Antimicrobial Susceptibility and Toxin Gene Profiles of Commensal Clostridium perfringens Isolates from Turkeys in Hungarian Poultry Farms (2022–2023)
Abstract
:1. Introduction
2. Results
2.1. Regional Distribution and Origin of Strains Received
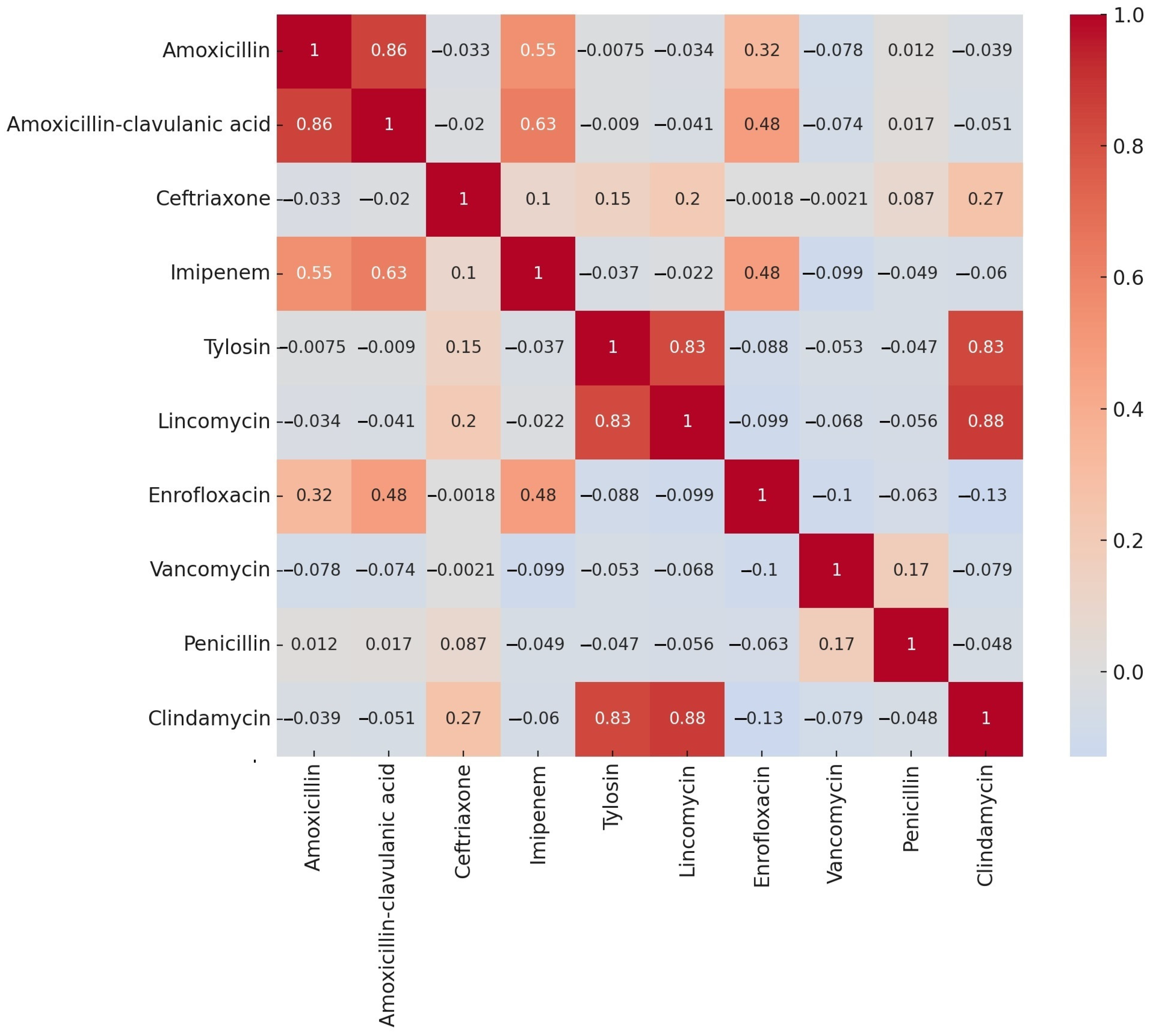
2.2. Antimicrobial Susceptibility Testing
3. Discussion
4. Materials and Methods
4.1. The Origin of the Strains
4.2. Minimum Inhibitory Concentration (MIC) Determination
4.3. PCR Tests
4.4. Statistic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- World Bank. People, Pathogens and Our Planet: The Economics of One Health; World Bank: Washington, DC, USA, 2012. [Google Scholar]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Dehove, A.; Commault, J.; Petitclerc, M.; Teissier, M.; Macé, J. Economic Analysis and Costing of Animal Health: A Literature Review of Methods and Importance. Rev. Sci. Tech. 2012, 31, 605–617+591–604. [Google Scholar] [CrossRef]
- Prescott, J.F. The Resistance Tsunami, Antimicrobial Stewardship, and the Golden Age of Microbiology. Vet. Microbiol. 2014, 171, 273–278. [Google Scholar] [CrossRef]
- Samreen; Ahmad, I.; Malak, H.A.; Abulreesh, H.H. Environmental Antimicrobial Resistance and Its Drivers: A Potential Threat to Public Health. J. Glob. Antimicrob. Resist. 2021, 27, 101–111. [Google Scholar] [CrossRef]
- Gibson, M.K.; Forsberg, K.J.; Dantas, G. Improved Annotation of Antibiotic Resistance Determinants Reveals Microbial Resistomes Cluster by Ecology. ISME J. 2015, 9, 207. [Google Scholar] [CrossRef]
- Timbermont, L.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F. Necrotic Enteritis in Broilers: An Updated Review on the Pathogenesis. Avian Pathol. 2011, 40, 341–347. [Google Scholar] [CrossRef]
- Kiu, R.; Hall, L.J. An Update on the Human and Animal Enteric Pathogen Clostridium perfringens. Emerg. Microbes Infect. 2018, 7, 1–15. [Google Scholar] [CrossRef]
- Komatsu, H.; Inui, A.; Sogo, T.; Fujisawa, T. Clostridium perfringens. Nihon rinsho. Jpn. J. Clin. Med. 2012, 70, 1357–1361. [Google Scholar]
- Mehdizadeh Gohari, I.; Navarro, M.A.; Li, J.; Shrestha, A.; Uzal, F.; McClane, B.A. Pathogenicity and Virulence of Clostridium perfringens. Virulence 2021, 12, 723–753. [Google Scholar] [CrossRef]
- Joshy, L.; Chaudhry, R.; Dhawan, B.; Kumar, L.; Das, B.K. Incidence and Characterization of Clostridium perfringens Isolated from Antibiotic-Associated Diarrhoeal Patients: A Prospective Study in an Indian Hospital. J. Hosp. Infect. 2006, 63, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Rood, J.I.; Adams, V.; Lacey, J.; Lyras, D.; McClane, B.A.; Melville, S.B.; Moore, R.J.; Popoff, M.R.; Sarker, M.R.; Songer, J.G.; et al. Expansion of the Clostridium perfringens Toxin-Based Typing Scheme. Anaerobe 2018, 53, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.; Ramírez, J.D.; Kiu, R.; Hall, L.J.; Muñoz, M. Unveiling the Pathogenic Mechanisms of Clostridium perfringens Toxins and Virulence Factors. Emerg. Microbes Infect. 2024, 13, 2341968. [Google Scholar] [CrossRef]
- Ronco, T.; Stegger, M.; Ng, K.L.; Lilje, B.; Lyhs, U.; Andersen, P.S.; Pedersen, K. Genome Analysis of Clostridium perfringens Isolates from Healthy and Necrotic Enteritis Infected Chickens and Turkeys. BMC Res. Notes 2017, 10, 270. [Google Scholar] [CrossRef]
- Slavić, D.; Boerlin, P.; Fabri, M.; Klotins, K.C.; Zoethout, J.K.; Weir, P.E.; Bateman, D. Antimicrobial Susceptibility of Clostridium perfringens Isolates of Bovine, Chicken, Porcine, and Turkey Origin from Ontario. Can. J. Vet. Res. 2011, 75, 89–97. [Google Scholar]
- Gazdzinski, P.; Julian, R.J. Necrotic Enteritis in Turkeys. Avian Dis. 1992, 36, 792–798. [Google Scholar] [CrossRef]
- Smyth, J.A.; Mishra, N.; Shivaprasad, H.L. Toxinotyping of Clostridium perfringens Strains Recovered from U.S. Turkeys with Necrotic Enteritis. Avian Dis. 2022, 66, 193–196. [Google Scholar] [CrossRef]
- Hardy, S.P.; Benestad, S.L.; Hamnes, I.S.; Moldal, T.; David, B.; Barta, J.R.; Reperant, J.-M.; Kaldhusdal, M. Developing an Experimental Necrotic Enteritis Model in Turkeys—The Impact of Clostridium perfringens, Eimeria meleagrimitis and Host Age on Frequency of Severe Intestinal Lesions. BMC Vet. Res. 2020, 16, 63. [Google Scholar] [CrossRef]
- Thachil, A.J.; McComb, B.; Andersen, M.M.; Shaw, D.P.; Halvorson, D.A.; Nagaraja, K.V. Role of Clostridium perfringens and Clostridium septicum in Causing Turkey Cellulitis. Avian Dis. 2010, 54, 795–801. [Google Scholar] [CrossRef]
- Clark, S.; Porter, R.; McComb, B.; Lipper, R.; Olson, S.; Nohner, S.; Shivaprasad, H.L. Clostridial Dermatitis and Cellulitis: An Emerging Disease of Turkeys. Avian Dis. 2010, 54, 788–794. [Google Scholar] [CrossRef]
- Forga, A.; Robbins, K.; Smith, A.; Coles, M.; Tellez-Isaias, G.; Vuong, C.N.; Hargis, B.; Graham, D. Evaluation of Clostridium septicum Hemolytic Activity, Administration Route, and Dosage Volume of a Clostridial Dermatitis (Cellulitis) Bacterin-Toxoid on Humoral Immune Response in Commercial Turkeys. Poult. Sci. 2023, 102, 102873. [Google Scholar] [CrossRef] [PubMed]
- Huff, G.R.; Huff, W.E.; Rath, N.C. Dexamethasone Immunosuppression Resulting in Turkey Clostridial Dermatitis: A Retrospective Analysis of Seven Studies, 1998–2009. Avian Dis. 2013, 57, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Agunos, A.; Carson, C.; Léger, D. Antimicrobial Therapy of Selected Diseases in Turkeys, Laying Hens, and Minor Poultry Species in Canada. Can. Vet. J. 2013, 54, 1041–1052. [Google Scholar]
- Yadav, J.P.; Das, S.C.; Dhaka, P.; Vijay, D.; Kumar, M.; Mukhopadhyay, A.K.; Chowdhury, G.; Chauhan, P.; Singh, R.; Dhama, K.; et al. Molecular Characterization and Antimicrobial Resistance Profile of Clostridium perfringens Type A Isolates from Humans, Animals, Fish and Their Environment. Anaerobe 2017, 47, 120–124. [Google Scholar] [CrossRef]
- Grass, J.E.; Gould, L.H.; Mahon, B.E. Epidemiology of Foodborne Disease Outbreaks Caused by Clostridium perfringens, United States, 1998–2010. Foodborne Pathog. Dis. 2013, 10, 131–136. [Google Scholar] [CrossRef]
- Scharff, R.L. Economic Burden from Health Losses Due to Foodborne Illness in the United States. J. Food Prot. 2012, 75, 123–131. [Google Scholar] [CrossRef]
- Mohiuddin, M.; Song, Z.; Liao, S.; Qi, N.; Li, J.; Lv, M.; Lin, X.; Cai, H.; Hu, J.; Liu, S.; et al. Animal Model Studies, Antibiotic Resistance and Toxin Gene Profile of NE Reproducing Clostridium perfringens Type A and Type G Strains Isolated from Commercial Poultry Farms in China. Microorganisms 2023, 11, 622. [Google Scholar] [CrossRef]
- Hassani, S.; Pakbin, B.; Brück, W.M.; Mahmoudi, R.; Mousavi, S. Prevalence, Antibiotic Resistance, Toxin-Typing and Genotyping of Clostridium perfringens in Raw Beef Meats Obtained from Qazvin City, Iran. Antibiotics 2022, 11, 340. [Google Scholar] [CrossRef]
- Johansson, A.; Greko, C.; Engström, B.E.; Karlsson, M. Antimicrobial Susceptibility of Swedish, Norwegian and Danish Isolates of Clostridium perfringens from Poultry, and Distribution of Tetracycline Resistance Genes. Vet. Microbiol. 2004, 99, 251–257. [Google Scholar] [CrossRef]
- Hetényi, N.; Bersényi, A.; Hullár, I. Physiological Effects of Medium-Chain Fatty Acids and Triglycerides, and Their Potential Use in Poultry and Swine Nutrition: A Literature Review. Magy. Állatorvosok Lapja 2024, 146, 651–659. [Google Scholar] [CrossRef]
- Sebők, C.; Márton, R.A.; Meckei, M.; Neogrády, Z.; Mátis, G. Antimicrobial Peptides as New Tools to Combat Infectious Diseases. Magy. Állatorvosok Lapja 2024, 146, 181–191. [Google Scholar] [CrossRef]
- Jerzsele, Á.; Somogyi, Z.; Szalai, M.; Kovács, D. Effects of Fermented Wheat Germ Extract on Artificial Salmonella Typhimurium Infection in Broiler Chickens. Magy. Állatorvosok Lapja 2020, 142, 77–85. [Google Scholar]
- Kovács, L.; Nagy, D.; Könyves, L.; Jerzsele, Á.; Kerek, Á. Antimicrobial Properties of Essential Oils—Animal Health Aspects. Magy. Állatorvosok Lapja 2023, 145, 497–510. [Google Scholar] [CrossRef]
- Olasz, Á.; Jerzsele, Á.; Balta, L.; Dobra, P.F.; Kerek, Á. In Vivo Efficacy of Different Extracts of Propolis in Broiler Salmonellosis. Magy. Állatorvosok Lapja 2023, 145, 461–475. [Google Scholar] [CrossRef]
- Kerek, Á.; Csanády, P.; Jerzsele, Á. Antibacterial Efficiency of Propolis—Part 1. Magy. Állatorvosok Lapja 2022, 144, 285–298. [Google Scholar]
- Farkas, M.; Könyves, L.; Csorba, S.; Farkas, Z.; Józwiák, Á.; Süth, M.; Kovács, L. Biosecurity Situation of Large-Scale Poultry Farms in Hungary According to the Databases of National Food Chain Safety Office Centre for Disease Control and Biosecurity Audit System of Poultry Product Board of Hungary in the Period of 2021–2022. Magy. Állatorvosok Lapja 2024, 146, 723–742. [Google Scholar] [CrossRef]
- Mag, P.; Németh, K.; Somogyi, Z.; Jerzsele, Á. Antibacterial therapy based on pharmacokinetic/ pharmacodynamic models in small animal medicine-1. Literature review. Magy. Állatorvosok Lapja 2023, 145, 419–438. [Google Scholar] [CrossRef]
- Essősy, M.; Fodor, I.; Ihnáth, Z.; Karancsi, Z.; Kovács, D.; Szalai, K.V.; Szentmiklósi, D.; Jerzsele, Á. The Possibilities of Antibiotic-Free Broiler-Hen Fattening, with Special Reference to the Use of Pre- and Probiotics. Magy. Állatorvosok Lapja 2020, 142, 397–407. [Google Scholar]
- Kovács, D.; Palkovicsné Pézsa, N.; Farkas, O.; Jerzsele, Á. Usage of Antibiotic Alternatives in Pig Farming: Literature Review. Magy. Állatorvosok Lapja 2021, 143, 281–282. [Google Scholar]
- Silva, R.O.S.; Salvarani, F.M.; Assis, R.A.; Martins, N.R.S.; Pires, P.S.; Lobato, F.C.F. Antimicrobial Susceptibility of Clostridium perfringens Strains Isolated from Broiler Chickens. Braz. J. Microbiol. 2009, 40, 262–264. [Google Scholar] [CrossRef]
- Martel, A.; Devriese, L.A.; Cauwerts, K.; De Gussem, K.; Decostere, A.; Haesebrouck, F. Susceptibility of Clostridium perfringens Strains from Broiler Chickens to Antibiotics and Anticoccidials. Avian Pathol. 2004, 33, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Osman, K.M.; Elhariri, M. Antibiotic resistance of Clostridium perfringens isolates from broiler chickens in Egypt. Rev. Sci. Tech. 2013, 32, 841–850. [Google Scholar] [CrossRef]
- Akhi, M.T.; Asl, S.B.; Pirzadeh, T.; Naghili, B.; Yeganeh, F.; Memar, Y.; Mohammadzadeh, Y. Antibiotic Sensitivity of Clostridium perfringens Isolated From Faeces in Tabriz, Iran. Jundishapur J. Microbiol. 2015, 8, e20863. [Google Scholar] [CrossRef]
- Duc, H.M.; Hoa, T.T.K.; Ha, C.T.T.; Van Hung, L.; Van Thang, N.; Minh Son, H.; Flory, G.A. Prevalence and Antibiotic Resistance Profile of Clostridium perfringens Isolated from Pork and Chicken Meat in Vietnam. Pathogens 2024, 13, 400. [Google Scholar] [CrossRef]
- Archambault, M.; Rubin, J.E. Antimicrobial Resistance in Clostridium and Brachyspira spp. and Other Anaerobes. Microbiol. Spectr. 2020, 8. [Google Scholar] [CrossRef]
- Priya, G.B.; Srinivas, K.; Shilla, H.; Milton, A.A.P. High Prevalence of Multidrug-Resistant, Biofilm-Forming Virulent Clostridium perfringens in Broiler Chicken Retail Points in Northeast India. Foods 2023, 12, 4185. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Yang, D.; Zhang, S.; Sun, Z.; Wang, Y.; Wang, S.; Wu, C. Prevalence and Antimicrobial Susceptibility of Clostridium perfringens in Chickens and Pigs from Beijing and Shanxi, China. Vet. Microbiol. 2020, 252, 108932. [Google Scholar] [CrossRef]
- de Souza Barbosa, F.; Capra Pezzi, L.; Tsao, M.; Franco de Oliveira, T.; Manoela Dias Macedo, S.; Schapoval, E.E.S.; S L Mendez, A. Stability and Degradation Products of Imipenem Applying High-Resolution Mass Spectrometry: An Analytical Study Focused on Solutions for Infusion. Biomed. Chromatogr. 2019, 33, e4471. [Google Scholar] [CrossRef]
- Park, C.S.; Hwang, J.Y.; Cho, G.J. The First Identification and Antibiogram of Clostridium perfringens Type C Isolated from Soil and The Feces of Dead Foals in South Korea. Animals 2019, 9, 579. [Google Scholar] [CrossRef]
- Gholamiandehkordi, A.; Eeckhaut, V.; Lanckriet, A.; Timbermont, L.; Bjerrum, L.; Ducatelle, R.; Haesebrouck, F.; Van Immerseel, F. Antimicrobial Resistance in Clostridium Perfringens Isolates from Broilers in Belgium. Vet Res Commun 2009, 33, 1031–1037. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Elbestawy, A.R.; El-Shall, N.A.; Saad, A.M.; Salem, H.M.; El-Tahan, A.M.; Khafaga, A.F.; Taha, A.E.; AbuQamar, S.F.; et al. Necrotic Enteritis in Broiler Chickens: Disease Characteristics and Prevention Using Organic Antibiotic Alternatives—A Comprehensive Review. Poult. Sci. 2022, 101, 101590. [Google Scholar] [CrossRef] [PubMed]
- Beres, C.; Colobatiu, L.; Tabaran, A.; Mihaiu, R.; Mihaiu, M. Prevalence and Characterisation of Clostridium perfringens Isolates in Food-Producing Animals in Romania. Microorganisms 2023, 11, 1373. [Google Scholar] [CrossRef] [PubMed]
- Watkins, K.L.; Shryock, T.R.; Dearth, R.N.; Saif, Y.M. In-Vitro Antimicrobial Susceptibility of Clostridium perfringens from Commercial Turkey and Broiler Chicken Origin. Vet. Microbiol. 1997, 54, 195–200. [Google Scholar] [CrossRef]
- Gharaibeh, S.; Al Rifai, R.; Al-Majali, A. Molecular Typing and Antimicrobial Susceptibility of Clostridium perfringens from Broiler Chickens. Anaerobe 2010, 16, 586–589. [Google Scholar] [CrossRef]
- Eubank, T.A.; Gonzales-Luna, A.J.; Hurdle, J.G.; Garey, K.W. Genetic Mechanisms of Vancomycin Resistance in Clostridioides difficile: A Systematic Review. Antibiotics 2022, 11, 258. [Google Scholar] [CrossRef]
- Pu, M.; Cho, J.M.; Cunningham, S.A.; Behera, G.K.; Becker, S.; Amjad, T.; Greenwood-Quaintance, K.E.; Mendes-Soares, H.; Jones-Hall, Y.; Jeraldo, P.R.; et al. Plasmid Acquisition Alters Vancomycin Susceptibility in Clostridioides difficile. Gastroenterology 2021, 160, 941–945.e8. [Google Scholar] [CrossRef]
- Fujiya, Y.; Harada, T.; Sugawara, Y.; Akeda, Y.; Yasuda, M.; Masumi, A.; Hayashi, J.; Tanimura, N.; Tsujimoto, Y.; Shibata, W.; et al. Transmission Dynamics of a Linear VanA-Plasmid during a Nosocomial Multiclonal Outbreak of Vancomycin-Resistant Enterococci in a Non-Endemic Area, Japan. Sci. Rep. 2021, 11, 14780. [Google Scholar] [CrossRef]
- Lyhs, U.; Perko-Mäkelä, P.; Kallio, H.; Brockmann, A.; Heinikainen, S.; Tuuri, H.; Pedersen, K. Characterization of Clostridium perfringens Isolates from Healthy Turkeys and from Turkeys with Necrotic Enteritis. Poult. Sci. 2013, 92, 1750–1757. [Google Scholar] [CrossRef]
- Wei, B.; Cha, S.-Y.; Zhang, J.-F.; Shang, K.; Park, H.-C.; Kang, J.; Lee, K.-J.; Kang, M.; Jang, H.-K. Antimicrobial Susceptibility and Association with Toxin Determinants in Clostridium perfringens Isolates from Chickens. Microorganisms 2020, 8, 1825. [Google Scholar] [CrossRef]
- Darcie, E.C. M11 Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria, 9th ed.; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Dubraska, D.-C.; Claire, B. VET01SEd7; Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 7th ed.; CLSI: Wayne, PA, USA, 2024. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; Volume CLSI standards M07. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Habibzadeh, F. Data Distribution: Normal or Abnormal? J. Korean Med. Sci. 2024, 39, e35. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Fay, M.P.; Proschan, M.A. Wilcoxon-Mann-Whitney or t-Test? On Assumptions for Hypothesis Tests and Multiple Interpretations of Decision Rules. Stat. Surv. 2010, 4, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Connelly, L.M. T-Tests. Medsurg Nurs. 2011, 20, 341. [Google Scholar]
- Dunn, O.J. Multiple Comparisons among Means. J. Am. Stat. Assoc. 1961, 56, 52–64. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal Component Analysis: A Review and Recent Developments. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Sibson, R. SLINK: An Optimally Efficient Algorithm for the Single-Link Cluster Method. Comput. J. 1973, 16, 30–34. [Google Scholar] [CrossRef]


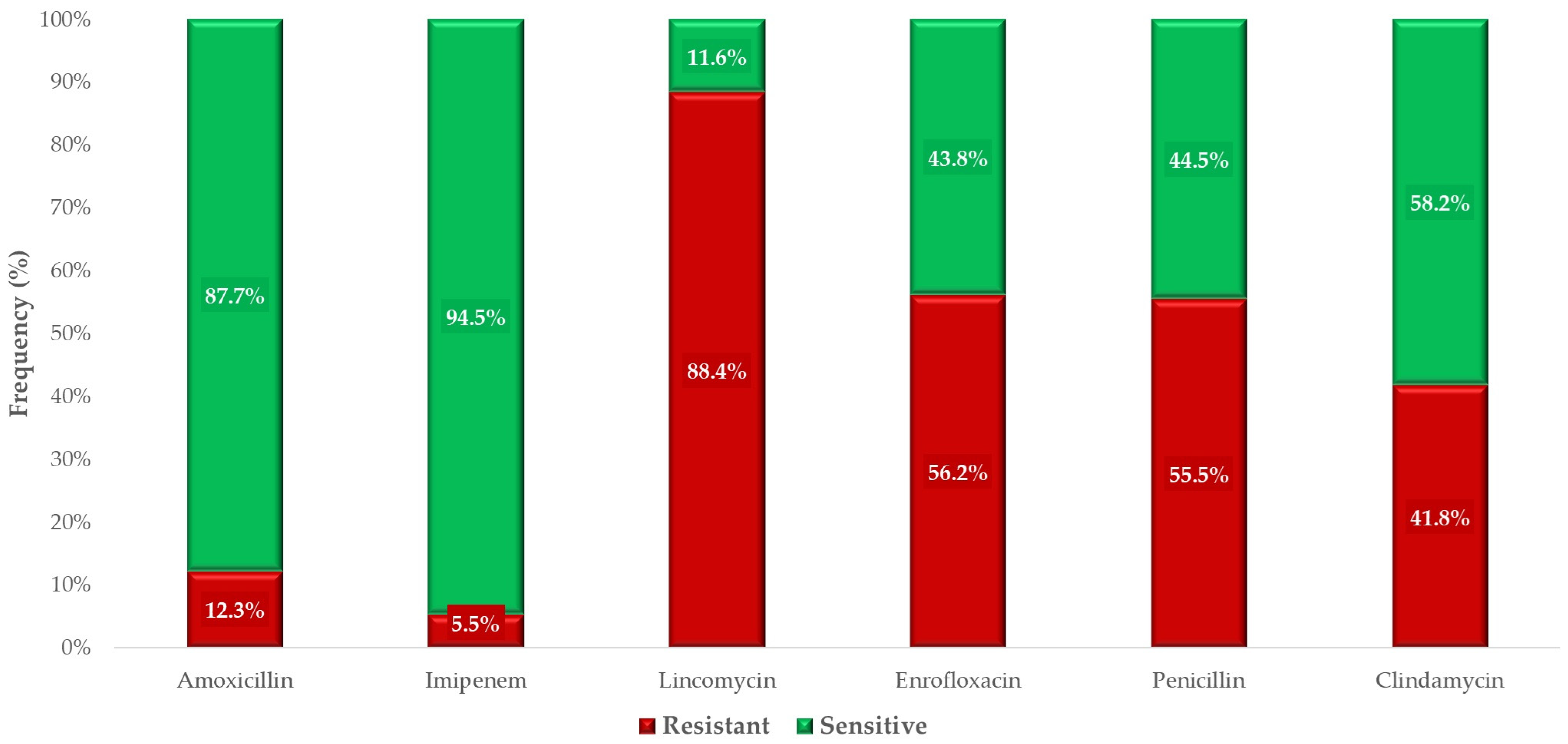
| Antibiotics | Broiler–Breeding | 1 Young–2 Adult | 3 Small–4 Medium |
|---|---|---|---|
| Amoxicillin | 0.0093 * | 0.0093 * | 0.0915 |
| Imipenem | 0.0973 | 0.0973 | 0.5595 |
| Lincomycin | 0.6313 | 0.6313 | 0.7438 |
| Enrofloxacin | 0.4941 | 0.4941 | 0.0499 * |
| Penicillin | 0.6939 | 0.6939 | 0.8216 |
| Clindamycin | 0.0043 * | 0.0043 * | 0.0084 * |
| Antibiotic | 1 BP * | 0.001 | 0.002 | 0.004 | 0.008 | 0.016 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 | MIC50 | MIC90 | 2 ECOFF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| µg/mL | µg/mL | ||||||||||||||||||||||||
| Amoxicillin | 16 | 1 | 0 | 5 | 6 | 9 | 6 | 8 | 19 | 18 | 26 | 15 | 12 | 3 | 0 | 0 | 18 | 0.5 | 32 | - | |||||
| 0.7% | 0.0% | 3.4% | 4.1% | 6.2% | 4.1% | 5.5% | 13.0% | 12.3% | 17.8% | 10.3% | 8.2% | 2.1% | 0.0% | 0.0% | 12.3% | ||||||||||
| Imipenem | 16 | 4 | 0 | 0 | 3 | 5 | 21 | 10 | 14 | 13 | 16 | 10 | 18 | 3 | 21 | 8 | 0.5 | 8 | - | ||||||
| 2.7% | 0.0% | 0.0% | 2.1% | 3.4% | 14.4% | 6.8% | 9.6% | 8.9% | 11.0% | 6.8% | 12.3% | 2.1% | 14.5% | 5.5% | |||||||||||
| Lincomycin | 1 | 3 | 0 | 0 | 0 | 2 | 1 | 11 | 5 | 32 | 11 | 6 | 8 | 10 | 46 | 2 | 0 | 4 | 5 | 16 | 64 | - | |||
| 2.1% | 0.0% | 0.0% | 0.0% | 1.4% | 0.7% | 7.5% | 3.4% | 21.9% | 7.5% | 4.1% | 5.5% | 6.8% | 31.5% | 1.4% | 0.0% | 2.7% | 3.4% | ||||||||
| Enrofloxacin | 2 | 1 | 0 | 0 | 6 | 14 | 15 | 28 | 23 | 9 | 12 | 38 | 2 | 16 | - | ||||||||||
| 0.7% | 0.0% | 0.0% | 4.1% | 9.6% | 10.3% | 19.2% | 15.8% | 6.2% | 8.2% | 26.0% | |||||||||||||||
| Penicillin | 1 | 1 | 3 | 5 | 4 | 6 | 20 | 26 | 28 | 18 | 8 | 5 | 2 | 13 | 0 | 1 | 3 | 3 | 1 | 32 | - | ||||
| 0.7% | 2.1% | 3.4% | 2.7% | 4.1% | 13.7% | 17.8% | 19.2% | 12.3% | 5.5% | 3.4% | 1.4% | 8.9% | 0.0% | 0.7% | 2.1% | 2.1% | |||||||||
| Clindamycin | 8 | 1 | 16 | 1 | 3 | 12 | 8 | 12 | 8 | 5 | 15 | 4 | 5 | 6 | 39 | 1 | 2 | 1 | 6 | 1 | 2 | 32 | 0.125 | ||
| 0.7% | 11.0% | 0.7% | 2.1% | 8.2% | 5.5% | 8.2% | 5.5% | 3.4% | 10.3% | 2.7% | 3.4% | 4.1% | 26.7% | 0.7% | 1.4% | 0.7% | 4.1% | 0.7% | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerek, Á.; Szabó, Á.; Barnácz, F.; Csirmaz, B.; Kovács, L.; Jerzsele, Á. Antimicrobial Susceptibility and Toxin Gene Profiles of Commensal Clostridium perfringens Isolates from Turkeys in Hungarian Poultry Farms (2022–2023). Antibiotics 2025, 14, 413. https://doi.org/10.3390/antibiotics14040413
Kerek Á, Szabó Á, Barnácz F, Csirmaz B, Kovács L, Jerzsele Á. Antimicrobial Susceptibility and Toxin Gene Profiles of Commensal Clostridium perfringens Isolates from Turkeys in Hungarian Poultry Farms (2022–2023). Antibiotics. 2025; 14(4):413. https://doi.org/10.3390/antibiotics14040413
Chicago/Turabian StyleKerek, Ádám, Ábel Szabó, Franciska Barnácz, Bence Csirmaz, László Kovács, and Ákos Jerzsele. 2025. "Antimicrobial Susceptibility and Toxin Gene Profiles of Commensal Clostridium perfringens Isolates from Turkeys in Hungarian Poultry Farms (2022–2023)" Antibiotics 14, no. 4: 413. https://doi.org/10.3390/antibiotics14040413
APA StyleKerek, Á., Szabó, Á., Barnácz, F., Csirmaz, B., Kovács, L., & Jerzsele, Á. (2025). Antimicrobial Susceptibility and Toxin Gene Profiles of Commensal Clostridium perfringens Isolates from Turkeys in Hungarian Poultry Farms (2022–2023). Antibiotics, 14(4), 413. https://doi.org/10.3390/antibiotics14040413







