Prevalence of Antibiotic-Resistant Pulmonary Tuberculosis in Bangladesh: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Results
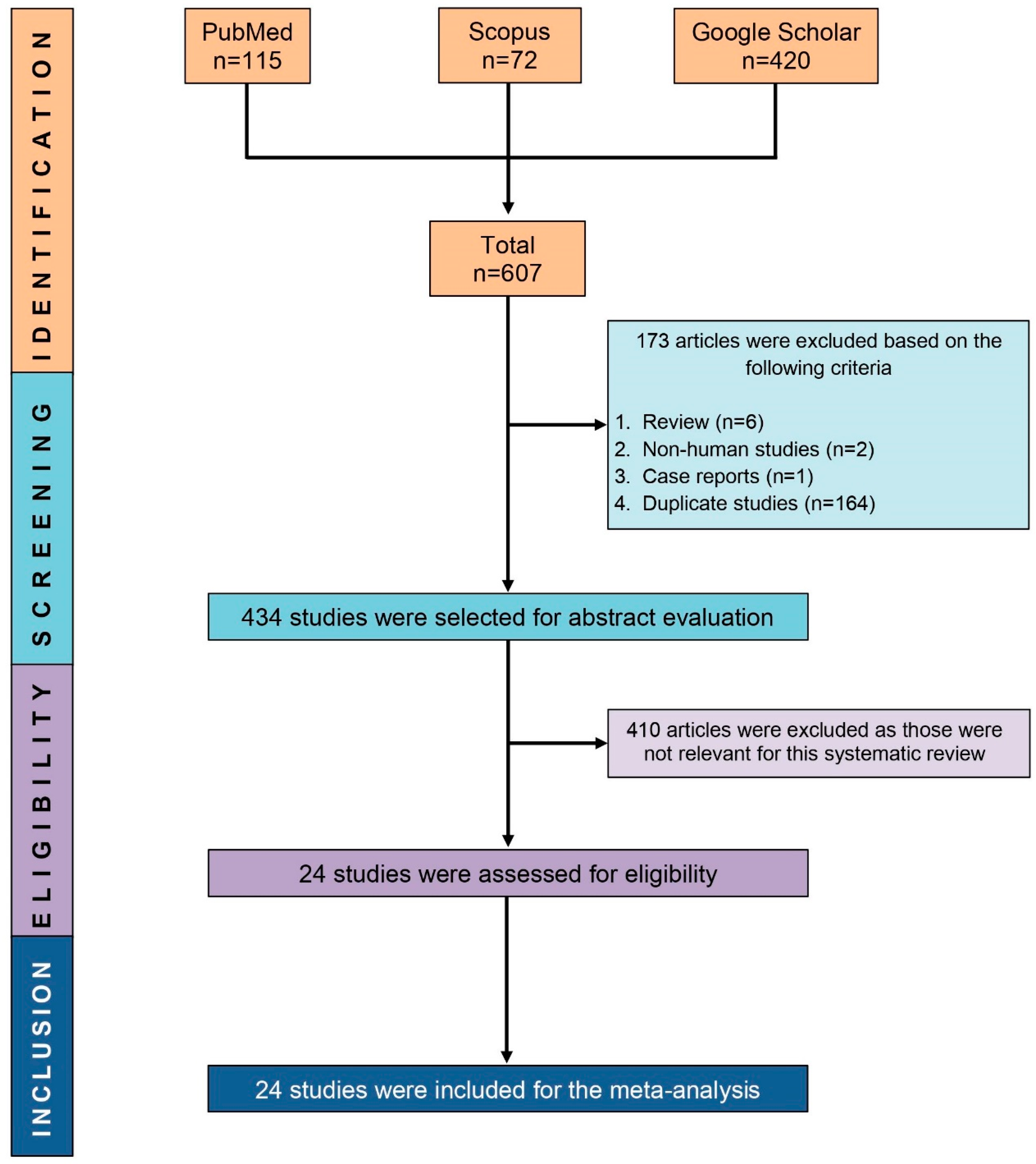
2.1. Study Selection
2.2. Study Characteristics
2.3. Quality Assessment
2.4. Outcomes
2.5. Sensitivity Analyses
3. Discussion
3.1. Prevalence of Any and Mono-Resistance to First-Line Anti-TB Drugs
3.2. The Molecular Basis and Influential Factors for the Resistance to First-Line Anti-TB Drugs
3.3. Prevalence of MDR-TB and Its Influential Factors
3.4. Prevalence of Poly-DR and XDR-TB and Its Influential Factors
3.5. Prevalence of Second-Line Anti-TB-DR and Its Associated Factors
3.6. Time-Trend of Anti-TB-DR in Bangladesh Compared to Other Countries
3.7. Recommendations to Prevent Further Acceleration of Anti-TB-DR in Bangladesh
3.8. Strength and Limitations
4. Materials and Methods
4.1. Systematic Review Protocol
4.2. Eligibility Criteria
4.3. Search Strategy
4.4. Study Selection
4.5. Definitions and Data extraction
4.6. Quality Assessment
4.7. Data Analyses
4.8. Subgroup and Sensitivity Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Global Tuberculosis Report 2019; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Khatun, U.F.; Amin, R.; Islam, M.; Rob, A.; Rahim, A. Socio-demographic Profile and Drug Sensitivity Pattern of Suspected Drug Resistant Tuberculosis among Patients in Regional Tuberculosis Reference Laboratory (RTRL) of a Tertiary Hospital. J. Med. 2017, 18, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Heysell, S.K.; Ahmed, S.; Ferdous, S.S.; Khan, M.S.R.; Rahman, S.M.; Gratz, J.; Rahman, M.T.; Mahmud, A.M.; Houpt, E.R.; Banu, S. Quantitative drug-susceptibility in patients treated for multidrug-resistant tuberculosis in Bangladesh: Implications for regimen choice. PLoS ONE 2015, 10, e0116795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aung, K.; Van Deun, A.; Declercq, E.; Sarker, M.; Das, P.; Hossain, M.; Rieder, H.L. Successful ‘9-month Bangladesh regimen’ for multidrug-resistant tuberculosis among over 500 consecutive patients. Int. J. Tuberc. Lung Dis. 2014, 18, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, S.D.; Ashkin, D.; Avendano, M.; Banerjee, R.; Bauer, M.; Bayona, J.N.; Becerra, M.C.; Benedetti, A.; Burgos, M.; Centis, R. Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: An individual patient data meta-analysis of 9,153 patients. PLoS Med. 2012, 9, e1001300. [Google Scholar] [CrossRef]
- Sharma, A.; Hill, A.; Kurbatova, E.; van der Walt, M.; Kvasnovsky, C.; Tupasi, T.E.; Caoili, J.C.; Gler, M.T.; Volchenkov, G.V.; Kazennyy, B.Y. Estimating the future burden of multidrug-resistant and extensively drug-resistant tuberculosis in India, the Philippines, Russia, and South Africa: A mathematical modelling study. Lancet Infect. Dis. 2017, 17, 707–715. [Google Scholar] [CrossRef]
- Lew, W.; Pai, M.; Oxlade, O.; Martin, D.; Menzies, D. Initial drug resistance and tuberculosis treatment outcomes: Systematic review and meta-analysis. Ann. Intern. Med. 2008, 149, 123–134. [Google Scholar] [CrossRef]
- Ali, M.H.; Alrasheedy, A.A.; Hassali, M.A.; Kibuule, D.; Godman, B. Predictors of multidrug-resistant tuberculosis (MDR-TB) in Sudan. Antibiotics 2019, 8, 90. [Google Scholar] [CrossRef] [Green Version]
- Kamal, S.; Hossain, A.; Sultana, S.; Begum, V.; Haque, N.; Ahmed, J.; Rahman, T.; Hyder, K.; Hossain, S.; Rahman, M. Anti-tuberculosis drug resistance in Bangladesh: Reflections from the first nationwide survey. Int. J. Tuberc. Lung Dis. 2015, 19, 151–156. [Google Scholar] [CrossRef]
- Banu, S.; Rahman, M.; Ahmed, S.; Khatun, R.; Ferdous, S.; Hosen, B.; Rahman, M.; Ahmed, T.; Cavanaugh, J.; Heffelfinger, J. Multidrug-resistant tuberculosis in Bangladesh: Results from a sentinel surveillance system. Int. J. Tuberc. Lung Dis. 2017, 21, 12–17. [Google Scholar] [CrossRef]
- Tuberculosis Control in Bangladesh. National Tuberculosis Control Programme (NTP). 2018. Available online: https://www.ntp.gov.bd/ntp_dashboard/magazines_image/NTP%20Annual%20Report-2018.pdf (accessed on 28 August 2020).
- Goyal, V.; Kadam, V.; Narang, P.; Singh, V. Prevalence of drug-resistant pulmonary tuberculosis in India: Systematic review and meta-analysis. BMC Public Health 2017, 17, 817. [Google Scholar] [CrossRef] [Green Version]
- Nasiri, M.J.; Dabiri, H.; Darban-Sarokhalil, D.; Rezadehbashi, M.; Zamani, S. Prevalence of drug-resistant tuberculosis in Iran: Systematic review and meta-analysis. Am. J. Infect. Control 2014, 42, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Onyedum, C.C.; Alobu, I.; Ukwaja, K.N. Prevalence of drug-resistant tuberculosis in Nigeria: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0180996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, Q.; Chen, Z.; Chen, C.; Zhang, Z.; Lu, Z.; Yang, Y.; Zhang, L. The prevalence of drug-resistant tuberculosis in mainland China: An updated systematic review and meta-analysis. PLoS ONE 2016, 11, e0148041. [Google Scholar] [CrossRef] [PubMed]
- Van Deun, A.; Maug, A.K.J.; Salim, M.A.H.; Das, P.K.; Sarker, M.R.; Daru, P.; Rieder, H.L. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am. J. Respir. Crit. Care Med. 2010, 182, 684–692. [Google Scholar] [CrossRef]
- Afroz, H.; Ali, M.; Fakruddin, M.; Kamrunnahar; Datta, S. Prevalence of Multi-Drug Resistant (MDR) pulmonary tuberculosis in a tertiary care hospital of Narshingdi, Bangladesh. Int. J. Biomed. Adv. Res. 2013, 4, 98–104. [Google Scholar]
- Aurin, T.H.; Munshi, S.K.; Kamal, S.M.; Rahman, M.M.; Hossain, M.S.; Marma, T.; Rahman, F.; Noor, R. Molecular approaches for detection of the multi-drug resistant tuberculosis (MDR-TB) in Bangladesh. PLoS ONE 2014, 9, e99810. [Google Scholar] [CrossRef]
- Banu, S.; Hossain, A.; Uddin, M.K.M.; Uddin, M.R.; Ahmed, T.; Khatun, R.; Mahmud, A.M.; Hyder, K.A.; Lutfor, A.B.; Karim, M.S. Pulmonary tuberculosis and drug resistance in Dhaka central jail, the largest prison in Bangladesh. PLoS ONE 2010, 5, e10759. [Google Scholar] [CrossRef] [Green Version]
- Banu, S.; Mahmud, A.M.; Rahman, M.T.; Hossain, A.; Uddin, M.K.M.; Ahmed, T.; Khatun, R.; Akhanda, W.; Brosch, R. Multidrug-resistant tuberculosis in admitted patients at a tertiary referral hospital of Bangladesh. PLoS ONE 2012, 7, e40545. [Google Scholar] [CrossRef]
- Banu, S.; Rahman, M.T.; Uddin, M.K.M.; Khatun, R.; Ahmed, T.; Rahman, M.M.; Husain, M.A.; van Leth, F. Epidemiology of tuberculosis in an urban slum of Dhaka City, Bangladesh. PLoS ONE 2013, 8, e77721. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.W.; Haque, M.A.; Ekram, A.S.; Hossain, M.; Rahman, M.F. Multi-drug resistant tuberculosis in Rajshahi district. J. Teach. Assoc. 2005, 18, 113–117. [Google Scholar] [CrossRef]
- Iqbal, H.; Mamun, K.; Shamsuzzaman, S.; Nahar, M.N.; Kamal, S.M. XDR and MDR tuberculosis from a tertiary chest hospital in Bangladesh. Bangladesh J. Med. Microbiol. 2013, 7, 17–20. [Google Scholar] [CrossRef] [Green Version]
- Mohiuddin, M.; Haq, J.A. First Line Anti-Tubercular Drug Resistance Pattern of Mycobacterium Tuberculosis Isolated From Specialized Hospitals of Dhaka City. Ibrahim Med. Coll. J. 2014, 8, 41–46. [Google Scholar] [CrossRef]
- Mottalib, A.; Hossain, M.; Khalil, I.; Islam, S.; Hossain, A. Drug susceptibility pattern of Mycobacterium tuberculosis isolates against conventional anti-tuberculosis drugs in Dhaka, Bangladesh. Saudi Med. J. 2011, 32, 484–488. [Google Scholar]
- Noor, R.; Akhter, S.; Rahman, F.; Munshi, S.K.; Kamal, S.M.; Feroz, F. Frequency of extensively drug-resistant tuberculosis (XDR-TB) among re-treatment cases in NIDCH, Dhaka, Bangladesh. J. Infect. Chemother. 2013, 19, 243–248. [Google Scholar] [CrossRef]
- Noor, R.; Hossain, A.; Munshi, S.K.; Rahman, F.; Kamal, S.M. Slide drug susceptibility test for the detection of multidrug-resistant tuberculosis in Bangladesh. J. Infect. Chemother. 2013, 19, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Kamal, S.M.; Mohammed, F.R.; Alam, M.B.; Ahasan, H.N. Anti-tuberculosis drug resistance pattern among different category of tuberculosis patients. J. Med. 2009, 10, 45–47. [Google Scholar] [CrossRef]
- Gundersen Storla, D.; Rahim, Z.; Akramul Islam, M.; Plettner, S.; Begum, V.; Myrvang, B.; Bjune, G.; Rønnild, E.; Dahle, U.R.; Mannsåker, T. Drug resistance of Mycobacterium tuberculosis in the Sunamganj District of Bangladesh. Scand. J. Infect. Dis. 2007, 39, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.K.M.; Ahmed, M.; Islam, M.R.; Rahman, A.; Khatun, R.; Hossain, M.A.; Maug, A.K.J.; Banu, S. Molecular characterization and drug susceptibility profile of Mycobacterium tuberculosis isolates from Northeast Bangladesh. Infect. Genet. Evol. 2018, 65, 136–143. [Google Scholar] [CrossRef]
- Van Deun, A.; Aung, K.; Chowdhury, S.; Saha, S.; Pankaj, A.; Ashraf, A.; Rigouts, L.; Fissette, K.; Portaels, F. Drug susceptibility of Mycobacterium tuberculosis in a rural area of Bangladesh and its relevance to the national treatment regimens. Int. J. Tuberc. Lung Dis. 1999, 3, 143–148. [Google Scholar]
- Wadud, A.A.; Rahman, A.M.; Miah, M.R.A.; Saleh, A.A. Drug resistance pattern of Mycobacterium tuberculosis isolated from patients attending a referral hospital. Bangladesh J. Med. Microbiol. 2009, 3, 13–17. [Google Scholar] [CrossRef]
- Zaman, K.; Rahim, Z.; Yunus, M.; Arifeen, S.E.; Baqui, A.H.; Sack, D.A.; Hossain, S.; Banu, S.; Islam, M.A.; Ahmed, J. Drug resistance of Mycobacterium tuberculosis in selected urban and rural areas in Bangladesh. Scand. J. Infect. Dis. 2005, 37, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Zignol, M.; Dean, A.S.; Alikhanova, N.; Andres, S.; Cabibbe, A.M.; Cirillo, D.M.; Dadu, A.; Dreyer, A.; Driesen, M.; Gilpin, C. Population-based resistance of Mycobacterium tuberculosis isolates to pyrazinamide and fluoroquinolones: Results from a multicountry surveillance project. Lancet Infect. Dis. 2016, 16, 1185–1192. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Yang, J.; Tan, G.; Liu, H.; Liu, Y.; Guo, Y.; Gao, R.; Wan, B.; Yu, F. Drug resistance characteristics of Mycobacterium tuberculosis isolates from patients with tuberculosis to twelve antituberculous drugs in China. Front. Cell. Infect. Microbiol. 2019, 9, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javaid, A.; Hasan, R.; Zafar, A.; Chaudry, M.; Qayyum, S.; Qadeer, E.; Shaheen, Z.; Agha, N.; Rizvi, N.; Afridi, M. Pattern of first- and second-line drug resistance among pulmonary tuberculosis retreatment cases in Pakistan. Int. J. Tuberc. Lung Dis. 2017, 21, 303–308. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, G.; Wu, L.; Lu, M.; Liu, W.; Wu, Y.; Wang, L.; Wang, K.; Qian, H.-Z.; Xie, L. Prevalence and patterns of drug resistance among pulmonary tuberculosis patients in Hangzhou, China. Antimicrob. Resist. Infect. Control 2018, 7, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, P.; Srivastava, G.; Gupta, A.; Anupurba, S. Association of risk factors and drug resistance pattern in tuberculosis patients in North India. J. Glob. Infect. Dis. 2017, 9, 139–145. [Google Scholar]
- Paramasivan, C.; Rehman, F.; Wares, F.; Sundar Mohan, N.; Sundar, S.; Devi, S.; Narayanan, P. First-and second-line drug resistance patterns among previously treated tuberculosis patients in India. Int. J. Tuberc. Lung Dis. 2010, 14, 243–246. [Google Scholar]
- Ismail, N.A.; Mvusi, L.; Nanoo, A.; Dreyer, A.; Omar, S.V.; Babatunde, S.; Molebatsi, T.; Van der Walt, M.; Adelekan, A.; Deyde, V. Prevalence of drug-resistant tuberculosis and imputed burden in South Africa: A national and sub-national cross-sectional survey. Lancet Infect. Dis. 2018, 18, 779–787. [Google Scholar] [CrossRef]
- Shamaei, M.; Marjani, M.; Chitsaz, E.; Kazempour, M.; Esmaeili, M.; Farnia, P.; Tabarsi, P.; Amiri, M.V.; Mirsaeidi, M.; Mansouri, D. First-line anti-tuberculosis drug resistance patterns and trends at the national TB referral center in Iran—Eight years of surveillance. Int. J. Infect. Dis. 2009, 13, e236–e240. [Google Scholar] [CrossRef] [Green Version]
- Villegas, L.; Otero, L.; Sterling, T.R.; Huaman, M.A.; Van der Stuyft, P.; Gotuzzo, E.; Seas, C. Prevalence, risk factors, and treatment outcomes of isoniazid-and rifampicin-mono-resistant pulmonary tuberculosis in Lima, Peru. PLoS ONE 2016, 11, e0152933. [Google Scholar] [CrossRef] [Green Version]
- Somoskovi, A.; Parsons, L.M.; Salfinger, M. The molecular basis of resistance to isoniazid, rifampin, and pyrazinamide in Mycobacterium tuberculosis. Respir. Res. 2001, 2, 164. [Google Scholar] [CrossRef] [Green Version]
- Machado, D.; Perdigão, J.; Portugal, I.; Pieroni, M.; Silva, P.A.; Couto, I.; Viveiros, M. Efflux activity differentially modulates the levels of isoniazid and rifampicin resistance among multidrug resistant and monoresistant Mycobacterium tuberculosis strains. Antibiotics 2018, 7, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, R.; Itagaki, N.; Sugawara, I. Overview of anti-tuberculosis (TB) drugs and their resistance mechanisms. Mini Rev. Med. Chem. 2007, 7, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Javaid, A.; Tahir, Z.; Ullah, O.; Shah, A.A.; Hasan, F.; Ayub, N. Pattern of drug resistance and risk factors associated with development of drug resistant Mycobacterium tuberculosis in Pakistan. PLoS ONE 2016, 11, e0147529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cervantes, J.; Yokobori, N.; Hong, B.-Y. Genetic Identification and Drug-Resistance Characterization of Mycobacterium tuberculosis Using a Portable Sequencing Device. A Pilot Study. Antibiotics 2020, 9, 548. [Google Scholar] [CrossRef] [PubMed]
- Flora, M.; Amin, M.; Karim, M.; Afroz, S.; Islam, S.; Alam, A.; Hossain, M. Risk factors of multi-drug-resistant tuberculosis in Bangladeshi population: A case control study. Bangladesh Med. Res. Counc. Bull. 2013, 39, 34–41. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Shi, G.; Han, W.; Zhao, H.; Zhang, H.; Xi, X. Determinants of multidrug-resistant tuberculosis in Henan province in China: A case control study. BMC Public Health 2015, 16, 42. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Wang, Y.; Wang, J.; Jing, H.; Yu, C.; Wang, H.; Liu, Z.; Graviss, E.A.; Ma, X. Laboratory-based surveillance of extensively drug-resistant tuberculosis, China. Emerg. Infect. Dis. 2011, 17, 495. [Google Scholar] [CrossRef]
- Adam, M.A.M.; Ali, H.M.H.; Khalil, E.A.G. First-line drug resistance patterns of Mycobacterium tuberculosis complex isolates from re-treatment patients from Sudan. J. Tuberc. Res. 2016, 4, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Gallant, V.; Vachon, J.; Siu, W. Tuberculosis drug resistance in Canada: 2006–2016. Can. Commun. Dis. Rep. 2017, 43, 236–241. [Google Scholar] [CrossRef]
- Günther, G.; Van Leth, F.; Alexandru, S.; Altet, N.; Avsar, K.; Bang, D.; Barbuta, R.; Bothamley, G.; Ciobanu, A.; Crudu, V. Multidrug-resistant tuberculosis in Europe, 2010–2011. Emerg. Infect. Dis. 2015, 21, 409–416. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Lee, L.-N.; Lai, H.-C.; Wang, S.-K.; Jan, I.-S.; Yu, C.-J.; Hsueh, P.-R.; Yang, P.-C. Fluoroquinolone resistance in Mycobacterium tuberculosis isolates: Associated genetic mutations and relationship to antimicrobial exposure. J. Antimicrob. Chemother. 2007, 59, 860–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, T.D.; Ziora, Z.M.; Blaskovich, M.A. Quinolone antibiotics. Medchemcomm 2019, 10, 1719–1739. [Google Scholar] [CrossRef] [PubMed]
- Van Ingen, J.; Boeree, M.; Wright, A.; van der Laan, T.; Dekhuijzen, P.; van Soolingen, D. Second-line drug resistance in multidrug-resistant tuberculosis cases of various origins in the Netherlands. Int. J. Tuberc. Lung Dis. 2008, 12, 1295–1299. [Google Scholar] [PubMed]
- Prammananan, T.; Arjratanakool, W.; Chaiprasert, A.; Tingtoy, N.; Leechawengwong, M.; Asawapokee, N.; Leelarasamee, A.; Dhiraputra, C. Second-line drug susceptibilities of Thai multidrug-resistant Mycobacterium tuberculosis isolates. Int. J. Tuberc. Lung Dis. 2005, 9, 216–219. [Google Scholar]
- Yin, Q.-Q.; Jiao, W.-W.; Li, Q.-J.; Xu, F.; Li, J.-Q.; Sun, L.; Li, Y.-J.; Huang, H.-R.; Shen, A.-D. Prevalence and molecular characteristics of drug-resistant Mycobacterium tuberculosis in Beijing, China: 2006 versus 2012. BMC Microbiol. 2016, 16, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, M.N.; Rahman, M.A.; Flora, M.S.; Azad, M.A.K. Factors Associated with Multidrup-Resistant Tuberculosis. Ibrahim Med. Coll. J. 2009, 3, 29–33. [Google Scholar] [CrossRef]
- Hossain, S.T.; Isaakidis, P.; Sagili, K.D.; Islam, S.; Islam, M.A.; Shewade, H.D.; Kamal, S.M.; Husain, A. The multi-drug resistant tuberculosis diagnosis and treatment cascade in Bangladesh. PLoS ONE 2015, 10, e0129155. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef] [Green Version]





| Study ID [References] | Study Design | Location | Patient Enrollment Time | Number of TB Patients Tested for Antibiotic Resistance (Female) | Antibiotic Susceptibility Testing Method/Medium | Age in Years (Mean ± SD/Range) | Anti-TB-DR Evaluated |
|---|---|---|---|---|---|---|---|
| Afroz 2013 [17] | Cohort | Narsingdi | February 2011 to November 2011 | 264 (89) | Conventional DST | 0.0–≥64.0 | SM, INH, RMP, and EMB |
| Aung 2014 [4] | Cohort | NR | March 2005 to June 2011 | 515 (151) | Proportion method/Middlebrook 7H11 agar | 12.0–76.0 | KM, PTN, and PZ |
| Aurin 2014 [18] | Cross-sectional | Dhaka | May 2012 to April 2013 | 277 (NR) | LPA | NR | RMP and INH |
| Banu 2010 [19] | Cross-sectional | Dhaka | October 2005 to September 2007 | 245 (4) | Proportion method/LJ | 10.0–≥40.0 | SM, INH, RMP, and EMB |
| Banu 2012 [20] | Cross-sectional | Dhaka | February 2002 to September 2005 | 189 (47) | Proportion method/LJ | 12.0–70.0 | SM, INH, RMP, and EMB |
| Banu 2013 [21] | Cross-sectional | Dhaka | August 2009 to December 2010 | 20 (NR) | Proportion method/LJ | 0.0–≥65.0 | SM, INH, RMP, and EMB |
| Banu 2017 [10] | Cross-sectional | Nationwide | August 2011 to December 2014 | 1906 (548) | Proportion method/LJ | 8.0–90.0 | SM, INH, RMP, and EMB |
| Heysell 2015 [3] | Cohort | Nationwide | 2011 to 2013 | 74 (23) | MIC/MYCOTB plate | 35.0 ± 15.0 | KM, OFX, MFX, ETN, AMK, PAS, and CS |
| Hussain 2005 [22] | Cross-sectional | Rajshahi | January 1998 to December 2004 | 4390 (NR) | NR | NR | SM, INH, RMP, and EMB |
| Iqbal 2013 [23] | Cross-sectional | Dhaka | NR | 100 (NR) | Slide DST/SLM | NR | SM, INH, RMP, EMB, GFX, KM, and OFX |
| Kamal 2015 [9] | Cross-sectional | Nationwide | December 2010 to November 2011 | 1340 (NR) | Proportion method/LJ | ≤14.0–≥65.0 | SM, INH, RMP, and EMB |
| Khatun 2017 [2] | Cross-sectional | Chattogram | July 2012 to July 2013 | 100 (22) | Gene Xpert MTB/RIF (RMP) and conventional DST (LJ) | 15.0–76.0 | SM, INH, RMP, and PZ |
| Mohiuddin 2014 [24] | Cross-sectional | Dhaka | April 2005 to September 2010 | 192 (NR) | Proportion method/LJ | NR | SM, INH, RMP, and EMB |
| Mottalib 2011 [25] | Cross-sectional | Dhaka | October 2008 to November 2009 | 50 (NR) | Proportion method/LJ | 14.0–70.0 | SM, INH, RMP, and EMB |
| Noor 2012 [26] | Cross-sectional | Dhaka | January 2011 to April 2012 | 84 (NR) | Proportion method/LJ | NR | OFX, GFX, and KM |
| Noor 2013 [27] | Cross-sectional | Dhaka | April 2011 to April 2012 | 109 (NR) | Conventional DST/LJ | NR | INH, RMP, OFX, GFX, and KM |
| Rahman 2009 [28] | Cross-sectional | Dhaka | January 2008 to December 2008 | 363 (76) | Proportion method/LJ | ≥10.0 | SM, INH, RMP and EMB |
| Storla 2007 [29] | Cross-sectional | Sunamganj | November 2003 to December 2004 | 95 (NR) | Bactec MGIT 960 System | NR | SM, INH, RMP, and EMB |
| Uddin 2018 [30] | Cross-sectional | Mymensingh and Netrokona | 2004 | 244 (90) | Proportion method/LJ | 11.0–90.0 | SM, INH, RMP, and EMB |
| Van Deun 2010 [16] | Cohort | NR | May 1997 to December 2007 | 427 (109) | NR | 33.8 | OFX, KM, and PTN |
| Van Deun 1999 [31] | Cross-sectional | Mymensingh | December 1994 to June 1995 | 645 (NR) | Proportion method/LJ | 0.0–≥64.0 | SM, INH, RMP, and EMB |
| Wadud 2009 [32] | Cross-sectional | Dhaka | February 2003 to January 2004 | 95 (NR) | Proportion method/LJ | 6.0–68.0 | SM, INH, RMP, EMB, and PZ |
| Zaman 2005 [33] | Cross-sectional | Dhaka and Matlab | June 2001 to June 2003 | 657 (214) | Proportion method/LJ | 15.0–≥45.0 | SM, INH, RMP, and EMB |
| Zignol 2016 [34] | Cross-sectional | NR | 2011 | 955 (NR) | Proportion method/LJ and MGIT 960 System | NR | PZ, OFX, LFX, MFX, and GFX |
| Drug-Resistance Patterns | Antibiotics | Number of Analyzed Studies | Total Number of Tuberculosis Patients | Prevalence of Antibiotic Resistance [95% CIs] (%) | Heterogeneity | Publication Bias, Egger’s Test (p-Value) | ||
|---|---|---|---|---|---|---|---|---|
| I2 | p-Value | |||||||
| Any-DR | First line drugs | Streptomycin | 10 | 4990 | 29.2 [17.8–40.6] | 99.0% | <0.0001 | 0.88 |
| Isoniazid | 13 | 6018 | 35.0 [23.1–46.8] | 99.0% | <0.0001 | 0.57 | ||
| Rifampicin | 13 | 6018 | 27.6 [19.9–35.4] | 99.0% | <0.0001 | 0.12 | ||
| Ethambutol | 10 | 4990 | 16.2 [10.1–22.4] | 99.0% | <0.0001 | 0.41 | ||
| Pyrazinamide | 3 | 1296 | 18.9 [0.0–39.9] | 98.0% | <0.0001 | NA | ||
| Second line drugs | Kanamycin | 6 | 1251 | 0.5 [0.1–1.0] | 5.0% | 0.47 | NA | |
| Amikacin | 1 | 74 | 1.4 [0.0–4.0] | - | NA | NA | ||
| Gatifloxacin | 4 | 1165 | 0.2 [0.0–1.1] | 44.0% | 0.11 | NA | ||
| Ofloxacin | 6 | 1674 | 7.3 [5.0–9.6] | 53.0% | 0.06 | NA | ||
| Moxifloxacin | 2 | 999 | 5.8 [0.0–17.0] | 89.0% | 0.002 | NA | ||
| Ethionamide | 1 | 74 | 35.1 [24.3–46.0] | - | NA | NA | ||
| Prothionamide | 2 | 904 | 15.7 [12.4–19.0] | 48.0% | 0.17 | NA | ||
| Cycloserine | 1 | 74 | 44.6 [33.3–55.9] | - | NA | NA | ||
| Para-aminosalicylic acid | 1 | 74 | 12.2 [4.7–19.6] | - | NA | NA | ||
| Levofloxacin | 1 | 921 | 3.8 [2.6–5.0] | - | NA | NA | ||
| Mono-DR | First line drugs | Streptomycin | 11 | 5090 | 6.1 [3.3–8.9] | 94.0% | <0.0001 | 0.91 |
| Isoniazid | 12 | 5199 | 4.2 [2.8–5.7] | 88.0% | <0.0001 | 0.0004 | ||
| Rifampicin | 12 | 5199 | 0.7 [0.3–1.1] | 52.0% | 0.02 | 0.0004 | ||
| Ethambutol | 10 | 4990 | 0.8 [0.3–1.3] | 64.0% | 0.002 | 0.001 | ||
| Pyrazinamide | 1 | 100 | 0.0 [0.0–1.4] | - | NA | NA | ||
| Drug-Resistance Patterns | Antibiotics | Number of Analyzed Studies | Total Number of Tuberculosis Patients | Prevalence of Antibiotic-Resistance [95% CIs] (%) | Heterogeneity | ||
|---|---|---|---|---|---|---|---|
| I2 | p-Value | ||||||
| Newly diagnosed tuberculosis patients | |||||||
| Any-DR | First line drugs | Streptomycin | 4 | 2909 | 21.4 [12.9–29.9] | 96.0% | <0.0001 |
| Isoniazid | 4 | 2909 | 10.8 [6.2–15.4] | 92.0% | <0.0001 | ||
| Rifampicin | 4 | 2909 | 2.8 [0.6–5.1] | 88.0% | <0.0001 | ||
| Ethambutol | 4 | 2909 | 3.7 [1.0–6.5] | 93.0% | <0.0001 | ||
| Pyrazinamide | 1 | 751 | 2.8 [1.6–4.0] | - | NA | ||
| Second line drugs | Gatifloxacin | 2 | 779 | 0.0 [0.0–0.2] | 0.0% | 0.50 | |
| Kanamycin | 1 | 50 | 0.0 [0.0–2.7] | - | NA | ||
| Levofloxacin | 1 | 729 | 3.3 [2.0–4.6] | - | NA | ||
| Moxifloxacin | 1 | 732 | 0.4 [0.0–0.9] | - | NA | ||
| Ofloxacin | 2 | 786 | 5.7 [1.2–10.1] | 39.0% | 0.20 | ||
| Mono-DR | First line drugs | Streptomycin | 6 | 3505 | 9.3 [5.4–13.1] | 91.0% | <0.0001 |
| Isoniazid | 6 | 3505 | 2.9 [1.4–4.5] | 83.0% | <0.0001 | ||
| Rifampicin | 6 | 3505 | 0.3 [0.1–0.5] | 8.0% | 0.32 | ||
| Ethambutol | 6 | 3505 | 0.3 [0.0–0.6] | 58.0% | 0.06 | ||
| Previously treated tuberculosis patients | |||||||
| Any-DR | First line drugs | Streptomycin | 4 | 621 | 42.5 [13.8–71.3] | 98.0% | <0.0001 |
| Isoniazid | 5 | 727 | 49.9 [20.5–79.3] | 99.0% | <0.0001 | ||
| Rifampicin | 5 | 727 | 40.9 [10.8–71.0] | 99.0% | <0.0001 | ||
| Ethambutol | 4 | 621 | 27.6 [0.0–61.0] | 99.0% | <0.0001 | ||
| Pyrazinamide | 1 | 192 | 14.6 [9.6–19.6] | - | NA | ||
| Second line drugs | Kanamycin | 6 | 1251 | 0.5 [0.1–1.0] | 5.0% | 0.47 | |
| Amikacin | 1 | 74 | 1.4 [0.0–4.0] | - | NA | ||
| Gatifloxacin | 2 | 242 | 0.0 [0.0–0.7] | 0.0% | 0.61 | ||
| Ofloxacin | 3 | 352 | 9.4 [6.3–12.4] | 0.0% | 0.98 | ||
| Moxifloxacin | 2 | 266 | 6.2 [0.0–16.5] | 86.0% | 0.006 | ||
| Ethionamide | 1 | 74 | 35.1 [24.3–46.0] | - | NA | ||
| Prothionamide | 2 | 904 | 15.7 [12.4–19.0] | 48.0% | 0.17 | ||
| Cycloserine | 1 | 74 | 44.6 [33.3–55.9] | - | NA | ||
| Para-aminosalicylic acid | 1 | 74 | 12.2 [4.7–19.6] | - | NA | ||
| Levofloxacin | 1 | 192 | 5.2 [2.1–8.4] | - | NA | ||
| Mono-DR | First line drugs | Streptomycin | 6 | 862 | 6.5 [3.9–9.1] | 52.0% | 0.06 |
| Isoniazid | 7 | 971 | 3.5 [1.1–5.9] | 84.0% | <0.0001 | ||
| Rifampicin | 7 | 971 | 0.7 [0.1–1.2] | 0.0% | 0.77 | ||
| Ethambutol | 6 | 862 | 0.7 [0.0–1.5] | 36.0% | 0.23 | ||
| Second line drugs | Ofloxacin | 1 | 109 | 0.0 [0.0–1.3] | - | NA | |
| Strategies of Sensitivity Analyses | Prevalence of Antibiotic Resistance [95% CIs] (%) | Difference of Pooled Prevalence Compared to the Main Result | Number of Studies Analyzed | Total Number of TB Patients | Heterogeneity | |
|---|---|---|---|---|---|---|
| I2 | p-Value | |||||
| Any-DR | ||||||
| Excluding small studies | 48.0 [34.2–61.8] | 6.0% higher | 12 | 6168 | 99.0% | <0.0001 |
| Excluding low-quality studies | 43.5 [31.7–55.3] | 4.0% lower | 15 | 6151 | 99.0% | <0.0001 |
| Mono-DR | ||||||
| Excluding small studies | 14.1 [11.0–17.3] | 1.4% lower | 10 | 5234 | 90.0% | <0.0001 |
| Excluding low-quality studies | 14.3 [11.4–17.2] | 0% change | 13 | 5444 | 88.0% | <0.0001 |
| Multi-DR | ||||||
| Excluding small studies | 22.9 [19.0–26.7] | 3.2% higher | 14 | 10,921 | 99.0% | <0.0001 |
| Excluding low-quality studies | 18.8 [14.7–23.0] | 15.3% lower | 16 | 6235 | 99.0% | <0.0001 |
| Poly-DR | ||||||
| Excluding small studies | 7.7 [5.5–9.9] | 0% change | 8 | 5025 | 90.0% | <0.0001 |
| Excluding low-quality studies | 7.7 [5.6–9.7] | 0% change | 10 | 5235 | 88.0% | <0.0001 |
| Extensive-DR | ||||||
| Excluding small studies | 0.1 [0.0–0.4] | 66.7% | 3 | 1051 | 0.0% | 0.64 |
| Excluding low-quality studies | 0.1 [0.0–0.5] | 66.7% | 4 | 1135 | 11.0% | 0.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kundu, S.; Marzan, M.; Gan, S.H.; Islam, M.A. Prevalence of Antibiotic-Resistant Pulmonary Tuberculosis in Bangladesh: A Systematic Review and Meta-Analysis. Antibiotics 2020, 9, 710. https://doi.org/10.3390/antibiotics9100710
Kundu S, Marzan M, Gan SH, Islam MA. Prevalence of Antibiotic-Resistant Pulmonary Tuberculosis in Bangladesh: A Systematic Review and Meta-Analysis. Antibiotics. 2020; 9(10):710. https://doi.org/10.3390/antibiotics9100710
Chicago/Turabian StyleKundu, Shoumik, Mahfuza Marzan, Siew Hua Gan, and Md Asiful Islam. 2020. "Prevalence of Antibiotic-Resistant Pulmonary Tuberculosis in Bangladesh: A Systematic Review and Meta-Analysis" Antibiotics 9, no. 10: 710. https://doi.org/10.3390/antibiotics9100710
APA StyleKundu, S., Marzan, M., Gan, S. H., & Islam, M. A. (2020). Prevalence of Antibiotic-Resistant Pulmonary Tuberculosis in Bangladesh: A Systematic Review and Meta-Analysis. Antibiotics, 9(10), 710. https://doi.org/10.3390/antibiotics9100710







