Emergence and Clonal Spread of Extended-Spectrum β-Lactamase-Producing Salmonella Infantis Carrying pESI Megaplasmids in Korean Retail Poultry Meat
Abstract
:1. Introduction
2. Results
2.1. Prevalence, Serotype, and Sequence Type Distribution of Salmonella in Poultry Meats
2.2. Comparisons of AMR Profiles of Salmonella enterica Isolates from Chicken and Duck Meat
2.3. ESBL-Producing Salmonella
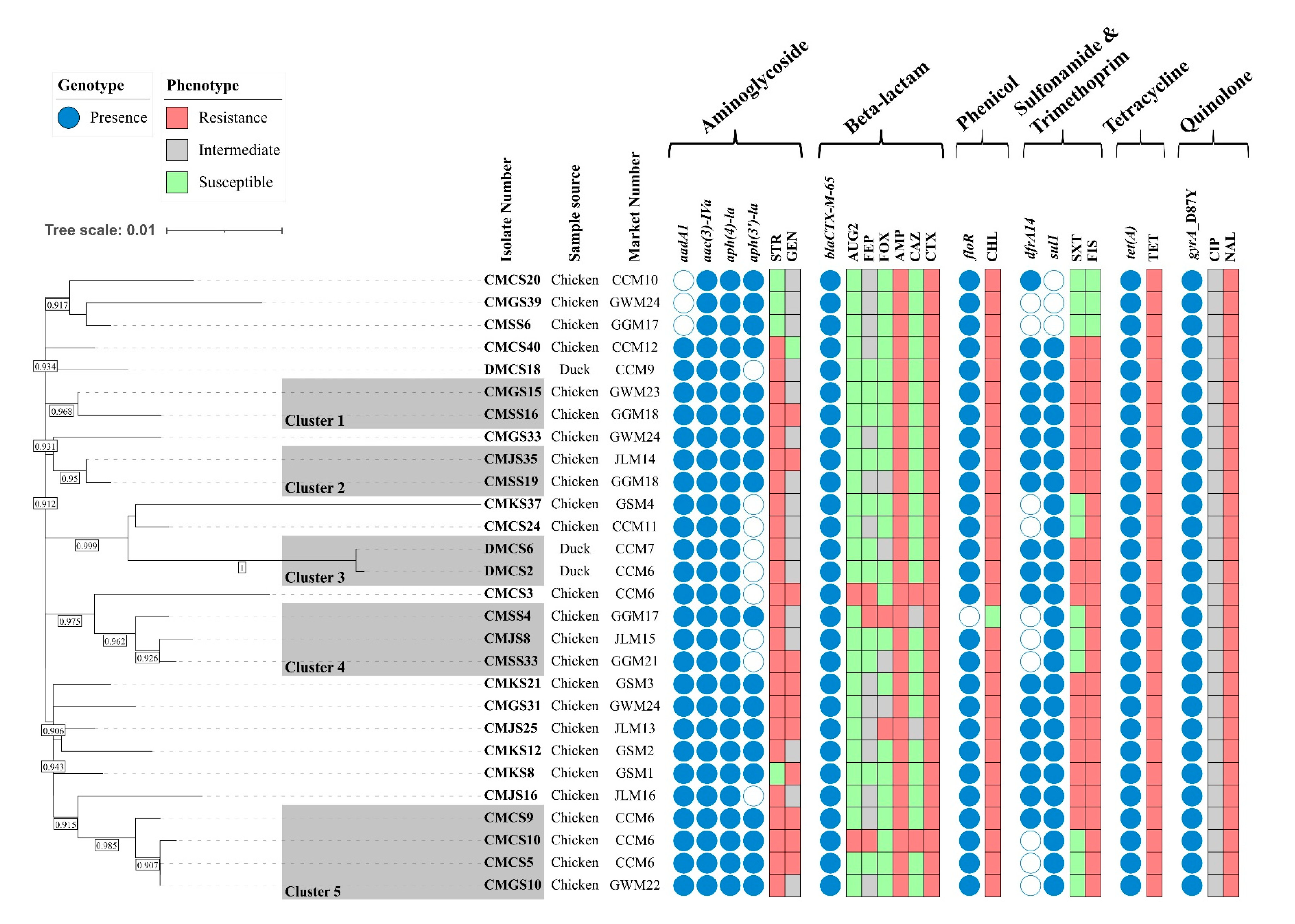
2.4. Genomic Characteristics of ESBL-Producing S. Infantis
2.5. Characterization of pESI-like Plasmids
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Isolation and Identification of Salmonella spp.
4.3. Serotyping and Multi-Locus Sequence Typing
4.4. Antimicrobial Susceptibility Test
4.5. Characterization of the β-Lactamase-Encoding Genes
4.6. Whole-Genome Sequencing Analysis
4.7. Detection and Reconstruction of pESI-like Plasmids
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeng, Y.B.; Xiong, L.G.; Tan, M.F.; Li, H.Q.; Yan, H.; Zhang, L.; Yin, D.F.; Kang, Z.F.; Wei, Q.P.; Luo, L.G. Prevalence and Antimicrobial Resistance of Salmonella in Pork, Chicken, and Duck from Retail Markets of China. Foodborne Pathog. Dis. 2019, 16, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Chlebicz, A.; Śliżewska, K. Campylobacteriosis, Salmonellosis, Yersiniosis, and Listeriosis as Zoonotic Foodborne Diseases: A Review. Int. J. Environ. Res. Public Health 2018, 15, 863. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Food and Drug Administration. Food poisoning statistics. Available online: https://www.foodsafetykorea.go.kr/portal/healthyfoodlife/foodPoisoningStat.do?menu_no=4425&menu_grp=MENU_NEW02 (accessed on 19 January 2024).
- Lamichhane, B.; Mawad, A.M.M.; Saleh, M.; Kelley, W.G.; Harrington, P.J., 2nd; Lovestad, C.W.; Amezcua, J.; Sarhan, M.M.; El Zowalaty, M.E.; Ramadan, H.; et al. Salmonellosis: An Overview of Epidemiology, Pathogenesis, and Innovative Approaches to Mitigate the Antimicrobial Resistant Infections. Antibiotics 2024, 13, 76. [Google Scholar] [CrossRef]
- Naushad, S.; Huang, H.; Ogunremi, D. Salmonella: A Brief Review. In Salmonella—Perspectives for Low-Cost Prevention, Control and Treatment; Huang, H., Naushad, S., Eds.; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar]
- Cheng, R.A.; Eade, C.R.; Wiedmann, M. Embracing Diversity: Differences in Virulence Mechanisms, Disease Severity, and Host Adaptations Contribute to the Success of Nontyphoidal Salmonella as a Foodborne Pathogen. Front. Microbiol. 2019, 10, 1368. [Google Scholar] [CrossRef]
- Ferrari, R.G.; Rosario, D.K.A.; Cunha-Neto, A.; Mano, S.B.; Figueiredo, E.E.S.; Conte-Junior, C.A. Worldwide Epidemiology of Salmonella Serovars in Animal-Based Foods: A Meta-analysis. Appl. Environ. Microbiol. 2019, 85, e00591-19. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef]
- Hur, J.; Jawale, C.; Lee, J.H. Antimicrobial resistance of Salmonella isolated from food animals: A review. Food Res. Int. 2012, 45, 819–830. [Google Scholar] [CrossRef]
- Nazeer, N.; Uribe-Diaz, S.; Rodriguez-Lecompte, J.C.; Ahmed, M. Antimicrobial peptides as an alternative to relieve antimicrobial growth promoters in poultry. Br. Poult. Sci. 2021, 62, 672–685. [Google Scholar] [CrossRef]
- Salim, H.M.; Huque, K.S.; Kamaruddin, K.M.; Beg, M. Global restriction of using antibiotic growth promoters and alternative strategies in poultry production. Sci. Prog. 2018, 101, 52–75. [Google Scholar] [CrossRef]
- Almansour, A.M.; Alhadlaq, M.A.; Alzahrani, K.O.; Mukhtar, L.E.; Alharbi, A.L.; Alajel, S.M. The Silent Threat: Antimicrobial-Resistant Pathogens in Food-Producing Animals and Their Impact on Public Health. Microorganisms 2023, 11, 2127. [Google Scholar] [CrossRef]
- World Health Organization. Critically Important Antimicrobials for Human Medicine: 6th Revision; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Alvarez, D.M.; Barrón-Montenegro, R.; Conejeros, J.; Rivera, D.; Undurraga, E.A.; Moreno-Switt, A.I. A review of the global emergence of multidrug-resistant Salmonella enterica subsp. enterica Serovar Infantis. Int. J. Food Microbiol. 2023, 403, 110297. [Google Scholar] [CrossRef]
- McMillan, E.A.; Wasilenko, J.L.; Tagg, K.A.; Chen, J.C.; Simmons, M.; Gupta, S.K.; Tillman, G.E.; Folster, J.; Jackson, C.R.; Frye, J.G. Carriage and Gene Content Variability of the pESI-Like Plasmid Associated with Salmonella Infantis Recently Established in United States Poultry Production. Genes 2020, 11, 1516. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.; Leekitcharoenphon, P.; Feltrin, F.; Alba, P.; Cordaro, G.; Iurescia, M.; Tolli, R.; D’Incau, M.; Staffolani, M.; Di Giannatale, E.; et al. Emergence of a Clonal Lineage of Multidrug-Resistant ESBL-Producing Salmonella Infantis Transmitted from Broilers and Broiler Meat to Humans in Italy between 2011 and 2014. PLoS ONE 2015, 10, e0144802. [Google Scholar] [CrossRef] [PubMed]
- McMillan, E.A.; Weinroth, M.D.; Frye, J.G. Increased Prevalence of Salmonella Infantis Isolated from Raw Chicken and Turkey Products in the United States Is Due to a Single Clonal Lineage Carrying the pESI Plasmid. Microorganisms 2022, 10, 1478. [Google Scholar] [CrossRef]
- Tyson, G.H.; Li, C.; Harrison, L.B.; Martin, G.; Hsu, C.H.; Tate, H.; Tran, T.T.; Strain, E.; Zhao, S. A Multidrug-Resistant Salmonella Infantis Clone is Spreading and Recombining in the United States. Microb. Drug Resist. 2021, 27, 792–799. [Google Scholar] [CrossRef]
- Kang, H.S.; Ali, M.S.; Na, S.H.; Moon, B.Y.; Kim, J.I.; Hwang, Y.J.; Yoon, S.S.; Park, S.C.; Lim, S.K. Nationwide surveillance and characterization of the third-generation cephalosporin-resistant Salmonella enterica serovar infantis isolated from chickens in South Korea between 2010 and 2022. Heliyon 2024, 10, e37124. [Google Scholar] [CrossRef]
- Kim, M.B.; Jung, H.R.; Lee, Y.J. Emergence of Salmonella Infantis carrying the pESI megaplasmid in commercial farms of five major integrated broiler operations in Korea. Poult. Sci. 2024, 103, 103516. [Google Scholar] [CrossRef]
- Kim, M.B.; Lee, Y.J. Emergence of Salmonella Infantis carrying the pESI-like plasmid from eggs in egg grading and packing plants in Korea. Food Microbiol. 2024, 122, 104568. [Google Scholar] [CrossRef]
- Yeh, Y.; Purushothaman, P.; Gupta, N.; Ragnone, M.; Verma, S.C.; de Mello, A.S. Bacteriophage application on red meats and poultry: Effects on Salmonella population in final ground products. Meat Sci. 2017, 127, 30–34. [Google Scholar] [CrossRef]
- Hyeon, J.Y.; Chon, J.W.; Hwang, I.G.; Kwak, H.S.; Kim, M.S.; Kim, S.K.; Choi, I.S.; Song, C.S.; Park, C.; Seo, K.H. Prevalence, antibiotic resistance, and molecular characterization of Salmonella serovars in retail meat products. J. Food Prot. 2011, 74, 161–166. [Google Scholar] [CrossRef]
- Kim, M.S.; Lim, T.H.; Jang, J.H.; Lee, D.H.; Kim, B.Y.; Kwon, J.H.; Choi, S.W.; Noh, J.Y.; Hong, Y.H.; Lee, S.B.; et al. Prevalence and antimicrobial resistance of Salmonella species isolated from chicken meats produced by different integrated broiler operations in Korea. Poult. Sci. 2012, 91, 2370–2375. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.; Bae, Y.; Lee, Y.S.; Kang, D.H.; Kim, S.H. Prevalence and Characteristics of Salmonella spp. Isolated from Raw Chicken Meat in the Republic of Korea. J. Microbiol. Biotechnol. 2022, 32, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Yoon, R.H.; Cha, S.Y.; Wei, B.; Roh, J.H.; Seo, H.S.; Oh, J.Y.; Jang, H.K. Prevalence of Salmonella isolates and antimicrobial resistance in poultry meat from South Korea. J. Food Prot. 2014, 77, 1579–1582. [Google Scholar] [CrossRef]
- Lunara Santos Pavelquesi, S.; Carolina Almeida de Oliveira Ferreira, A.; Fernandes Silva Rodrigues, L.; Maria de Souza Silva, C.; Cristina Rodrigues da Silva, I.; Castilho Orsi, D. Prevalence and Antimicrobial Resistance of Salmonella spp. Isolated From Chilled Chicken Meat Commercialized at Retail in Federal District, Brazil. J. Food Prot. 2023, 86, 100130. [Google Scholar] [CrossRef]
- Bolkenov, B.; Lee, K.Y.; Atwill, E.R.; Pitesky, M.; Rickard, M.; Hung-Fan, M.; Shafii, M.; Lavelle, K.; Huang, A.; Sebti, J.; et al. Phenotypic and genotypic characterization of antimicrobial resistance of non-typhoidal Salmonella from retail meat in California. Int. J. Food Microbiol. 2024, 421, 110785. [Google Scholar] [CrossRef]
- Maddocks, S.; Olma, T.; Chen, S. Comparison of CHROMagar Salmonella medium and xylose-lysine-desoxycholate and Salmonella-Shigella agars for isolation of Salmonella strains from stool samples. J. Clin. Microbiol. 2002, 40, 2999–3003. [Google Scholar] [CrossRef]
- Su, L.H.; Chiu, C.H.; Chu, C.; Ou, J.T. Antimicrobial resistance in nontyphoid Salmonella serotypes: A global challenge. Clin. Infect. Dis. 2004, 39, 546–551. [Google Scholar] [CrossRef]
- Korea Disease Control and Prevention Agency. One Health AMR. Available online: https://nih.go.kr/nohas/common/main.do (accessed on 20 March 2025).
- World Health Organization. Integrated Surveillance of Antimicrobial Resistance in Foodborne Bacteria: Application of a One Health Approach: Guidance from the WHO Advisory Group on Integrated Surveillanec of Antimicrobial Resistance (AGISAR); World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Alzahrani, K.O.; Al-Reshoodi, F.M.; Alshdokhi, E.A.; Alhamed, A.S.; Al Hadlaq, M.A.; Mujallad, M.I.; Mukhtar, L.E.; Alsufyani, A.T.; Alajlan, A.A.; Al Rashidy, M.S.; et al. Antimicrobial resistance and genomic characterization of Salmonella enterica isolates from chicken meat. Front. Microbiol. 2023, 14, 1104164. [Google Scholar] [CrossRef]
- Castello, A.; Piraino, C.; Butera, G.; Alio, V.; Cardamone, C.; Oliveri, G.; Cascone, G.; Ciravolo, C.; Costa, A. Prevalence and antimicrobial resistance profiles of Salmonella spp. in poultry meat. Ital. J. Food Saf. 2023, 12, 11135. [Google Scholar] [CrossRef]
- Perin, A.P.; Martins, B.T.F.; Barreiros, M.A.B.; Yamatogi, R.S.; Nero, L.A.; Dos Santos Bersot, L. Occurrence, quantification, pulse types, and antimicrobial susceptibility of Salmonella sp. isolated from chicken meat in the state of Paraná, Brazil. Braz. J. Microbiol. 2020, 51, 335–345. [Google Scholar] [CrossRef]
- Jeon, H.Y.; Kim, Y.B.; Lim, S.K.; Lee, Y.J.; Seo, K.W. Characteristics of cephalosporin-resistant Salmonella isolates from poultry in Korea, 2010-2017. Poult. Sci. 2019, 98, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Tate, H.; Folster, J.P.; Hsu, C.H.; Chen, J.; Hoffmann, M.; Li, C.; Morales, C.; Tyson, G.H.; Mukherjee, S.; Brown, A.C.; et al. Comparative Analysis of Extended-Spectrum-β-Lactamase CTX-M-65-Producing Salmonella enterica Serovar Infantis Isolates from Humans, Food Animals, and Retail Chickens in the United States. Antimicrob. Agents Chemother. 2017, 61, e00488-17. [Google Scholar] [CrossRef] [PubMed]
- Aviv, G.; Tsyba, K.; Steck, N.; Salmon-Divon, M.; Cornelius, A.; Rahav, G.; Grassl, G.A.; Gal-Mor, O. A unique megaplasmid contributes to stress tolerance and pathogenicity of an emergent Salmonella enterica serovar Infantis strain. Environ. Microbiol. 2014, 16, 977–994. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A.; Bertini, A.; Villa, L.; Falbo, V.; Hopkins, K.L.; Threlfall, E.J. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 2005, 63, 219–228. [Google Scholar] [CrossRef]
- Vázquez, X.; Fernández, J.; Rodríguez-Lozano, J.; Calvo, J.; Rodicio, R.; Rodicio, M.R. Genomic Analysis of Two MDR Isolates of Salmonella enterica Serovar Infantis from a Spanish Hospital Bearing the blaCTX-M-65 Gene with or without fosA3 in pESI-like Plasmids. Antibiotics 2022, 11, 786. [Google Scholar] [CrossRef]
- Xu, Y.; Jing, Y.; Hu, L.; Cheng, Q.; Gao, H.; Zhang, Z.; Yang, H.; Zhao, Y.; Zhou, D.; Yin, Z.; et al. IncFIB-4.1 and IncFIB-4.2 Single-Replicon Plasmids: Small Backbones with Large Accessory Regions. Infect. Drug Resist. 2022, 15, 1191–1203. [Google Scholar] [CrossRef]
- García-Soto, S.; Abdel-Glil, M.Y.; Tomaso, H.; Linde, J.; Methner, U. Emergence of Multidrug-Resistant Salmonella enterica Subspecies enterica Serovar Infantis of Multilocus Sequence Type 2283 in German Broiler Farms. Front. Microbiol. 2020, 11, 1741. [Google Scholar] [CrossRef]
- Diamant, I.; Adani, B.; Sylman, M.; Rahav, G.; Gal-Mor, O. The transcriptional regulation of the horizontally acquired iron uptake system, yersiniabactin and its contribution to oxidative stress tolerance and pathogenicity of globally emerging salmonella strains. Gut Microbes 2024, 16, 2369339. [Google Scholar] [CrossRef]
- Alba, P.; Carfora, V.; Feltrin, F.; Diaconu, E.L.; Sorbara, L.; Dell’Aira, E.; Cerci, T.; Ianzano, A.; Donati, V.; Franco, A.; et al. Evidence of structural rearrangements in ESBL-positive pESI(like) megaplasmids of S. Infantis. FEMS Microbiol. Lett. 2023, 370, fnad014. [Google Scholar] [CrossRef]
- Cohen, E.; Kriger, O.; Amit, S.; Davidovich, M.; Rahav, G.; Gal-Mor, O. The emergence of a multidrug resistant Salmonella Muenchen in Israel is associated with horizontal acquisition of the epidemic pESI plasmid. Clin. Microbiol. Infect. 2022, 28, 1499.e7–1499.e14. [Google Scholar] [CrossRef]
- Li, C.; Tate, H.; Huang, X.; Hsu, C.H.; Harrison, L.B.; Zhao, S.; Fortenberry, G.Z.; Dessai, U.; McDermott, P.F.; Strain, E.A. The spread of pESI-mediated extended-spectrum cephalosporin resistance in Salmonella serovars-Infantis, Senftenberg, and Alachua isolated from food animal sources in the United States. PLoS ONE 2024, 19, e0299354. [Google Scholar] [CrossRef]
- Dos Santos, A.M.P.; Panzenhagen, P.; Ferrari, R.G.; Conte-Junior, C.A. Large-scale genomic analysis reveals the pESI-like megaplasmid presence in Salmonella Agona, Muenchen, Schwarzengrund, and Senftenberg. Food Microbiol. 2022, 108, 104112. [Google Scholar] [CrossRef] [PubMed]
- Kasturi, K.N.; Drgon, T. Real-Time PCR Method for Detection of Salmonella spp. in Environmental Samples. Appl. Environ. Microbiol. 2017, 83, e00644-17. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Frye, J.G.; Hu, J.; Fedorka-Cray, P.J.; Gautom, R.; Boyle, D.S. Multiplex PCR-based method for identification of common clinical serotypes of Salmonella enterica subsp. enterica. J. Clin. Microbiol. 2006, 44, 3608–3615. [Google Scholar] [CrossRef]
- Kang, H.; Kim, H.; Lee, J.; Jeon, J.H.; Kim, S.; Park, Y.; Joo, I.; Kim, H. Genetic Characteristics of Multidrug-Resistant Salmonella Isolated from Poultry Meat in South Korea. Microorganisms 2024, 12, 1646. [Google Scholar] [CrossRef]
- Animal and Plant Quarantine Agency, Ministry of Agriculture Food and Rural Affairs, Ministry of Food and Drug Safety. National Antimicrobial Usage and Resistance Monitoring in Animals and Animal Products, 2023; Animal and Plant Quarantine Agency, Ministry of Agriculture Food and Rural Affairs, Ministry of Food and Drug Safety: Seoul, Republic of Korea, 2023. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021. [Google Scholar]
- National Antimicrobial Resistance Monitoring System. The National Antimicrobial Resistance Monitoring System: Enteric Bacteria; National Antimicrobial Resistance Monitoring System: Atlanta, GA, USA, 2021. [Google Scholar]
- Kim, D.; Yoon, E.J.; Hong, J.S.; Choi, M.H.; Kim, H.S.; Kim, Y.R.; Kim, Y.A.; Uh, Y.; Shin, K.S.; Shin, J.H.; et al. Major Bloodstream Infection-Causing Bacterial Pathogens and Their Antimicrobial Resistance in South Korea, 2017-2019: Phase I Report From Kor-GLASS. Front. Microbiol. 2021, 12, 799084. [Google Scholar] [CrossRef]
- Kang, J.H.; Bae, I.K.; Kwon, S.B.; Jeong, S.H.; Lee, J.; Lee, W.G.; Kang, J.O.; Ahn, J.Y.; Hong, S.G.; Shin, J.H.; et al. Prevalence of Ambler Class A Extended-Spectrum beta-Lactamase-Producing Escherichia coli and Klebsiella pneumoniae Isolates in Korea. Korean J. Clin. Microbiol. 2005, 8, 17–25. [Google Scholar]
- Kim, Y.; Cho, H.; Jang, B.; Lee, M.; Park, K.T. Molecular characterization of emerging multi-drug resistant Clostridium perfringens isolated from pork production chains in Korea. Food Microbiol. 2025, 128, 104729. [Google Scholar] [CrossRef]
- Zhang, S.; den Bakker, H.C.; Li, S.; Chen, J.; Dinsmore, B.A.; Lane, C.; Lauer, A.C.; Fields, P.I.; Deng, X. SeqSero2: Rapid and Improved Salmonella Serotype Determination Using Whole-Genome Sequencing Data. Appl. Environ. Microbiol. 2019, 85, e01746-19. [Google Scholar] [CrossRef]
- Davis, S.; Pettengill, J.B.; Luo, Y.; Payne, J.; Shpuntoff, A.; Rand, H.; Strain, E. CFSAN SNP Pipeline: An automated method for constructing SNP matrices from next-generation sequence data. PeerJ Comput. Sci. 2015, 1, e20. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Pightling, A.W.; Pettengill, J.B.; Luo, Y.; Baugher, J.D.; Rand, H.; Strain, E. Interpreting Whole-Genome Sequence Analyses of Foodborne Bacteria for Regulatory Applications and Outbreak Investigations. Front. Microbiol. 2018, 9, 1482. [Google Scholar] [CrossRef]
- Feldgarden, M.; Brover, V.; Gonzalez-Escalona, N.; Frye, J.G.; Haendiges, J.; Haft, D.H.; Hoffmann, M.; Pettengill, J.B.; Prasad, A.B.; Tillman, G.E.; et al. AMRFinderPlus and the Reference Gene Catalog facilitate examination of the genomic links among antimicrobial resistance, stress response, and virulence. Sci. Rep. 2021, 11, 12728. [Google Scholar] [CrossRef]
- Carattoli, A.; Zankari, E.; García-Fernández, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Møller Aarestrup, F.; Hasman, H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef]
- Robertson, J.; Nash, J.H.E. MOB-suite: Software tools for clustering, reconstruction and typing of plasmids from draft assemblies. Microb. Genom. 2018, 4, e000206. [Google Scholar] [CrossRef]
- Cho, H.; Kim, Y.; Hassan, A.; Park, K.T. Whole-genome sequence-based comparison of antimicrobial resistant diarrheagenic Escherichia coli in pork and chicken production chains in Korea. Int. J. Food Microbiol. 2025, 431, 111085. [Google Scholar] [CrossRef]
- Lee, I.; Ouk Kim, Y.; Park, S.C.; Chun, J. OrthoANI: An improved algorithm and software for calculating average nucleotide identity. Int. J. Syst. Evol. Microbiol. 2016, 66, 1100–1103. [Google Scholar] [CrossRef]
- Alikhan, N.F.; Petty, N.K.; Ben Zakour, N.L.; Beatson, S.A. BLAST Ring Image Generator (BRIG): Simple prokaryote genome comparisons. BMC Genom. 2011, 12, 402. [Google Scholar] [CrossRef]

| Serotype | ST 2 | Chicken Meat (%) | Duck Meat (%) | Total (%) |
|---|---|---|---|---|
| Infantis | ST32 * | 106/200 (53.0) | 7/100 (7.0) | 113/300 (37.7) |
| Typhimurium | ST19 * | 2/200 (1.0) | 39/100 (39.0) | 41/300 (13.7) |
| Enteritidis | ST11 | 7/200 (3.5) | 0/100 (0.0) | 7/300 (2.3) |
| Agona | ST13 * | 21/200 (10.5) | 1/100 (1.0) | 22/300 (7.3) |
| Montevideo | ST4 | 4/200 (2.0) | 0/100 (0.0) | 4/300 (1.3) |
| Thompson | ST292 * | 0/200 (0.0) | 6/100 (6.0) | 6/300 (2.0) |
| Stanley | ST321 | 0/200 (0.0) | 1/100 (1.0) | 1/300 (0.3) |
| Mbandaka | ST2133 | 3/200 (1.5) | 0/100 (0.0) | 3/300 (1.0) |
| Brandenburg | ST1954 * | 0/200 (0.0) | 7/100 (7.0) | 7/300 (2.3) |
| Westhampton | ST14 | 3/200 (1.5) | 0/100 (0.0) | 3/300 (1.0) |
| Javiana | ST684 * | 0/200 (0.0) | 3/100 (3.0) | 3/300 (1.0) |
| NT 1 | ST17 * | 0/200 (0.0) | 7/100 (7.0) | 7/300 (2.3) |
| ST26 | 4/200 (2.0) | 0/100 (0.0) | 4/300 (1.3) | |
| ST33 * | 0/200 (0.0) | 13/100 (13.0) | 13/300 (4.3) | |
| ST48 | 4/200 (2.0) | 0/100 (0.0) | 4/300 (1.3) | |
| ST203 | 3/200 (1.5) | 0/100 (0.0) | 3/300 (1.0) | |
| ST316 | 0/200 (0.0) | 1/100 (1.0) | 1/300 (0.3) | |
| ST543 | 1/200 (0.5) | 1/100 (1.0) | 2/300 (0.7) | |
| Total | 158/200 (79.0) | 86/100 (86.0) | 244/300 (81.3) | |
| Antimicrobial Agents 1 | MIC Break Point(μg/mL) | References 2 | Origin of Isolates | |||
|---|---|---|---|---|---|---|
| Chicken Meat (n = 158) | Duck Meat (n = 86) | p-Value | Total (n = 244) | |||
| AMP | 32 | CLSI | 103/158 (65.2) 4 | 14/86 (16.3) | <0.001 | 117/244 (48.0) |
| AUG2 | 32/16 | CLSI | 2/158 (1.3) | 0/86 (0.0) | 0.295 | 2/244 (0.8) |
| CAZ | 16 | CLSI | 4/158 (2.5) | 0/86 (0.0) | 0.137 | 4/244 (1.6) |
| CHL | 32 | CLSI | 121/158 (76.6) | 11/86 (12.8) | <0.001 | 132/244 (54.1) |
| CIP | 1 | CLSI | 2/158 (1.3) | 3/86 (3.5) | 0.242 | 5/244 (2.0) |
| COL | 4 | CLSI | 1/158 (0.6) | 1/86 (1.2) | - 3 | 2/244 (0.8) |
| CTX | 4 | CLSI | 100/158 (63.3) | 7/86 (8.1) | <0.001 | 107/244 (43.9) 5 |
| FEP | 16 | CLSI | 3/158 (1.9) | 0/86 (0.0) | 0.199 | 3/244 (1.2) |
| FIS | 512 | CLSI | 120/158 (75.9) | 13/86 (15.1) | <0.001 | 133/244 (54.5) |
| FOX | 32 | CLSI | 6/158 (3.8) | 0/86 (0.0) | 0.067 | 6/244 (2.5) |
| GEN | 16 | CLSI | 29/158 (18.4) | 0/86 (0.0) | <0.001 | 29/244 (11.9) |
| MERO | 4 | CLSI | 0/158 (0.0) | 0/86 (0.0) | - | 0/244 (0.0) |
| NAL | 32 | CLSI | 122/158 (77.2) | 44/86 (51.2) | <0.001 | 166/244 (68.0) |
| STR | 32 | NARMS | 112/158 (75.9) | 21/86 (24.4) | <0.001 | 133/244 (54.5) |
| SXT | 4/76 | CLSI | 67/158 (42.4) | 12/86 (14.0) | <0.001 | 79/244 (32.4) |
| TET | 16 | CLSI | 121/158 (76.6) | 13/86 (15.1) | <0.001 | 134/244 (54.9) |
| Multidrug resistance | 126/158 (79.7) | 16/86 (18.6) | <0.001 | 142/244 (58.2) | ||
| AMR Phenotype * | Chicken Meat (%) | Duck Meat (%) | Total (%) |
|---|---|---|---|
| AMP-AUG2-CAZ-CHL-CTX-FEP-FIS-GEN-NAL-STR-SXT-TET | 1 (1.0) | 0 (0.0) | 1 (0.9) |
| AMP-AUG2-CAZ-CHL-CTX-FEP-FIS-GEN-NAL-STR-TET | 1 (1.0) | 0 (0.0) | 1 (0.9) |
| AMP-CAZ-CHL-CTX-FIS-GEN-NAL-STR-SXT-TET | 1 (1.0) | 0 (0.0) | 1 (0.9) |
| AMP-CHL-CTX-FIS-FOX-GEN-NAL-STR-SXT-TET | 2 (2.0) | 0 (0.0) | 2 (1.9) |
| AMP-CHL-CTX-FIS-FOX-NAL-STR-SXT-TET | 1 (1.0) | 0 (0.0) | 1 (0.9) |
| AMP-CHL-CTX-FIS-GEN-STR-SXT-NAL-TET | 17 (17.0) | 0 (0.0) | 17 (15.9) |
| AMP-CAZ-CHL-CTX-FIS-NAL-STR-TET | 1 (1.0) | 0 (0.0) | 1 (0.9) |
| AMP-CHL-CTX-FIS-FOX-NAL-STR-TET | 1 (1.0) | 0 (0.0) | 1 (0.9) |
| AMP-CHL-CTX-FIS-GEN-NAL-STR-TET | 4 (4.0) | 0 (0.0) | 4 (3.7) |
| AMP-CHL-CTX-FIS-GEN-NAL-SXT-TET | 2 (2.0) | 0 (0.0) | 2 (1.9) |
| AMP-CHL-CTX-FIS-STR-SXT-NAL-TET | 37 (37.0) | 7 (100.0) | 44 (41.1) |
| AMP-CTX-FEP-FIS-FOX-NAL-STR-TET | 1 (1.0) | 0 (0.0) | 1 (0.9) |
| AMP-CHL-CTX-FIS-NAL-STR-SXT | 2 (2.0) | 0 (0.0) | 2 (1.9) |
| AMP-CHL-CTX-FIS-NAL-STR-TET | 17 (17.0) | 0 (0.0) | 17 (15.9) |
| AMP-CHL-CTX-FIS-NAL-SXT-TET | 1 (1.0) | 0 (0.0) | 1 (0.9) |
| AMP-CHL-CTX-NAL-STR-SXT-TET | 1 (1.0) | 0 (0.0) | 1 (0.9) |
| AMP-CHL-CTX-GEN-NAL-TET | 1 (1.0) | 0 (0.0) | 1 (0.9) |
| AMP-CHL-CTX-FIS-NAL-TET | 2 (2.0) | 0 (0.0) | 2 (1.9) |
| AMP-CHL-CTX-FIS-NAL-STR | 1 (1.0) | 0 (0.0) | 1 (0.9) |
| AMP-CHL-CTX-FOX-NAL | 1 (1.0) | 0 (0.0) | 1 (0.9) |
| AMP-CHL-CTX-GEN-NAL | 1 (1.0) | 0 (0.0) | 1 (0.9) |
| AMP-CHL-CTX-NAL-TET | 4 (4.0) | 0 (0.0) | 4 (3.7) |
| Total | 100/107 (93.5) | 7/107 (6.5) | 107/107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Cho, H.; Lee, M.; Hassan, A.; Yang, S.-J.; Chae, J.-C.; Park, K.T. Emergence and Clonal Spread of Extended-Spectrum β-Lactamase-Producing Salmonella Infantis Carrying pESI Megaplasmids in Korean Retail Poultry Meat. Antibiotics 2025, 14, 366. https://doi.org/10.3390/antibiotics14040366
Kim Y, Cho H, Lee M, Hassan A, Yang S-J, Chae J-C, Park KT. Emergence and Clonal Spread of Extended-Spectrum β-Lactamase-Producing Salmonella Infantis Carrying pESI Megaplasmids in Korean Retail Poultry Meat. Antibiotics. 2025; 14(4):366. https://doi.org/10.3390/antibiotics14040366
Chicago/Turabian StyleKim, Yeona, Hyeonwoo Cho, Miru Lee, Amany Hassan, Soo-Jin Yang, Jong-Chan Chae, and Kun Taek Park. 2025. "Emergence and Clonal Spread of Extended-Spectrum β-Lactamase-Producing Salmonella Infantis Carrying pESI Megaplasmids in Korean Retail Poultry Meat" Antibiotics 14, no. 4: 366. https://doi.org/10.3390/antibiotics14040366
APA StyleKim, Y., Cho, H., Lee, M., Hassan, A., Yang, S.-J., Chae, J.-C., & Park, K. T. (2025). Emergence and Clonal Spread of Extended-Spectrum β-Lactamase-Producing Salmonella Infantis Carrying pESI Megaplasmids in Korean Retail Poultry Meat. Antibiotics, 14(4), 366. https://doi.org/10.3390/antibiotics14040366





