcus aureus to Antimicrobial Peptide Activity. Antibiotics 2025, 14, 349. https://doi.org/10.3390/antibiotics14040349
Vásquez A, Leidy C, Manrique-Moreno M. Lysyl-Phosphatidylglycerol: A Lipid Involved in the Resistance of Staphylococcus aureus to Antimicrobial Peptide Activity. Antibiotics. 2025; 14(4):349. https://doi.org/10.3390/antibiotics14040349
Chicago/Turabian StyleVásquez, Andrea, Chad Leidy, and Marcela Manrique-Moreno. 2025. "Lysyl-Phosphatidylglycerol: A Lipid Involved in the Resistance of Staphylococcus aureus to Antimicrobial Peptide Activity" Antibiotics 14, no. 4: 349. https://doi.org/10.3390/antibiotics14040349
APA StyleVásquez, A., Leidy, C., & Manrique-Moreno, M. (2025). Lysyl-Phosphatidylglycerol: A Lipid Involved in the Resistance of Staphylococcus aureus to Antimicrobial Peptide Activity. Antibiotics, 14(4), 349. https://doi.org/10.3390/antibiotics14040349








