Molecular Analysis of Tigecycline Resistance in Carbapenem-Resistant Enterobacterales (CRE) in Mthatha and Surrounding Hospitals
Abstract
:1. Introduction
2. Results
2.1. Carbapenem Resistant Enterobacterales Species
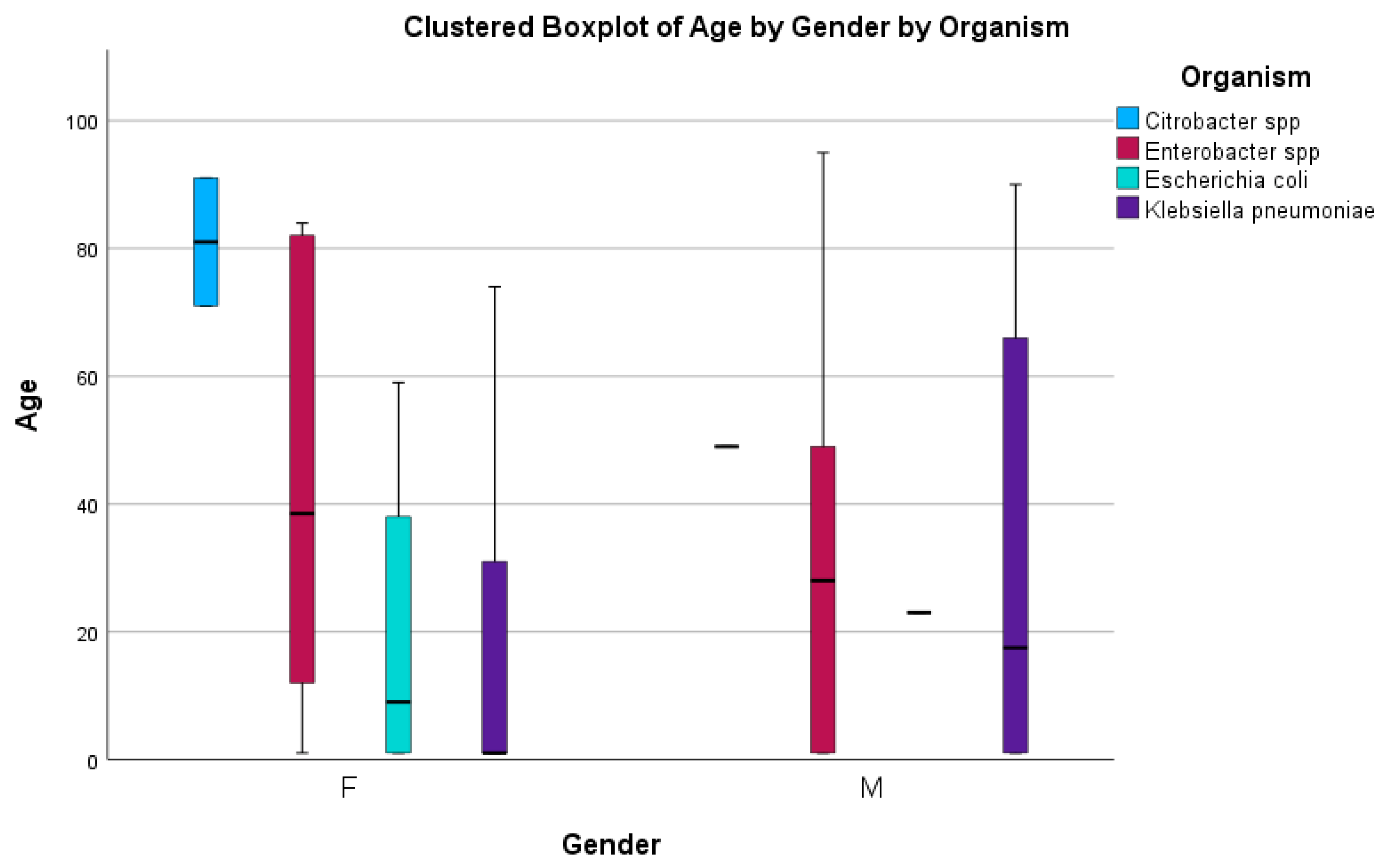
2.2. Tigecycline Susceptibility Using E-Test
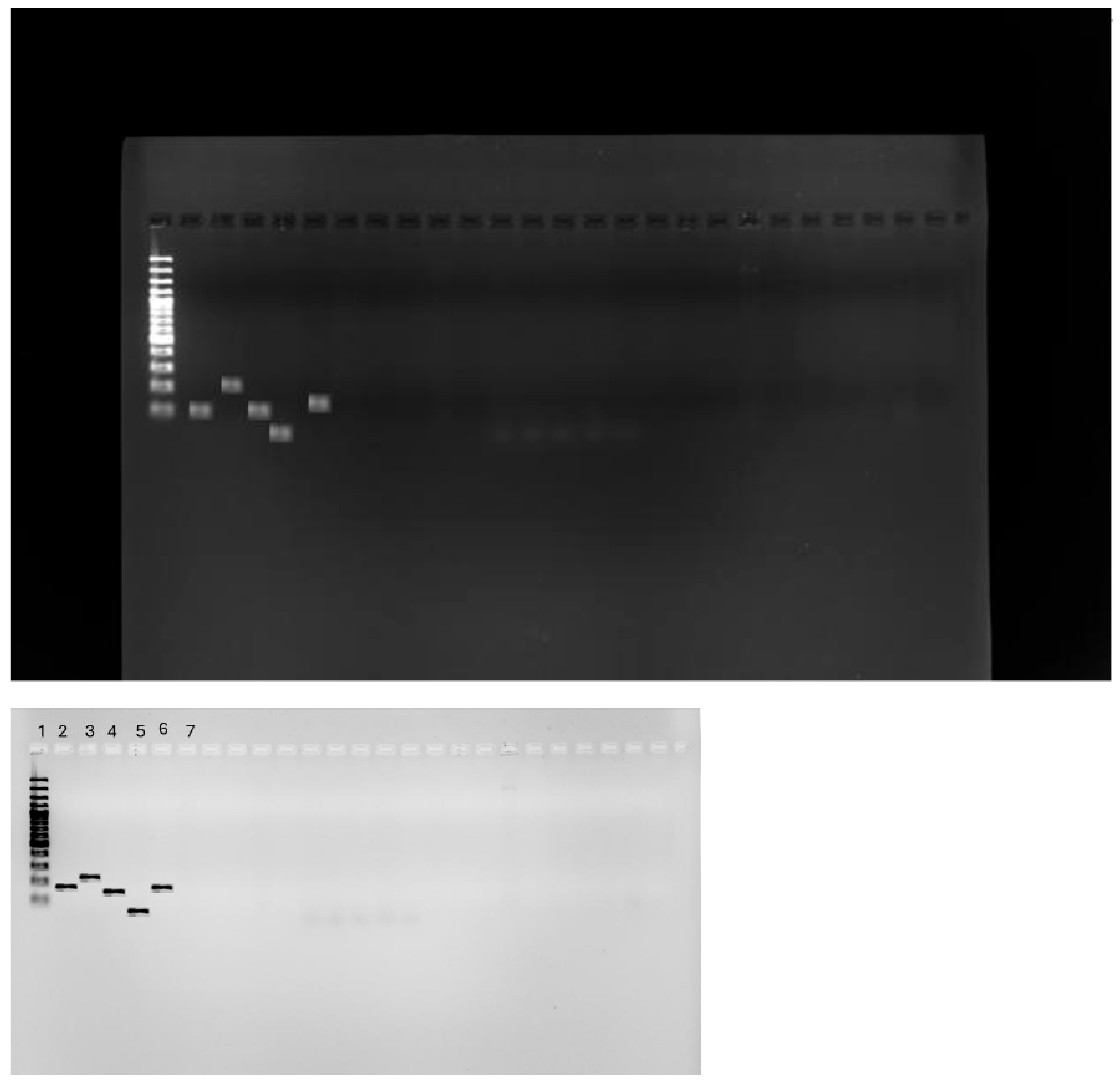
2.3. Detection of tet(X) Genes Using PCR
2.4. Risk Factors Associated with Tigecycline Resistance
3. Discussion
3.1. Determining the CRE Species
3.2. Tigecycline Susceptibility Using E-Test
3.3. PCR for tet(X) Genes
3.4. Risk Factors Associated with Tigecycline
4. Materials and Methods
4.1. Study Area
4.2. Study Design
4.3. Study Setting
4.4. Study Population and Sampling Method
4.5. Data Treatment and Analysis
4.6. Ethical Considerations
4.7. Methodological Design
4.7.1. Antibiotic Susceptibility Testing (E-Test)
4.7.2. DNA Extraction
4.7.3. tet(X) Gene Detection Using Conventional PCR
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lowe, M.; Shuping, L.; Perovic, O. Carbapenem-resistant Enterobacterales in patients with bacteremia at tertiary academic hospitals in South Africa, 2019–2020: An update. S. Afr. Med. J. 2022, 112, 545–552. [Google Scholar]
- Zeng, M.; Xia, J.; Zong, Z.; Shi, Y.; Ni, Y.; Hu, F.; Chen, Y.; Zhuo, C.; Hu, B.; Lv, X.; et al. Guidelines for the diagnosis, treatment, prevention, and control of infections caused by carbapenem-resistant gram-negative bacilli. J. Microbiol. Immunol. Infect. 2023, 56, 653–671. [Google Scholar] [CrossRef] [PubMed]
- Kaye, K.S.; Naas, T.; Pogue, J.M.; Rossolini, G.M. Cefiderocol, a siderophore cephalosporin, as a treatment option for infections caused by carbapenem-resistant Enterobacterales. Infect. Dis. Ther. 2023, 12, 777–806. [Google Scholar] [CrossRef]
- Campany-Herrero, D.; Larrosa-Garcia, M.; Lalueza-Broto, P.; Rivera-Sánchez, L.; Espinosa-Pereiro, J.; Mestre-Torres, J.; Pigrau-Serrallach, C. Tigecycline-associated hypofibrinogenemia in a real-world setting. Int. J. Clin. Pharm. 2020, 42, 1184–1189. [Google Scholar] [CrossRef] [PubMed]
- Hovan, M.R.; Narayanan, N.; Cedarbaum, V.; Bhowmick, T.; Kirn, T.J. Comparing mortality in patients with carbapenemase-producing carbapenem resistant Enterobacterales and non-carbapenemase-producing carbapenem resistant Enterobacterales bacteremia. Diagn. Microbiol. Infect. Dis. 2021, 101, 115505. [Google Scholar] [CrossRef]
- Mzimela, B.W.; Nkwanyana, N.M.; Singh, R. Clinical outcome of neonates with Carbapenem-resistant Enterobacteriaceae infections at the King Edward VIII Hospital’s neonatal unit, Durban, South Africa. S. Afr. J. Infect. Dis. 2021, 36, 223. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, M.; Cai, M.; Liu, K.; Wang, Y.; Zhou, C.; Chang, Z.; Zou, Q.; Xiao, S.; Cao, Y.; et al. An analysis of risk factors for carbapenem-resistant Enterobacteriaceae infection. J. Glob. Antimicrob. Resist. 2022, 30, 191–198. [Google Scholar] [CrossRef]
- Rempel, S.; Stanek, W.K.; Slotboom, D.J. ECF-type ATP-binding cassette transporters. Annu. Rev. Biochem. 2019, 88, 551–576. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Yang, S.; Zhang, Y.; Gao, Y.; Ji, Q.; Fu, L.; Wei, Q.; Sun, F.; Qu, S. Tigecycline-resistance mechanisms and biological characteristics of drug-resistant Salmonella typhimurium strains in vitro. Vet. Microbiol. 2024, 288, 109927. [Google Scholar] [CrossRef]
- Ni, W.; Han, Y.; Liu, J.; Wei, C.; Zhao, J.; Cui, J.; Wang, R.; Liu, Y. Tigecycline treatment for carbapenem-resistant Enterobacteriaceae infections: A systematic review and meta-analysis. Medicine 2016, 95, e3126. [Google Scholar] [CrossRef]
- Cassir, N.; Rolain, J.M.; Brouqui, P. A new strategy to fight antimicrobial resistance: The revival of old antibiotics. Front. Microbiol. 2014, 5, 109581. [Google Scholar] [CrossRef]
- Stein, G.E.; Babinchak, T. Tigecycline: An update. Diagn. Microbiol. Infect. Dis. 2013, 75, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, S.; Zekiy, A.O.; Krutova, M.; Gholami, M.; Kouhsari, E.; Sholeh, M.; Ghafouri, Z.; Maleki, F. Tigecycline antibacterial activity, clinical effectiveness, and mechanisms and epidemiology of resistance: Narrative review. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 1003–1022. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Quan, J.; Yang, Y.; Ji, J.; Liu, L.; Fu, Y.; Hua, X.; Chen, Y.; Pi, B.; Jiang, Y.; et al. Abrp, a new gene, confers reduced susceptibility to tetracycline, glycylcine, chloramphenicol, and fosfomycin classes in Acinetobacter baumannii. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Jenner, L.; Starosta, A.L.; Terry, D.S.; Mikolajka, A.; Filonava, L.; Yusupov, M.; Blanchard, S.C.; Wilson, D.N.; Yusupova, G. Structural basis for potent inhibitory activity of the antibiotic tigecycline during protein synthesis. Proc. Natl. Acad. Sci. USA 2013, 110, 3812–3816. [Google Scholar] [CrossRef]
- Lee, M.; Abbey, T.; Biagi, M.; Wenzler, E. Activity of aztreonam in combination with ceftazidime–avibactam against serine-and metallo-β-lactamase–producing Pseudomonas aeruginosa. Diagn. Microbiol. Infect. Dis. 2021, 99, 115227. [Google Scholar] [CrossRef]
- Li, Y.Y.; Wang, J.; Wang, R.; Cai, Y. Double-carbapenem therapy in the treatment of multidrug resistant Gram-negative bacterial infections: A systematic review and meta-analysis. BMC Infect. Dis. 2020, 20, 408. [Google Scholar] [CrossRef]
- Woodworth, K.R. Vital signs: Containment of novel multidrug-resistant organisms and resistance mechanisms—United States, 2006–2017. MMWR. Morb. Mortal. Wkly. Rep. 2018, 67, 396–401. [Google Scholar] [CrossRef]
- Zhang, R.M.; Sun, J.; Sun, R.Y.; Wang, M.G.; Cui, C.Y.; Fang, L.X.; Liao, M.N.; Lu, X.Q.; Liu, Y.X.; Liao, X.P.; et al. Source tracking and global distribution of the tigecycline non-susceptible tet (X). Microbiol. Spectr. 2021, 9, e0116421. [Google Scholar] [CrossRef]
- Turner, A.M.; Li, L.; Monk, I.R.; Lee, J.Y.H.; Ingle, D.J.; Duchene, S.; Sherry, N.L.; Stinear, T.P.; Kwong, J.C.; Gorrie, C.L.; et al. Rifaximin prophylaxis causes resistance to the last-resort antibiotic daptomycin. medRxiv 2023. [Google Scholar] [CrossRef]
- Rashid, F.A.; Sahlan, N.; Palanisamy, N.K.; Nawi, S.F.; Nor, F.M. Detection of carbapenemase-producing carbapenem resistant Enterobacterales (CP-CRE): A preliminary data from a tertiary hospital in Malaysia. Int. J. Infect. Dis. 2025, 152, 107545. [Google Scholar] [CrossRef]
- Kechagias, K.S.; Chorepsima, S.; Triarides, N.A.; Falagas, M.E. Tigecycline for the treatment of patients with Clostridium difficile infection: An update of the clinical evidence. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Liu, D.; Song, H.; Liu, Z.; Jiang, H.; Wang, Y. Development of a multiplex real-time PCR assay to rapidly detect tigecycline resistance gene tet (X) variants from bacterial, fecal, and environmental samples. Antimicrob. Agents Chemother. 2020, 64, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, J.S.; Kim, H.S.; Yu, J.K.; Han, S.H.; Kang, M.J.; Hong, C.K.; Lee, S.M.; Oh, Y.H. Prevalence of carbapenem-resistant Enterobacteriaceae in Seoul, Korea. J. Bacteriol. Virol. 2020, 50, 107–116. [Google Scholar] [CrossRef]
- Varma, M.; Reddy, L.R.; Vidyasagar, S.; Holla, A.; Bhat, N.K. Risk factors for carbapenem resistant enterobacteriaceae in a teritiary hospital—A case control study. Indian J. Med. Spec. 2018, 9, 178–183. [Google Scholar] [CrossRef]
- Korczak, L.; Majewski, P.; Iwaniuk, D.; Sacha, P.; Matulewicz, M.; Wieczorek, P.; Majewska, P.; Wieczorek, A.; Radziwon, P.; Tryniszewska, E. Molecular mechanisms of tigecycline-resistance among Enterobacterales. Front. Cell. Infect. Microbiol. 2024, 14, 1289396. [Google Scholar] [CrossRef]
- Saeed, N.K.; Alkhawaja, S.; Azam, N.F.A.E.M.; Alaradi, K.; Al-Biltagi, M. Epidemiology of carbapenem-resistant Enterobacteriaceae in a Tertiary Care Center in the Kingdom of Bahrain. J. Lab. Physicians 2019, 11, 111–117. [Google Scholar] [CrossRef]
- Alraddadi, B.M.; Heaphy, E.L.; Aljishi, Y.; Ahmed, W.; Eljaaly, K.; Al-Turkistani, H.H.; Alshukairi, A.N.; Qutub, M.O.; Alodini, K.; Alosaimi, R.; et al. Molecular epidemiology and outcome of carbapenem-resistant Enterobacterales in Saudi Arabia. BMC Infect. Dis. 2022, 22, 542. [Google Scholar] [CrossRef]
- Jiang, Y.; Jia, X.; Xia, Y. Risk factors with the development of infection with tigecycline-and carbapenem-resistant Enterobacter cloacae. Infect. Drug Resist. 2019, 12, 667–674. [Google Scholar] [CrossRef]
- Paveenkittiporn, W.; Lyman, M.; Biedron, C.; Chea, N.; Bunthi, C.; Kolwaite, A.; Janejai, N. Molecular epidemiology of carbapenem-resistant Enterobacterales in Thailand, 2016–2018. Antimicrob. Resist. Infect. Control. 2021, 10, 88. [Google Scholar] [CrossRef]
- Adesanya, O.A.; Igwe, H.A. Carbapenem-resistant Enterobacteriaceae (CRE) and gram-negative bacterial infections in south-west Nigeria: A retrospective epidemiological surveillance study. AIMS Public Health 2020, 7, 804. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, K.; Horwich-Scholefield, S.; Epson, E. Carbapenem and cephalosporin resistance among Enterobacteriaceae in healthcare-associated infections, California, USA. Emerg. Infect. Dis. 2019, 25, 1389. [Google Scholar] [CrossRef]
- Lim, J.; Sim, J.; Lee, H.; Hyun, J.; Lee, S.; Park, S. Characteristics of Carbapenem-resistant Enterobacteriaceae (CRE) in the Republic of Korea, 2022. Public Health Wkly. Rep. 2024, 17, 115–127. [Google Scholar]
- Aslam, B.; Rasool, M.; Muzammil, S.; Siddique, A.B.; Nawaz, Z.; Shafique, M.; Zahoor, M.A.; Binyamin, R.; Waseem, M.; Khurshid, M.; et al. Carbapenem resistance: Mechanisms and drivers of global menace. In Pathogenic Bacteria; IntechOpen: London, UK, 2020; pp. 1–11. [Google Scholar]
- Babaei, S.; Haeili, M. Evaluating the performance characteristics of different antimicrobial susceptibility testing methodologies for testing susceptibility of gram-negative bacteria to tigecycline. BMC Infect. Dis. 2021, 21, 709. [Google Scholar] [CrossRef] [PubMed]
- Hassoun-Kheir, N.; Hussien, K.; Karram, M.; Saffuri, M.; Badaan, S.; Peleg, S.; Aboelhega, W.; Warman, S.; Alon, T.; Pollak, D.; et al. Clinical significance and burden of carbapenem-resistant Enterobacterales (CRE) colonization acquisition in hospitalized patients. Antimicrob. Resist. Infect. Control 2023, 12, 129. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Jiang, Y.; Li, M.M.; Sun, Y.; Cao, J.M.; Zhou, C.; Zhang, X.X.; Qu, Y.; Zhou, T.L. Acquisition of Tigecycline Resistance by carbapenem-resistant Klebsiella pneumoniae confers collateral hypersensitivity to aminoglycosides. Front. Microbiol. 2021, 12, 674502. [Google Scholar] [CrossRef]
- Wang, L.; Tong, X.; Huang, J.; Zhang, L.; Wang, D.; Wu, M.; Liu, T.; Fan, H. Triple versus double therapy for the treatment of severe infections caused by carbapenem-resistant Enterobacteriaceae: A systematic review and meta-analysis. Front. Pharmacol. 2020, 10, 487865. [Google Scholar] [CrossRef]
- Sun, C.; Yu, Y.; Hua, X. Resistance mechanisms of tigecycline in Acinetobacter baumannii. Front. Cell. Infect. Microbiol. 2023, 13, 1141490. [Google Scholar] [CrossRef]
- Wilson, L.A.; Kuruvilla, T.S. Evaluation of in vitro activity of tigecycline against multidrug-resistant clinical isolates. APIK J. Intern. Med. 2023, 11, 150–153. [Google Scholar] [CrossRef]
- Yu, W.L.; Lee, N.Y.; Wang, J.T.; Ko, W.C.; Ho, C.H.; Chuang, Y.C. Tigecycline therapy for infections caused by extended-spectrum β-lactamase-producing Enterobacteriaceae in critically ill patients. Antibiotics 2020, 9, 231. [Google Scholar] [CrossRef]
- Heidary, M.; Sholeh, M.; Asadi, A.; Khah, S.M.; Kheirabadi, F.; Saeidi, P.; Darbandi, A.; Taheri, B.; Ghanavati, R. Prevalence of tigecycline resistance in methicillin-resistant Staphylococcus aureus: A systematic review and meta-analysis. Diagn. Microbiol. Infect. Dis. 2024, 108, 116088. [Google Scholar] [CrossRef] [PubMed]
- Viechtbauer, W.; Cheung, M.W.L. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods 2010, 1, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, A.; Mishra, B.; Loomba, P.S.; Thakur, A.; Sharma, A.; Rathod, P.G.; Das, M.; Bhasin, A. Tigecycline Susceptibility of Carbapenem Resistant Enterobacteriaceae and Acinetobacter spp. isolates from Respiratory Tract: A Tertiary Care Centre Study. J. Krishna Inst. Med. Sci. (JKIMSU) 2020, 9, 1. [Google Scholar]
- Zhang, S.; Wen, J.; Wang, Y.; Wang, M.; Jia, R.; Chen, S.; Liu, M.; Zhu, D.; Zhao, X.; Wu, Y.; et al. Dissemination and prevalence of plasmid-mediated high-level tigecycline resistance gene tet (X4). Front. Microbiol. 2022, 13, 969769. [Google Scholar] [CrossRef]
- Ji, K.; Xu, Y.; Sun, J.; Huang, M.; Jia, X.; Jiang, C.; Feng, Y. Harnessing efficient multiplex PCR methods to detect the expanding Tet (X) family of tigecycline resistance genes. Virulence 2020, 11, 49–56. [Google Scholar] [CrossRef]
- Zhou, C.C.; Huang, F.; Zhang, J.M.; Zhuang, Y.G. Population pharmacokinetics of tigecycline: A systematic review. Drug Des. Dev. Ther. 2023, 16, 1885–1896. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, F.; Jin, P. Clinical Manifestations and Risk Factors of Tigecycline-Associated Thrombocytopenia. Infect. Drug Resist. 2023, 16, 6225–6235. [Google Scholar] [CrossRef]
- Soraci, L.; Cherubini, A.; Paoletti, L.; Filippelli, G.; Luciani, F.; Laganà, P.; Gambuzza, M.E.; Filicetti, E.; Corsonello, A.; Lattanzio, F. Safety and tolerability of antimicrobial agents in the older patient. Drugs Aging 2023, 40, 499–526. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, Y.; Liu, D.; Yang, D.; Liu, Z.; Wang, Y.; Wang, J.; Wang, X.; Xu, X.; Li, X.; et al. Abundance of tigecycline resistance genes and association with antibiotic residues in Chinese livestock farms. J. Hazard. Mater. 2021, 409, 124921. [Google Scholar] [CrossRef]
- Kessel, J.; Bender, J.; Werner, G.; Griskaitis, M.; Herrmann, E.; Lehn, A.; Serve, H.; Zacharowski, K.; Zeuzem, S.; Vehreschild, M.J.; et al. Risk factors and outcomes associated with the carriage of tigecycline vancomycin-resistant Enterococcus faecium. J. Infect. 2021, 82, 227–234. [Google Scholar] [CrossRef]
- Vasaikar, S.D.; Hanise, P.; Abaver, D.T. Epidemiology, risk factors and molecular analysis of carbapenem-resistant Enterobacteriaceae (CRE) in Mthatha, Eastern Cape, South Africa. Int. J. Infect. Dis. 2020, 101, 54. [Google Scholar] [CrossRef]
- M100-S29; The Performance Stands for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2019.


| CRE | Percentage (%) |
|---|---|
| Klebsiella pneumoniae | 53.4% |
| Escherichia coli | 6.7% |
| Enterobacter spp. | 20.5% |
| Citrobacter spp. | 19.4% |
| Species | VIM | KPC | NDM | OXA-48 | Not Detected |
|---|---|---|---|---|---|
| Citrobacter spp. | 0 | 0 | 50% | 25% | 25% |
| Enterobacter spp. | 0 | 0 | 45.6% | 27.8% | 27.% |
| Escherichia coli | 0 | 0 | 60% | 40% | 0 |
| Klebsiella pneumoniae | 0 | 0 | 60.2% | 29.1% | 11.3% |
| Specimen | Type | ||||||
|---|---|---|---|---|---|---|---|
| Species | Abscess | Blood Culture | Pus Swab | Sputum | Urine | Tissue | Other |
| Citrobacter spp. | 0 | 0 | 8.3% | 14.3% | 5.9% | 0 | 0 |
| Enterobacter spp. | 0 | 13% | 33.3% | 33.3% | 41.2% | 0 | 80% |
| Escherichia coli | 0 | 4.35% | 50% | 14.3% | 5.9% | 0 | 0 |
| Klebsiella pneumoniae | 100% | 86.9% | 41.7 | 71.4% | 41.2% | 100% | 20% |
| Chi-Square Tests | |||
|---|---|---|---|
| Value | Df. | Asymptotic Significance | |
| Pearson Chi-square | 47.085 a | 64 | 0.944 |
| Likelihood Ratio | 34.491 | 64 | 0.999 |
| N of Valid Cases | 70 | ||
| Tigecycline Susceptibility | ||||||
|---|---|---|---|---|---|---|
| Tigecycline Resistance Risk Factor | No. of CRE (%) | S | R | I | p-Value | R-Value |
| Ward | 0.1631 | 0.4182 | ||||
| Accident and Emergency | 1.43 | 100 | 0 | 0 | ||
| Adult Care | 1.43 | 100 | 0 | 0 | ||
| Casualty | 2.86 | 100 | 0 | 0 | ||
| Female Surgical | 4.29 | 100 | 0 | 0 | ||
| Gyno | 1.43 | 100 | 0 | 0 | ||
| ICU | 5.71 | 75 | 25 | 0 | ||
| Male Surgical | 14.29 | 100 | 0 | 0 | ||
| Maternity | 8.57 | 100 | 0 | 0 | ||
| Neonatal | 24.29 | 94.11 | 0 | 5.88 | ||
| Trauma | 2.86 | 100 | 0 | 0 | ||
| Not stated (unknown) | 1.43 | 100 | 0 | 0 | ||
| OPD | 11.43 | 100 | 0 | 0 | ||
| Pediatrics | 20 | 100 | 0 | 0 | ||
| Age | 0.0011 | 0.9501 | ||||
| 0–1 | 42.86 | 98.57 | 0 | 1.43 | ||
| 2–15 | 2.8 | 100 | 0 | 0 | ||
| 16–29 | 11.42 | 100 | 0 | 0 | ||
| 30–43 | 8.57 | 98.57 | 1.43 | 0 | ||
| 44–56 | 8.57 | 100 | 0 | 0 | ||
| 57–70 | 11.42 | 100 | 0 | 0 | ||
| 71–83 | 7.14 | 100 | 0 | 0 | ||
| 84–96 | 5.71 | 100 | 0 | 0 | ||
| 97–109 | 0 | 0 | 0 | 0 | ||
| Gender | 0.001 | 0.991 | ||||
| Males | 47.88 | 97.05 | 2.94 | 100 | ||
| Females | 50.7 | 100 | 100 | 2.94 | ||
| Not stated | 1.43 | 100 | 100 | 100 | ||
| Prior exposureto antibiotics | 0.0478 | 0.967 | ||||
| Antibiotic intake | 75.71 | 98.11 | 1.88 | 0 | ||
| No antibiotics taken | 24.29 | 94.41 | 0 | 5.88 | ||
| Invasive procedure | ||||||
| Underwent procedure | 5.63 | 75 | 25 | 0 | <0.0001 | |
| No procedure | 94.37 | 98.51 | 1.49 | 0 | ||
| Hospitalization duration | 0.3679 | |||||
| Prolonged | 10 | 98.69 | 1.41 | 0 | ||
| Standardized | 90 | 98.68 | 0 | 1.41 | ||
| Gene | Sequences 5’-3’ | Band Size | Annealing Temperature (°C) | References |
|---|---|---|---|---|
| tet(X1) | F-CAGCGTTTCCGAGTTCTTGA R -GGACGATTACTCTTCCAAGGCT | 141 | 80.6 | [23] |
| tet(X2) | F -TGCGGCTAATGGCATCTCAC R- GCTGCTACACATGACAACGTCGT | 227 | 81.6 | [23] |
| tet(X3) | F- GTGGATGCTTTGCTATTGTCTGA R -TCTGTTGATTCGTCCTGCGTAT | 125 | 79.5 | [23] |
| tet(X4) | tet(X4)-F TTGGGACGAACGCTACAAAG tet(X4)-R CATCAACCCGCTGTTTACGC | 93 | 55 | [17] |
| tet(X5) | F- TGCCGTTGACCTACACAAAGG R -TGTCAAAACGATTTTCG GGTC | 161 | 80.9 | [23] |
| Step | Temperature (°C) | Time | Cycles |
|---|---|---|---|
| Activation | 50 | 2 min | 1 |
| Denaturation | 95 | 3 min | 40 |
| Annealing | 95 | 30 s | 1 |
| Elongation | 60 | 30 s | 1 |
| Final Elongation | 72 | 30 s | 1 |
| Storage | 4 | Indefinite | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vumba, L.; Singh, R.; Vasaikar, S. Molecular Analysis of Tigecycline Resistance in Carbapenem-Resistant Enterobacterales (CRE) in Mthatha and Surrounding Hospitals. Antibiotics 2025, 14, 407. https://doi.org/10.3390/antibiotics14040407
Vumba L, Singh R, Vasaikar S. Molecular Analysis of Tigecycline Resistance in Carbapenem-Resistant Enterobacterales (CRE) in Mthatha and Surrounding Hospitals. Antibiotics. 2025; 14(4):407. https://doi.org/10.3390/antibiotics14040407
Chicago/Turabian StyleVumba, Luyolo, Ravesh Singh, and Sandeep Vasaikar. 2025. "Molecular Analysis of Tigecycline Resistance in Carbapenem-Resistant Enterobacterales (CRE) in Mthatha and Surrounding Hospitals" Antibiotics 14, no. 4: 407. https://doi.org/10.3390/antibiotics14040407
APA StyleVumba, L., Singh, R., & Vasaikar, S. (2025). Molecular Analysis of Tigecycline Resistance in Carbapenem-Resistant Enterobacterales (CRE) in Mthatha and Surrounding Hospitals. Antibiotics, 14(4), 407. https://doi.org/10.3390/antibiotics14040407







