One Health Approach: Antibiotic Resistance Among Enterococcal Isolates in Dairy Farms in Selangor
Abstract
:1. Introduction
2. Results
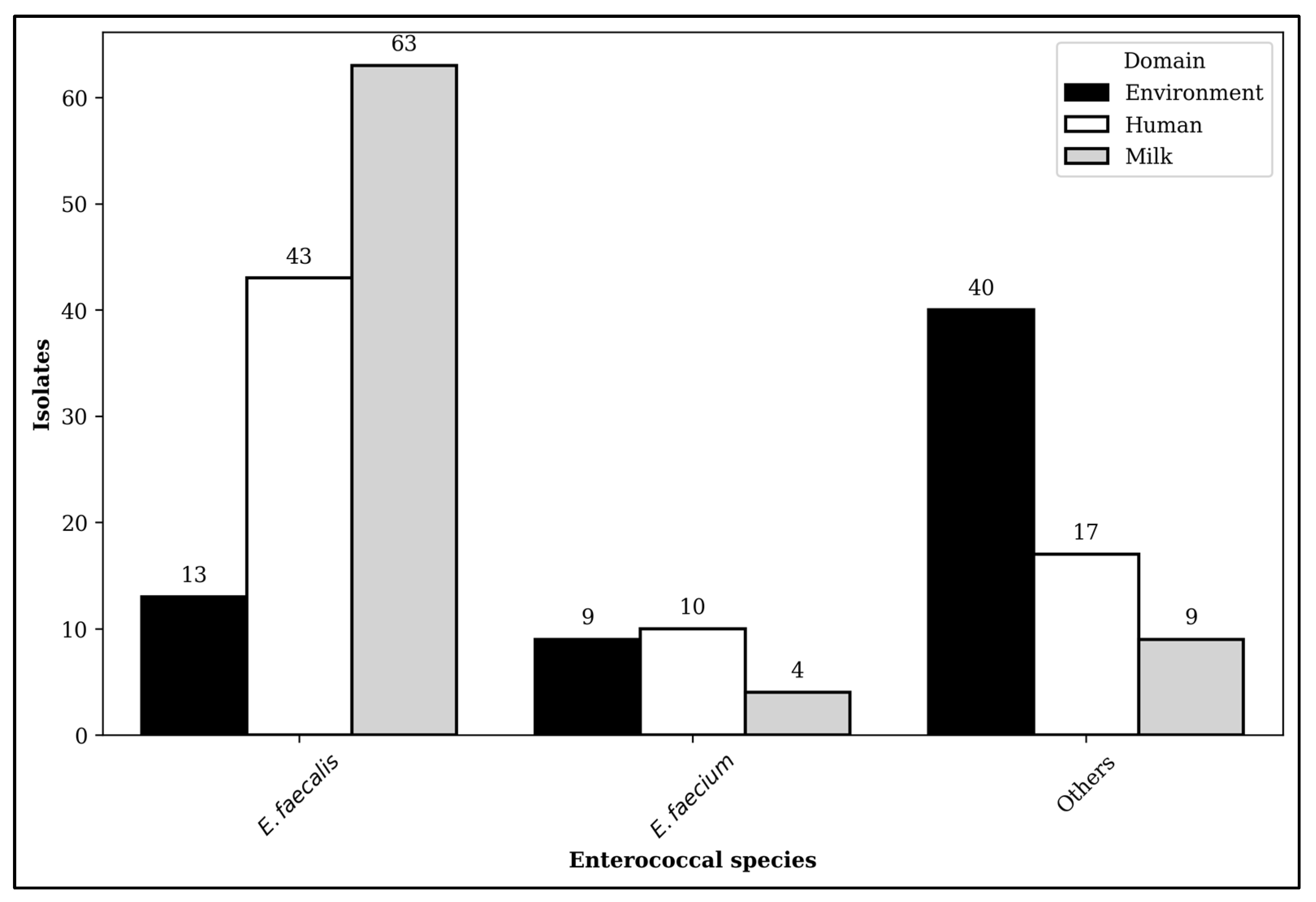
2.1. Distribution of Enterococcal Isolates
2.2. Antibiotic-Susceptible and -Resistant Isolates
2.3. Multidrug Resistance
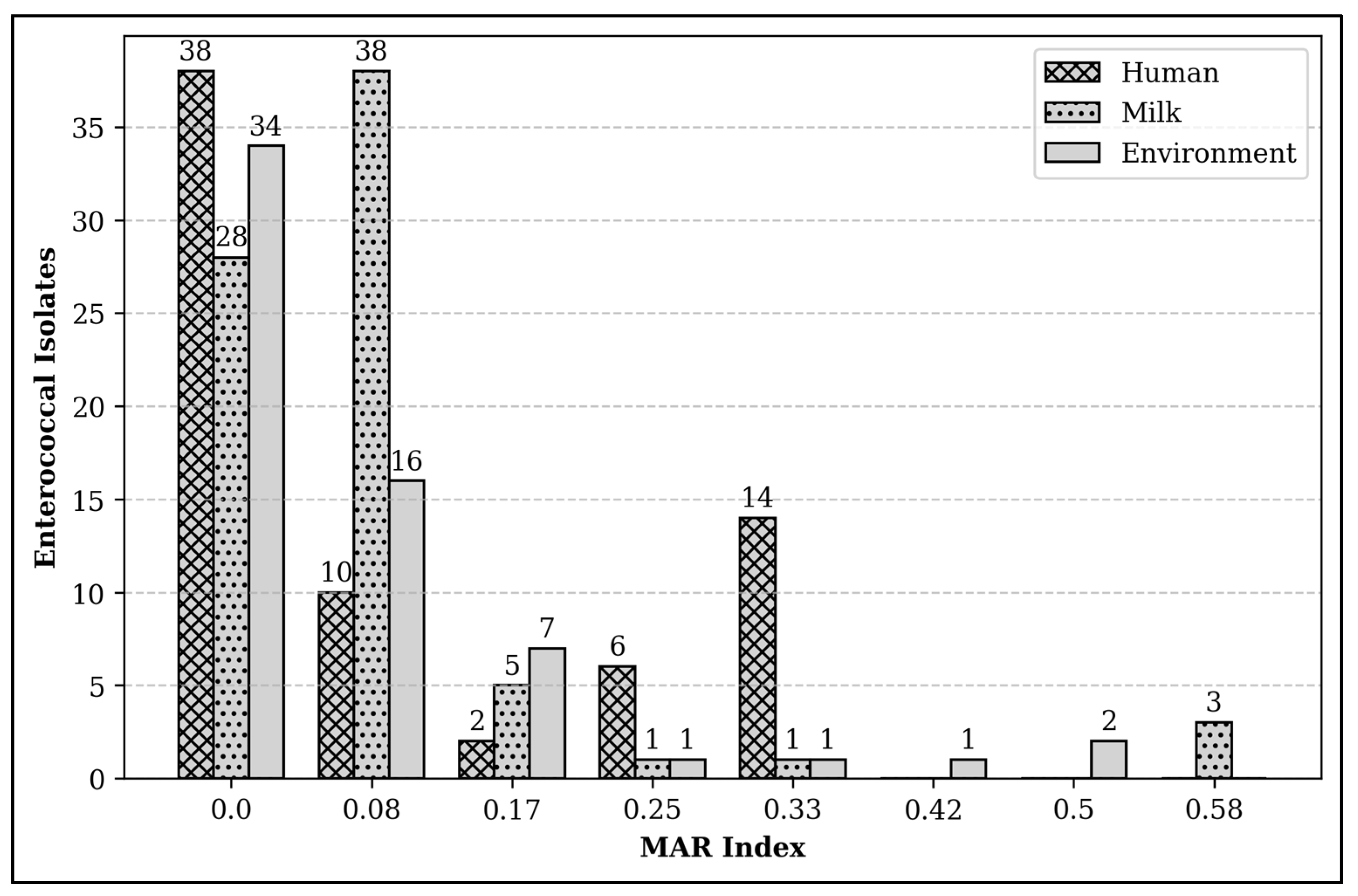
2.4. Multiple Antibiotic Resistance Index
3. Discussion
4. Materials and Methods
4.1. Study Design and Sample Sources
4.2. Sample Sources and Collection
4.3. Sample Preparation and Isolation of Enterococci
4.4. Identification of Enterococcal Species and Antimicrobial Susceptibility Testing
4.5. MAR Index
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AR | Antibiotic Resistance |
| DVS | Department of Veterinary Services |
| LRE | Linezolid-Resistant Enterococci |
| MAR | Multiple Antibiotic Resistance |
| MDR | Multidrug Resistance |
| VCIA | Veterinary Critically Important Antimicrobial |
| VRE | Vancomycin-Resistant Enterococci |
References
- Velazquez-Meza, M.E.; Galarde-López, M.; Carrillo-Quiróz, B.; Alpuche-Aranda, C.M. Antimicrobial Resistance: One Health Approach. Vet. World 2022, 15, 743–749. [Google Scholar] [CrossRef]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6, 521–547. [Google Scholar] [CrossRef] [PubMed]
- Rhouma, M.; Archambault, M.; Butaye, P. Antimicrobial Use and Resistance in Animals from a One Health Perspective. Vet. Sci. 2023, 10, 319. [Google Scholar] [CrossRef] [PubMed]
- OIE—World Organisation for Animal Health. OIE Standards, Guidelines and Resolution on Antimicrobial Resistance and the Use of Antimicrobial Agents, 2nd ed.; OIE: Paris, France, 2020; ISBN 978-92-95115-06-4. [Google Scholar]
- McKernan, C.; Benson, T.; Farrell, S.; Dean, M. Antimicrobial Use in Agriculture: Critical Review of the Factors Influencing Behaviour. JAC-Antimicrob. Resist. 2021, 3, dlab178. [Google Scholar] [CrossRef]
- Wen, R.; Li, C.; Zhao, M.; Wang, H.; Tang, Y. Withdrawal of Antibiotic Growth Promoters in China and Its Impact on the Foodborne Pathogen Campylobacter coli of Swine Origin. Front. Microbiol. 2022, 13, 1004725. [Google Scholar] [CrossRef]
- Cuong, N.V.; Kiet, B.T.; Hien, V.B.; Truong, B.D.; Phu, D.H.; Thwaites, G.; Choisy, M.; Carrique-Mas, J. Antimicrobial Use through Consumption of Medicated Feeds in Chicken Flocks in the Mekong Delta of Vietnam: A Three-Year Study before a Ban on Antimicrobial Growth Promoters. PLoS ONE 2021, 16, e0250082. [Google Scholar] [CrossRef] [PubMed]
- Rajendiran, S.; Veloo, Y.; Thahir, S.S.A.; Shaharudin, R. Resistance towards Critically Important Antimicrobials among Enterococcus Faecalis and E. Faecium in Poultry Farm Environments in Selangor, Malaysia. Antibiotics 2022, 11, 1118. [Google Scholar] [CrossRef]
- Noor, A.U.Z.; Kabir, H.; Chowdhury, M.A.A.; Ather, M.F.; Kamrul Hasan, M. An Emerging Route to Antibiotic Resistance in South Asia: A Correspondence. Ann. Med. Surg. 2023, 85, 335–336. [Google Scholar] [CrossRef]
- Lambraki, I.A.; Chadag, M.V.; Cousins, M.; Graells, T.; Léger, A.; Henriksson, P.J.G.; Troell, M.F.; Harbarth, S.; Wernli, D.; Jørgensen, P.S.; et al. Factors Impacting Antimicrobial Resistance in the South East Asian Food System and Potential Places to Intervene: A Participatory, One Health Study. Front. Microbiol. 2023, 13, 992507. [Google Scholar] [CrossRef]
- Coyne, L.; Arief, R.; Benigno, C.; Giang, V.N.; Huong, L.Q.; Jeamsripong, S.; Kalpravidh, W.; McGrane, J.; Padungtod, P.; Patrick, I.; et al. Characterizing Antimicrobial Use in the Livestock Sector in Three South East Asian Countries (Indonesia, Thailand, and Vietnam). Antibiotics 2019, 8, 33. [Google Scholar] [CrossRef]
- Malaysian Productivity Corporation. Potential Application of Circular Economy (CE) Concept in Livestock Production; Malaysian Productivity Corporation: Selangor, Malaysia, 2020; ISBN 978-967-2941-05-7. [Google Scholar]
- Faghiri, H.; Yusop, Z.; Hj Othman, M.; Eric Krauss, S. Structural Analysis of Factors Affecting Dairy Cattle Industry Development in Malaysia. Rev. Polit. Public Policy Emerg. Econ. 2019, 1, 23–42. [Google Scholar] [CrossRef]
- Ramos, S.; Silva, V.; Dapkevicius, M.; Igrejas, G.; Poeta, P. Enterococci, from Harmless Bacteria to a Pathogen. Microorganisms 2020, 8, 1118. [Google Scholar] [CrossRef]
- Ikhimiukor, O.O.; Okeke, I.N. A Snapshot Survey of Antimicrobial Resistance in Food-Animals in Low and Middle-Income Countries. One Health 2023, 16, 100489. [Google Scholar] [CrossRef] [PubMed]
- Dapkevicius, M.D.L.E.; Sgardioli, B.; Câmara, S.P.A.; Poeta, P.; Malcata, F.X. Current Trends of Enterococci in Dairy Products: A Comprehensive Review of Their Multiple Roles. Foods 2021, 10, 821. [Google Scholar] [CrossRef]
- García-Solache, M.; Rice, L.B. The Enterococcus: A Model of Adaptability to Its Environment. Clin. Microbiol. Rev. 2019, 32, e00058-18. [Google Scholar] [CrossRef]
- Gołaś-Prądzyńska, M.; Łuszczyńska, M.; Rola, J.G. Dairy Products: A Potential Source of Multidrug-Resistant Enterococcus Faecalis and Enterococcus Faecium Strains. Foods 2022, 11, 4116. [Google Scholar] [CrossRef]
- Said, M.S.; Tirthani, E.; Lesho, E. Enterococcus Infections. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Malaysian Veterinary Antimicrobials Guidelines. 2021. Available online: https://myohar.moh.gov.my/publications-department-of-veterinary-services-malaysia/ (accessed on 20 January 2025).
- World Health Organization. Critically Important Antimicrobials for Human Medicine, 6th ed.; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-151552-8. [Google Scholar]
- Greer, N.D. Tigecycline (Tygacil): The First in the Glycylcycline Class of Antibiotics. Bayl. Univ. Med. Cent. Proc. 2006, 19, 155–161. [Google Scholar] [CrossRef]
- Veterinary Dept Will Ban Six Antibiotics on Livestock on Aug 31. Available online: https://thesun.my/malaysia-news/veterinary-dept-will-ban-six-antibiotics-on-livestock-on-aug-31-XL1926743 (accessed on 24 January 2025).
- Daniel, D.S.; Lee, S.M.; Gan, H.M.; Dykes, G.A.; Rahman, S. Genetic Diversity of Enterococcus Faecalis Isolated from Environmental, Animal and Clinical Sources in Malaysia. J. Infect. Public Health 2017, 10, 617–623. [Google Scholar] [CrossRef]
- Chan, Y. Low Prevalence of Vancomycin- and Bifunctional Aminoglycoside-Resistant Enterococci Isolated from Poultry Farms in Malaysia. Int. J. Food Microbiol. 2008, 122, 221–226. [Google Scholar] [CrossRef]
- Paschoalini, B.R.; Nuñez, K.V.M.; Maffei, J.T.; Langoni, H.; Guimarães, F.F.; Gebara, C.; Freitas, N.E.; Dos Santos, M.V.; Fidelis, C.E.; Kappes, R.; et al. The Emergence of Antimicrobial Resistance and Virulence Characteristics in Enterococcus Species Isolated from Bovine Milk. Antibiotics 2023, 12, 1243. [Google Scholar] [CrossRef]
- Rowe, S.; Cunningham, C.; Ingenhoff, L.; Norris, J.; Zadoks, R. Low Prevalence of Antimicrobial Resistant Organisms (Methicillin Resistant Staphylococcus aureus, Extended Beta-lactamase Producing Enterobacteriaceae, and Vancomycin Resistant Enterococci) in Bulk Tank Milk in New South Wales, Australia. Aust. Vet. J. 2023, 101, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Morandi, S.; Silvetti, T.; Lopreiato, V.; Piccioli-Cappelli, F.; Trevisi, E.; Brasca, M. Biodiversity and Antibiotic Resistance Profile Provide New Evidence for a Different Origin of Enterococci in Bovine Raw Milk and Feces. Food Microbiol. 2024, 120, 104492. [Google Scholar] [CrossRef]
- Giraffa, G. Enterococci from Foods. FEMS Microbiol. Rev. 2002, 26, 163–171. [Google Scholar] [CrossRef]
- Zarzecka, U.; Zakrzewski, A.J.; Chajęcka-Wierzchowska, W.; Zadernowska, A. Linezolid-Resistant Enterococcus Spp. Isolates from Foods of Animal Origin—The Genetic Basis of Acquired Resistance. Foods 2022, 11, 975. [Google Scholar] [CrossRef]
- Driesen, M.; Timmermans, M.; Cargnel, M.; Simons, X.; Filippitzi, M.-E.; Catry, B.; Dal Pozzo, F.; Vanderhaeghen, W.; Callens, B.; Dispas, M.; et al. Risk Factor Analysis for Occurrence of Linezolid-Resistant Bacteria in the Digestive and Respiratory Tract of Food-Producing Animals in Belgium: A Pilot Study. Antibiotics 2024, 13, 707. [Google Scholar] [CrossRef] [PubMed]
- National Antibitoic Resistance Surveillance Report. 2023. Available online: https://imr.nih.gov.my/MyOHAR/index.php/site/archive_rpt (accessed on 23 January 2025).
- Wada, Y.; Afolabi, H.A.; Al-Mhanna, S.B.; Bello, K.E.; Irekeola, A.A.; Wada, M.; Ahmed, N.; Harun, A.; Yean, C.Y.; Mohamad Nasir, N.S.; et al. Global Occurrence of Linezolid-Resistant Enterococcus (LRE): The First Systematic Review and Meta-Analysis. Microbe 2024, 2, 100041. [Google Scholar] [CrossRef]
- Fukuda, A.; Nakajima, C.; Suzuki, Y.; Usui, M. Transferable Linezolid Resistance Genes (optrA and poxtA) in Enterococci Derived from Livestock Compost at Japanese Farms. J. Glob. Antimicrob. Resist. 2024, 36, 336–344. [Google Scholar] [CrossRef]
- Sun, D.; Jeannot, K.; Xiao, Y.; Knapp, C.W. Editorial: Horizontal Gene Transfer Mediated Bacterial Antibiotic Resistance. Front. Microbiol. 2019, 10, 1933. [Google Scholar] [CrossRef]
- Syed Abu Thahir, S.; Rajendiran, S.; Shaharudin, R.; Veloo, Y. Multidrug-Resistant Salmonella Species and Their Mobile Genetic Elements from Poultry Farm Environments in Malaysia. Antibiotics 2023, 12, 1330. [Google Scholar] [CrossRef]
- Kathleen, M.M.; Samuel, L.; Felecia, C.; Reagan, E.L.; Kasing, A.; Lesley, M.; Toh, S.C. Antibiotic Resistance of Diverse Bacteria from Aquaculture in Borneo. Int. J. Microbiol. 2016, 2016, 2164761. [Google Scholar] [CrossRef]
- Gautam, A.; Bastola, S.; Lamsal, K.; Kaphle, K.; Shrestha, P.; Shah, S.; Subedi, D. Prevalence and Risk Factors of Multidrug Resistant (MDR) Escherichia Coli Isolated from Milk of Small Scale Dairy Buffaloes in Rupandehi, Nepal. Zoonotic Dis. 2024, 4, 174–186. [Google Scholar] [CrossRef]
- Seidel, J.; Magzamen, S.; Wang, Y.H.; Neujahr, V.; Schaeffer, J.W. Lessons from Dairy Farmers for Occupational Allergy and Respiratory Disease. Curr. Allergy Asthma Rep. 2023, 23, 325–339. [Google Scholar] [CrossRef]
- Marshall, K.E.; Drehoff, C.C.; Alden, N.; Montoya, S.; Stringer, G.; Kohnen, A.; Mellis, A.; Ellington, S.; Singleton, J.; Reed, C.; et al. Personal Protective Equipment Use by Dairy Farmworkers Exposed to Cows Infected with Highly Pathogenic Avian Influenza A(H5N1) Viruses—Colorado, 2024. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Grout, L.; Baker, M.G.; French, N.; Hales, S. A Review of Potential Public Health Impacts Associated With the Global Dairy Sector. GeoHealth 2020, 4, e2019GH000213. [Google Scholar] [CrossRef]
- Garcia, S.N.; Osburn, B.I.; Cullor, J.S. A One Health Perspective on Dairy Production and Dairy Food Safety. One Health 2019, 7, 100086. [Google Scholar] [CrossRef]
- Chajęcka-Wierzchowska, W.; Zadernowska, A.; García-Solache, M. Ready-to-Eat Dairy Products as a Source of Multidrug-Resistant Enterococcus Strains: Phenotypic and Genotypic Characteristics. J. Dairy Sci. 2020, 103, 4068–4077. [Google Scholar] [CrossRef] [PubMed]
- Malaysian Action Plan on Antimicrobial Resistance (MyAP-AMR) 2022–2026. Available online: https://www.who.int/publications/m/item/malaysia--malaysian-second-action-plan-on-antimicrobial-resistance-(myap-amr) (accessed on 21 March 2025).
- Aini, N. Evaluation of Milk Production and Farm management Practices: The case of selected Dairy Cattle Farms in Johor, Malaysia. Malays. J. Vet. Res. 2023, 14, 27–34. [Google Scholar]
- Veloo, Y.; Rajendiran, S.; Zakaria, Z.; Ismail, R.; Rahman, S.A.; Mansor, R.; Thahir, S.S.A. Prevalence and Antimicrobial Resistance Patterns of Escherichia Coli in the Environment, Cow Dung, and Milk of Selangor Dairy Farms. Antibiotics 2025, 14, 137. [Google Scholar] [CrossRef]
- HiCromeTM Enterococcus Faecium Agar Base. Available online: https://www.himedialabs.com/us/m1580-hicrome-enterococcus-faecium-agar-base.html (accessed on 3 January 2025).
- Vitek2 Results You Can Trust: Microbiology with Confidence. Available online: https://www.biomerieux-usa.com/sites/subsidiary_us/files/prn_052571_rev_02.a_cln_idast_vitek2-cards-brochure_final_art_2.pdf (accessed on 30 December 2024).
- Riaz, S.; Faisal, M.; Hasnain, S. Antibiotic Susceptibility Pattern and Multiple Antibiotic Resistances (MAR) Calculation of Extended Spectrum β- Lactamase (ESBL) Producing Escherichia Coli and Klebsiella Species in Pakistan. Afr. J. Biotechnol. 2011, 10, 6325–6331. [Google Scholar]
- Krumperman, P.H. Multiple Antibiotic Resistance Indexing of Escherichia coli to Identify High-Risk Sources of Fecal Contamination of Foods. Appl. Environ. Microbiol. 1983, 46, 165–170. [Google Scholar] [CrossRef]
- Afunwa, R.A.; Ezeanyinka, J.; Afunwa, E.C.; Udeh, A.S.; Oli, A.N.; Unachukwu, M. Multiple Antibiotic Resistant Index of Gram-Negative Bacteria from Bird Droppings in Two Commercial Poultries in Enugu, Nigeria. Open J. Med. Microbiol. 2020, 10, 171–181. [Google Scholar] [CrossRef]
- Veloo, Y.; Thahir, S.S.A.; Shaharudin, R.; Rajendiran, S. Multidrug-Resistant Escherichia Coli in Broiler and Indigenous Farm Environments in Klang Valley, Malaysia. Antibiotics 2025, 14, 246. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, R.; Dahiya, S.; Sayal, P. Evaluation of Multiple Antibiotic Resistance (MAR) Index and Doxycycline Susceptibility of Acinetobacter Species among Inpatients. Indian J. Microbiol. Res. 2016, 3, 299. [Google Scholar] [CrossRef]
- Mir, R.; Salari, S.; Najimi, M.; Rashki, A. Determination of Frequency, Multiple Antibiotic Resistance Index and Resistotype of Salmonella Spp. in Chicken Meat Collected from Southeast of Iran. Vet. Med. Sci. 2022, 8, 229–236. [Google Scholar] [CrossRef]
- Kim, H.-Y. Statistical Notes for Clinical Researchers: Chi-Squared Test and Fisher’s Exact Test. Restor. Dent. Endod. 2017, 42, 152. [Google Scholar] [CrossRef]
- McHugh, M.L. The Chi-Square Test of Independence. Biochem. Medica 2013, 23, 143–149. [Google Scholar] [CrossRef]
| Antibiotic | Domain | Total Resistance, n (%) | Resistance by Species | ||
|---|---|---|---|---|---|
| E. faecalis, n (%) | E. faecium, n (%) | Others, n (%) | |||
| Ampicillin | Human | 0 | 0 | 0 | 0 |
| Milk | 0 | 0 | 0 | 0 | |
| Environment | 2 (3.2) | 0 | 0 | 2 (5.0) | |
| Benzylpenicillin | Human | 0 | 0 | 0 | 0 |
| Milk | 0 | 0 | 0 | 0 | |
| Environment | 2 (3.3) | 0 | 0 | 2 (5.0) | |
| Ciprofloxacin | Human | 0 | 0 | 0 | 0 |
| Milk | 4 (5.3) | 4 (6.3) | 0 | 0 | |
| Environment | 0 | 0 | 0 | 0 | |
| Erythromycin | Human | 22 (31.4) | 17 (39.5) | 3 (30.0) | 2 (11.8) |
| Milk | 9 (11.8) | 8 (12.7) | 0 | 1 (11.1) | |
| Environment | 12 (19.4) | 2 (15.4) | 7 (77.8) | 3 (7.5) | |
| Gentamicin | Human | 16 (22.9) | 16 37.2) | 0 | 0 |
| Milk | 3 (3.9) | 3 (4.8) | 0 | 0 | |
| Environment | 0 | 0 | 0 | 0 | |
| Streptomycin | Human | 15 (21.4) | 15 (34.9) | 0 | 0 |
| Milk | 10 (13.2) | 10 (15.9) | 0 | 0 | |
| Environment | 12 (19.4) | 5 (38.5) | 3 (33.3) | 4 (10.0) | |
| Levofloxacin | Human | 0 | 0 | 0 | 0 |
| Milk | 4 (5.3) | 4 (6.3) | 0 | 0 | |
| Environment | 0 | 0 | 0 | 0 | |
| Linezolid | Human | 3 (4.3) | 0 | 1 (10.0) | 2 (11.8) |
| Milk | 3 (3.9) | 3 (4.8) | 0 | 0 | |
| Environment | 2 (3.2) | 0 | 0 | 2 (5.0) | |
| Nitrofurantoin | Human | 3 (4.3) | 0 | 2 (20.0) | 1 (5.9) |
| Milk | 1 (1.3) | 1 (1.6) | 0 | 0 | |
| Environment | 2 (1.3) | 0 | 1 (11.1) | 1 (2.5) | |
| Tetracycline | Human | 29 (41.4) | 22 (51.2) | 3 (30.0) | 4 (30.0) |
| Milk | 40 (52.6) | 37 (58.7) | 0 | 3 (33.3) | |
| Environment | 20 (32.3) | 2 (15.4) | 6 (66.7) | 12 (30.0) | |
| Vancomycin | Human | 0 | 0 | 0 | 0 |
| Milk | 2 (2.6) | 1 (1.6) | 0 | 1 (11.1) | |
| Environment | 2 (3.2) | 0 | 2 (22.2) | 0 | |
| Number of Antibiotics | Phenotype | AR Profile | Total | H | M | E |
|---|---|---|---|---|---|---|
| 7 | P1 | TET/ERY/STR/GEN/LZD/CIP/LVX | 3 | 0 | 3 | 0 |
| 6 | P2 | TET/ERY/STR/LZD/AMP/PEN | 2 | 0 | 0 | 2 |
| 5 | P3 | TET/ERY/STR/NIT/VAN | 1 | 0 | 0 | 1 |
| 4 | P4 | TET/ERY/STR/GEN | 13 | 13 | 0 | 0 |
| P5 | TET/ERY/STR/VAN | 1 | 0 | 0 | 1 | |
| P6 | TET/ERY/LZD/NIT | 1 | 1 | 0 | 0 | |
| P7 | TET/ERY/CIP/LVX | 1 | 0 | 1 | 0 | |
| 3 | P8 | TET/ERY/GEN | 3 | 3 | 0 | 0 |
| P9 | TET/ERY/LZD | 2 | 2 | 0 | 0 | |
| P10 | TET/ERY/STR | 3 | 1 | 1 | 1 |
| Farm | Number of Lactating Cows | Farm Scale |
|---|---|---|
| A | 105 | Large |
| B | 21 | Small |
| C | 49 | Semi-commercial |
| D | 80 | Commercial |
| E | 5 | Small |
| F | 32 | Semi-commercial |
| G | 110 | Commercial |
| H | 25 | Small |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajendiran, S.; Veloo, Y.; Abdul Rahman, S.; Ismail, R.; Zakaria, Z.; Mansor, R.; Mohd Ali, M.; Khalil, H.; Syed Abu Thahir, S. One Health Approach: Antibiotic Resistance Among Enterococcal Isolates in Dairy Farms in Selangor. Antibiotics 2025, 14, 380. https://doi.org/10.3390/antibiotics14040380
Rajendiran S, Veloo Y, Abdul Rahman S, Ismail R, Zakaria Z, Mansor R, Mohd Ali M, Khalil H, Syed Abu Thahir S. One Health Approach: Antibiotic Resistance Among Enterococcal Isolates in Dairy Farms in Selangor. Antibiotics. 2025; 14(4):380. https://doi.org/10.3390/antibiotics14040380
Chicago/Turabian StyleRajendiran, Sakshaleni, Yuvaneswary Veloo, Salina Abdul Rahman, Rohaida Ismail, Zunita Zakaria, Rozaihan Mansor, Maslina Mohd Ali, Hassuzana Khalil, and Syahidiah Syed Abu Thahir. 2025. "One Health Approach: Antibiotic Resistance Among Enterococcal Isolates in Dairy Farms in Selangor" Antibiotics 14, no. 4: 380. https://doi.org/10.3390/antibiotics14040380
APA StyleRajendiran, S., Veloo, Y., Abdul Rahman, S., Ismail, R., Zakaria, Z., Mansor, R., Mohd Ali, M., Khalil, H., & Syed Abu Thahir, S. (2025). One Health Approach: Antibiotic Resistance Among Enterococcal Isolates in Dairy Farms in Selangor. Antibiotics, 14(4), 380. https://doi.org/10.3390/antibiotics14040380





