Nonthermal Plasma Treatment Improves Uniformity and Adherence of Cyclodextrin-Based Coatings on Hydrophobic Polymer Substrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plasma Cleaning and Activation of PP Substrate Surfaces
2.3. Effects of Plasma Treatment on PP Substrates
2.3.1. Wettability—Contact Angle Goniometry
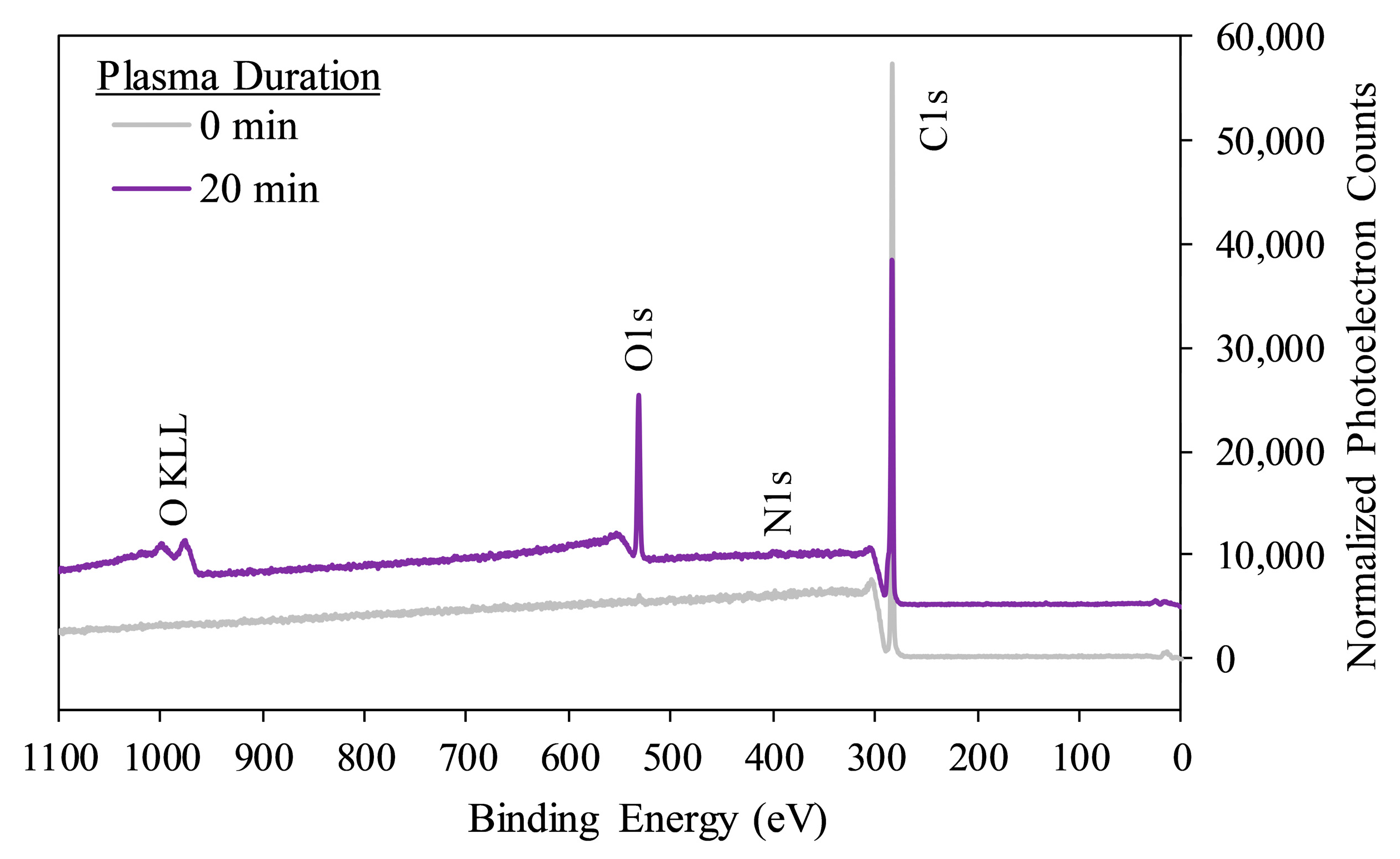
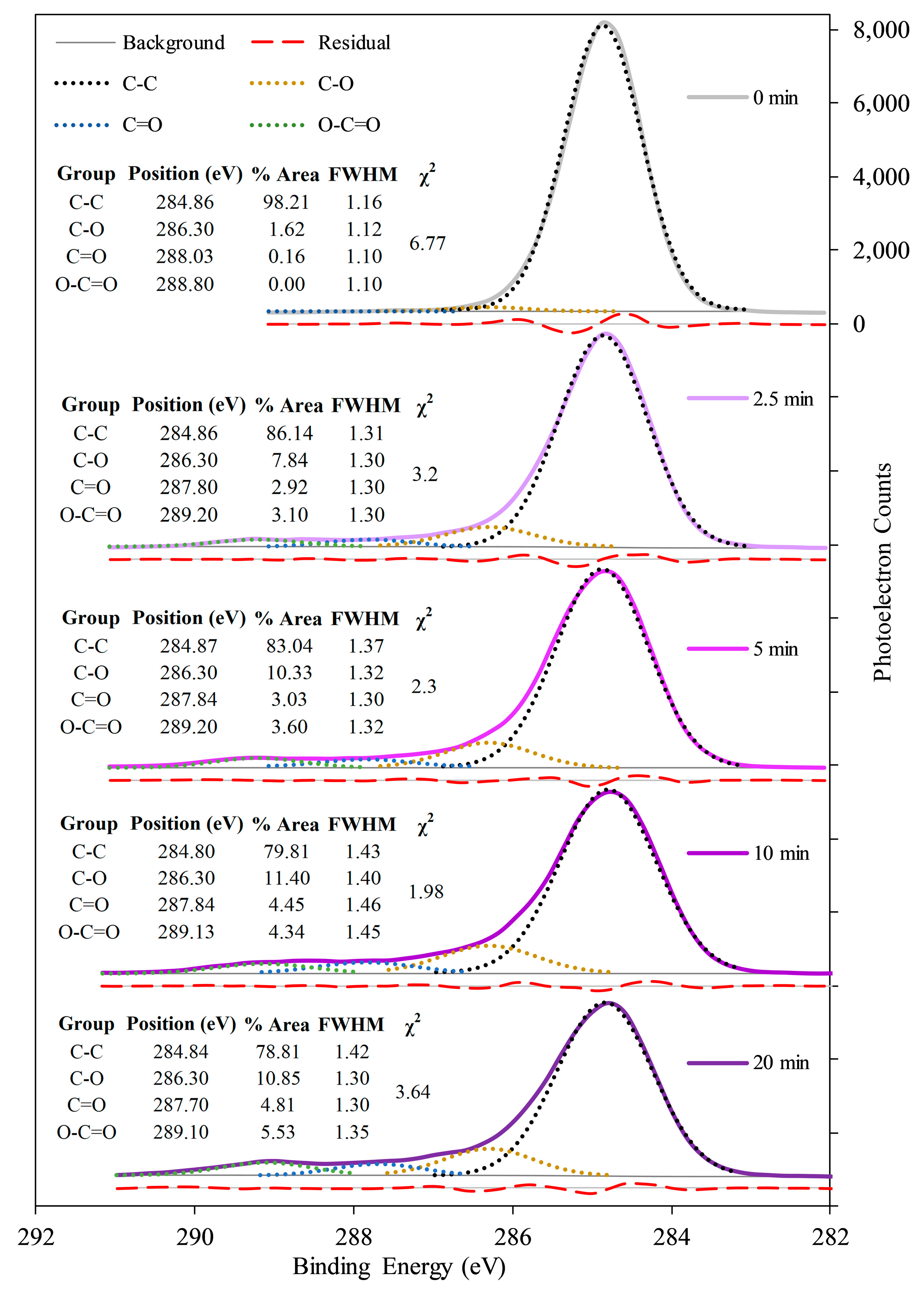
2.3.2. Surface Chemistry—XPS
2.4. pCD Synthesis and Coating onto Surfaces
2.5. Effects of Plasma Treatment on pCD Coatings
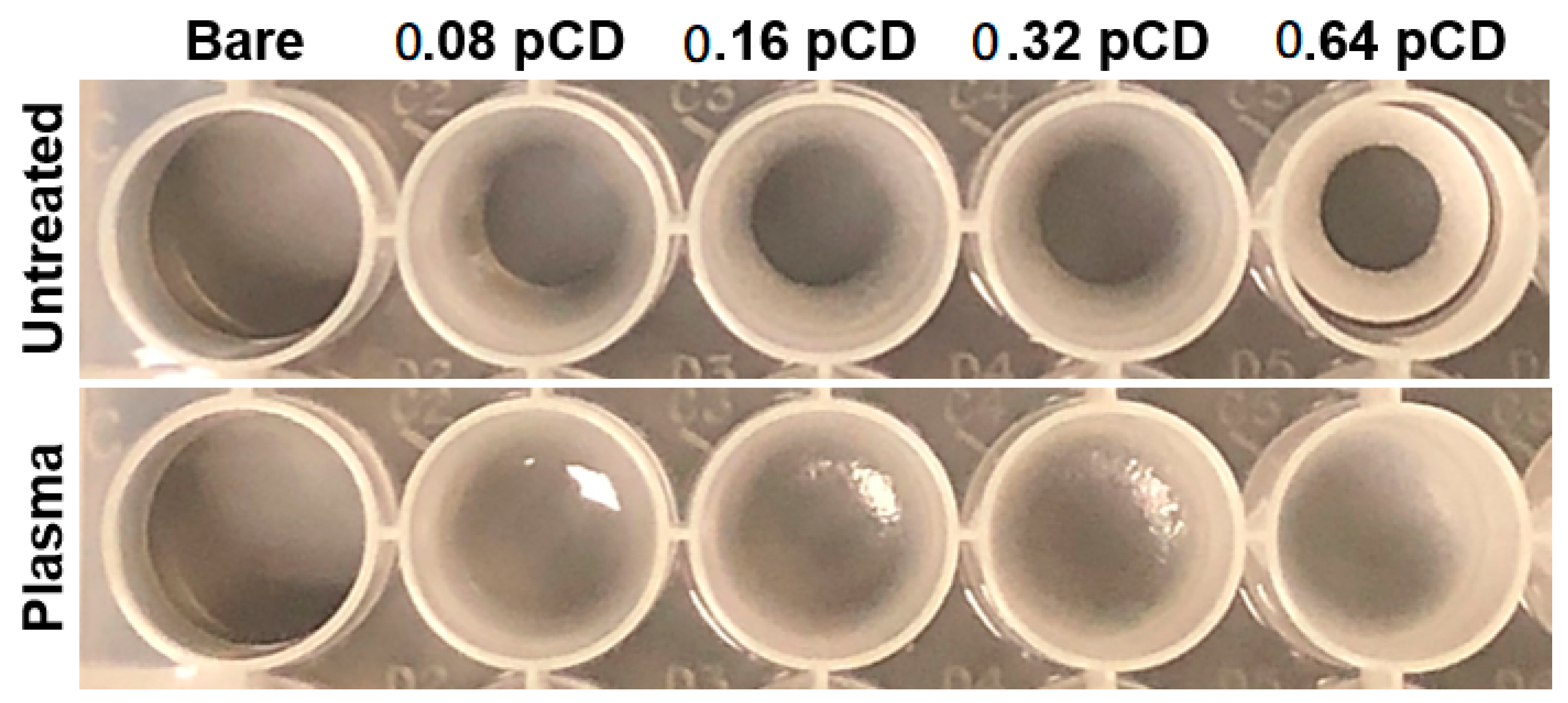
2.5.1. Qualitative Uniformity—Direct Visualization
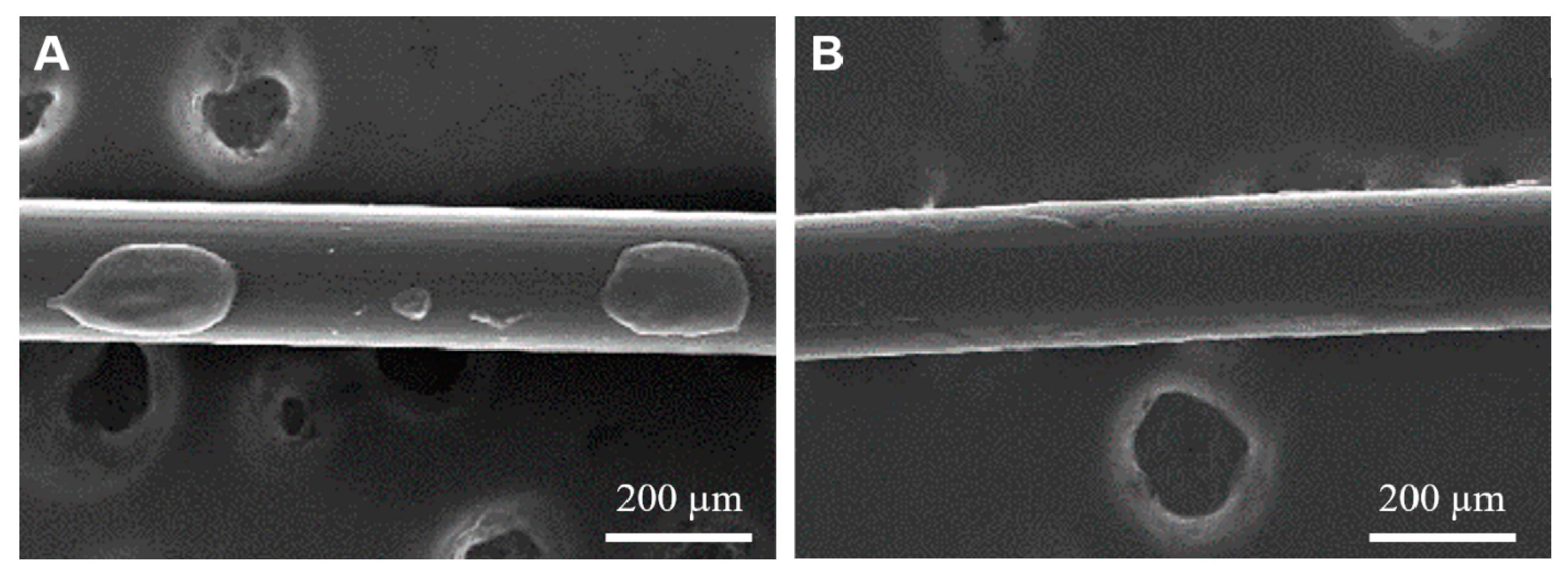
2.5.2. Qualitative Uniformity—SEM
2.5.3. Semi-Quantitative Uniformity—Direct Visualization
2.5.4. Adherence—Lap-Shear Testing
2.5.5. Interfacial Covalent Bonding—XPS
2.6. Statistical Analysis
3. Results
3.1. Effects of Plasma Treatment on PP Substrate Wettability and Surface Chemistry
3.2. Effects of Plasma Treatment on pCD Coating Uniformity, Adherence, and Interfacial Covalent Bonding
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maddah, H.A. Polypropylene as a promising plastic: A review. Am. J. Polym. Sci. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Zhang, S.; Horrocks, A.R. A review of flame retardant polypropylene fibres. Prog. Polym. Sci. 2003, 28, 1517–1538. [Google Scholar] [CrossRef]
- Yang, Y.-F.; Wan, L.-S.; Xu, Z.-K. Surface engineering of microporous polypropylene membrane for antifouling: A mini-review. J. Adhes. Sci. Technol. 2011, 25, 245–260. [Google Scholar] [CrossRef]
- Boone, J.; Lox, F.; Pottie, S. Deficiencies of polypropylene in its use as a food-packaging material—A review. Packag. Technol. Sci. 1993, 6, 277–281. [Google Scholar] [CrossRef]
- Ryntz, R.A. Coating adhesion to low surface free energy substrates. Prog. Org. Coatings 1994, 25, 73–83. [Google Scholar] [CrossRef]
- Hoffman, A. Non-Fouling Surface Technologies. J. Biomater. Sci. Polym. Ed. 1999, 10, 1011–1014. [Google Scholar] [CrossRef]
- Hu, W.J.; Eaton, J.W.; Ugarova, T.P.; Tang, L. Molecular basis of biomaterial-mediated foreign body reactions. Blood 2001, 98, 1231–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zdolsek, J.; Eaton, J.W.; Tang, L. Histamine release and fibrinogen adsorption mediate acute inflammatory responses to biomaterial implants in humans. J. Transl. Med. 2007, 5, 31. [Google Scholar] [CrossRef] [Green Version]
- Koo, J.; Rafailovich, M.H.; Medved, L.; Tsurupa, G.; Kudryk, B.J.; Liu, Y.; Galanakis, D.K. Evaluation of fibrinogen self-assembly: Role of its αC region. J. Thromb. Haemost. 2010, 8, 2727–2735. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.; Roddick, F.A.; Fan, L. Biofouling of water treatment membranes: A review of the underlying causes, monitoring techniques and control measures. Membranes (Basel) 2012, 2, 804–840. [Google Scholar] [CrossRef] [Green Version]
- Flemming, H.C. Biofouling in water systems—Cases, causes and countermeasures. Appl. Microbiol. Biotechnol. 2002, 59, 629–640. [Google Scholar] [CrossRef]
- Weon, J.-I.; Choi, K.-Y. Surface Characterization and morphology in Ar-plasma-treated polypropylene blend. Macromol. Res. 2009, 17, 886–893. [Google Scholar] [CrossRef]
- Harth, K.; Hibst, H. Surface modification of polypropylene in oxygen and nitrogen plasmas. Surf. Coatings Technol. 1993, 59, 350–355. [Google Scholar] [CrossRef]
- Desmet, T.; Morent, R.; De Geyter, N.; Leys, C.; Schacht, E.; Dubruel, P. Nonthermal plasma technology as a versatile strategy for polymeric biomaterials surface modification: A review. Biomacromolecules 2009, 10, 2351–2378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogaerts, A.; Neyts, E.; Gijbels, R.; van der Mullen, J. Gas discharge plasmas and their applications. Spectrochim. Acta Part B At. Spectrosc. 2002, 57, 609–658. [Google Scholar] [CrossRef]
- Navaneetha Pandiyaraj, K.; Deshmukh, R.R.; Arunkumar, A.; Ramkumar, M.C.; Ruzybayev, I.; Ismat Shah, S.; Su, P.-G.; Periayah, M.H.; Halim, A.S. Evaluation of mechanism of non-thermal plasma effect on the surface of polypropylene films for enhancement of adhesive and hemo compatible properties. Appl. Surf. Sci. 2015, 347, 336–346. [Google Scholar] [CrossRef]
- Learn, G.D.; Lai, E.J.; Wilson, E.J.; von Recum, H.A. Nonthermal plasma treatment of polymers modulates biological fouling but can cause material embrittlement. bioRxiv 2019, 842260. [Google Scholar] [CrossRef]
- Thatiparti, T.R.; Shoffstall, A.J.; von Recum, H.A. Cyclodextrin-based device coatings for affinity-based release of antibiotics. Biomaterials 2010, 31, 2335–2347. [Google Scholar] [CrossRef] [PubMed]
- Harth, K.C.; Rosen, M.J.; Thatiparti, T.R.; Jacobs, M.R.; Halaweish, I.; Bajaksouzian, S.; Furlan, J.; Von Recum, H.A. Antibiotic-releasing mesh coating to reduce prosthetic sepsis: An in vivo study. J. Surg. Res. 2010, 163, 337–343. [Google Scholar] [CrossRef]
- Cyphert, E.L.; Zuckerman, S.T.; Korley, J.N.; von Recum, H.A. Affinity interactions drive post-implantation drug filling, even in the presence of bacterial biofilm. Acta Biomater. 2017, 57, 95–102. [Google Scholar] [CrossRef]
- Blanchemain, N.; Laurent, T.; Chai, F.; Neut, C.; Haulon, S.; Krump-konvalinkova, V.; Morcellet, M.; Martel, B.; Kirkpatrick, C.J.; Hildebrand, H.F. Polyester vascular prostheses coated with a cyclodextrin polymer and activated with antibiotics: Cytotoxicity and microbiological evaluation. Acta Biomater. 2008, 4, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Haley, R.M.; Qian, V.R.; Learn, G.D.; von Recum, H.A. Use of affinity allows anti-inflammatory and anti-microbial dual release that matches suture wound resolution. J. Biomed. Mater. Res. A 2019, 107, 1434–1442. [Google Scholar] [CrossRef] [PubMed]
- Vermet, G.; Degoutin, S.; Chai, F.; Maton, M.; Bria, M.; Danel, C.; Hildebrand, H.F.; Blanchemain, N.; Martel, B. Visceral mesh modified with cyclodextrin for the local sustained delivery of ropivacaine. Int. J. Pharm. 2014, 476, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Chai, F.; Maton, M.; Degoutin, S.; Vermet, G.; Simon, N.; Rousseaux, C.; Martel, B.; Blanchemain, N. In vivo evaluation of post-operative pain reduction on rat model after implantation of intraperitoneal PET meshes functionalised with cyclodextrins and loaded with ropivacaine. Biomaterials 2019, 192, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Romi, R.; Nostro, P.L.; Bocci, E.; Ridi, F.; Baglioni, P. Bioengineering of a cellulosic fabric for insecticide delivery via grafted cyclodextrin. Biotechnol. Prog. 2005, 21, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, M.B.; Azizi, N.; Baffoun, A.; Chevalier, Y.; Majdoub, M. Fragrant microcapsules based on β-cyclodextrin for cosmetotextile application. J. Renew. Mater. 2019, 7, 1347–1362. [Google Scholar] [CrossRef] [Green Version]
- Martel, B.; Le Thuaut, P.; Bertini, S.; Crini, G.; Bacquet, M.; Torri, G.; Morcellet, M. Grafting of cyclodextrins onto polypropylene nonwoven fabrics for the manufacture of reactive filters. III. Study of the sorption properties. J. Appl. Polym. Sci. 2002, 85, 1771–1778. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, S.; Zhang, B.; Fang, J.; Zhu, L. Interfacially crosslinked β-cyclodextrin polymer composite porous membranes for fast removal of organic micropollutants from water by flow-through adsorption. J. Hazard. Mater. 2020, 384, 121187. [Google Scholar] [CrossRef]
- Fuhrer, R.; Herrmann, I.K.; Athanassiou, E.K.; Grass, R.N.; Stark, W.J. Immobilized β-cyclodextrin on surface-modified carbon-coated cobalt nanomagnets: Reversible organic contaminant adsorption and enrichment from water. Langmuir 2011, 27, 1924–1929. [Google Scholar] [CrossRef]
- Ghoul, Y.E.; Martel, B.; Achari, A.E.; Campagne, C.; Razafimahefa, L.; Vroman, I. Improved dyeability of polypropylene fabrics finished with β-cyclodextrin–citric acid polymer. Polym. J. 2010, 42, 804–811. [Google Scholar] [CrossRef] [Green Version]
- Park, J.S.; Kim, I.-S. Use of β-cyclodextrin in an antimigration coating for polyester fabric. Color. Technol. 2013, 129, 347–351. [Google Scholar] [CrossRef]
- Learn, G.D.; Lai, E.J.; von Recum, H.A. Cyclodextrin polymer coatings resist protein fouling, mammalian cell adhesion, and bacterial attachment. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Blatnik, J.A.; Thatiparti, T.R.; Krpata, D.M.; Zuckerman, S.T.; Rosen, M.J.; von Recum, H.A. Infection prevention using affinity polymer-coated, synthetic meshes in a pig hernia model. J. Surg. Res. 2017, 219, 5–10. [Google Scholar] [CrossRef]
- Grafmiller, K.T.; Zuckerman, S.T.; Petro, C.; Liu, L.; von Recum, H.A.; Rosen, M.J.; Korley, J.N. Antibiotic-releasing microspheres prevent mesh infection in vivo. J. Surg. Res. 2016, 206, 41–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cumpson, P.J. Estimation of inelastic mean free paths for polymers and other organic materials: Use of quantitative structure–property relationships. Surf. Interface Anal. 2001, 31, 23–34. [Google Scholar] [CrossRef]
- Ton-That, C.; Shard, A.G.; Bradley, R.H. Thickness of spin-cast polymer thin films determined by angle-resolved XPS and AFM tip-scratch methods. Langmuir 2000, 16, 2281–2284. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Leys, C.; Gengembre, L.; Payen, E. Comparison between XPS- and FTIR-analysis of plasma-treated polypropylene film surfaces. Surf. Interface Anal. 2008, 40, 597–600. [Google Scholar] [CrossRef]
- Wang, K.; Wang, W.; Yang, D.; Huo, Y.; Wang, D. Surface modification of polypropylene non-woven fabric using atmospheric nitrogen dielectric barrier discharge plasma. Appl. Surf. Sci. 2010, 256, 6859–6864. [Google Scholar] [CrossRef]
- Mutel, B.; Grimblot, J.; Dessaux, O.; Goudmand, P. XPS investigations of nitrogen-plasma-treated polypropylene in a reactor coupled to the spectrometer. Surf. Interface Anal. 2000, 30, 401–406. [Google Scholar] [CrossRef]
- Sarani, A.; Nikiforov, A.Y.; De Geyter, N.; Morent, R.; Leys, C. Surface modification of polypropylene with an atmospheric pressure plasma jet sustained in argon and an argon/water vapour mixture. Appl. Surf. Sci. 2011, 257, 8737–8741. [Google Scholar] [CrossRef]
- Boyd, R.D.; Kenwright, A.M.; Badyal, J.P.S.; Briggs, D. Atmospheric nonequilibrium plasma treatment of biaxially oriented polypropylene. Macromolecules 1997, 30, 5429–5436. [Google Scholar] [CrossRef]
- ASTM International. ASTM D3163-01(2014) Standard Test Method for Determining Strength of Adhesively Bonded Rigid Plastic Lap-Shear Joints in Shear by Tension Loading; ASTM: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Sanbhal, N.; Mao, Y.; Sun, G.; Xu, R.F.; Zhang, Q.; Wang, L. Surface modification of polypropylene mesh devices with cyclodextrin via cold plasma for hernia repair: Characterization and antibacterial properties. Appl. Surf. Sci. 2018, 439, 749–759. [Google Scholar] [CrossRef]
- Bao, L.; Fan, H.; Chen, Y.; Yan, J.; Yang, T.; Guo, Y. Effect of surface free energy and wettability on the adhesion property of waterborne polyurethane adhesive. RSC Adv. 2016, 6, 99346–99352. [Google Scholar] [CrossRef]
- Mühlhan, C.; Weidner, S.; Friedrich, J.; Nowack, H. Improvement of bonding properties of polypropylene by low-pressure plasma treatment. Surf. Coatings Technol. 1999, 116–119, 783–787. [Google Scholar] [CrossRef]
- Mehta, R.I.; Mehta, R.I. Hydrophilic polymer embolism: An update for physicians. Am. J. Med. 2017, 130, e287–e290. [Google Scholar] [CrossRef] [Green Version]
- Deflorian, F.; Rossi, S.; Prosseda, S. Improvement of corrosion protection system for aluminium body bus used in public transportation. Mater. Des. 2006, 27, 758–769. [Google Scholar] [CrossRef]
- Sørensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anticorrosive coatings:A review. J. Coatings Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Epstein, A.K.; Wong, T.-S.; Belisle, R.A.; Boggs, E.M.; Aizenberg, J. Liquid-infused structured surfaces with exceptional anti-biofouling performance. Proc. Natl. Acad. Sci. 2012, 109, 13182–13187. [Google Scholar] [CrossRef] [Green Version]
- Charnley, M.; Textor, M.; Acikgoz, C. Designed polymer structures with antifouling–antimicrobial properties. React. Funct. Polym. 2011, 71, 329–334. [Google Scholar] [CrossRef]
- Kingshott, P.; Griesser, H.J. Surfaces that resist bioadhesion. Curr. Opin. Solid State Mater. Sci. 1999, 4, 403–412. [Google Scholar] [CrossRef]
- Maestá Bezerra, F.; García Carmona, Ó.; García Carmona, C.; Souza Plath, A.M.; Lis, M. Biofunctional wool using β-cyclodextrins as vehiculizer of citronella oil. Process Biochem. 2019, 77, 151–158. [Google Scholar] [CrossRef]
- Peila, R.; Scordino, P.; Shanko, D.B.; Caldera, F.; Trotta, F.; Ferri, A. Synthesis and characterization of β-cyclodextrin nanosponges for N,N-diethyl-meta-toluamide complexation and their application on polyester fabrics. React. Funct. Polym. 2017, 119, 87–94. [Google Scholar] [CrossRef]
- Turan, A.C.; Özen, İ.; Gürakın, H.K.; Fatarella, E. Controlled release profile of imidacloprid-β-cyclodextrin inclusion complex embedded polypropylene filament yarns. J. Eng. Fiber. Fabr. 2017, 12. [Google Scholar] [CrossRef] [Green Version]
- Sricharussin, W.; Sopajaree, C.; Maneerung, T.; Sangsuriya, N. Modification of cotton fabrics with β-cyclodextrin derivative for aroma finishing. J. Text. Inst. 2009, 100, 682–687. [Google Scholar] [CrossRef]
- Xiao, Z.; Deng, J.; Niu, Y.; Zhu, G.; Zhu, J.; Liu, M.; Liu, S. Preparation of sustained-release fragrance based on the cavity structure of β-cyclodextrin and its application in cotton fabric. Text. Res. J. 2018, 89, 3466–3474. [Google Scholar] [CrossRef]
- Wang, C.X.; Chen, S.L. Fragrance-release property of β-cyclodextrin inclusion compounds and their application in aromatherapy. J. Ind. Text. 2005, 34, 157–166. [Google Scholar] [CrossRef]
- El-Tahlawy, K.; El-Nagar, K.; Elhendawy, A.G. Cyclodextrin-4 hydroxy benzophenone inclusion complex for UV protective cotton fabric. J. Text. Inst. 2007, 98, 453–462. [Google Scholar] [CrossRef]
- Liu, J.; Ma, X.; Shi, W.; Xing, J.; Ma, C.; Li, S.; Huang, Y. Anti-ultraviolet properties of β-cyclodextrin-grafted cotton fabrics dyed by broadleaf holly leaf extract. Text. Res. J. 2020, 90. [Google Scholar] [CrossRef]
- Schoolenberg, G.E. A fracture mechanics approach to the effects of UV-degradation on polypropylene. J. Mater. Sci. 1988, 23, 1580–1590. [Google Scholar] [CrossRef]
- Mhlanga, S.D.; Mamba, B.B.; Krause, R.W.; Malefetse, T.J. Removal of organic contaminants from water using nanosponge cyclodextrin polyurethanes. J. Chem. Technol. Biotechnol. 2007, 82, 382–388. [Google Scholar] [CrossRef]
- Yamasaki, H.; Makihata, Y.; Fukunaga, K. Efficient phenol removal of wastewater from phenolic resin plants using crosslinked cyclodextrin particles. J. Chem. Technol. Biotechnol. 2006, 81, 1271–1276. [Google Scholar] [CrossRef]
- Chen, H.; Li, L.; Ma, Y.; Mcdonald, T.P.; Wang, Y. Development of active packaging film containing bioactive components encapsulated in β-cyclodextrin and its application. Food Hydrocoll. 2019, 90, 360–366. [Google Scholar] [CrossRef]
- Siró, I.; Fenyvesi, É.; Szente, L.; De Meulenaer, B.; Devlieghere, F.; Orgoványi, J.; Sényi, J.; Barta, J. Release of alpha-tocopherol from antioxidative low-density polyethylene film into fatty food simulant: Influence of complexation in beta-cyclodextrin. Food Addit. Contam. 2006, 23, 845–853. [Google Scholar] [CrossRef] [PubMed]
- López-de-Dicastillo, C.; Catalá, R.; Gavara, R.; Hernández-Muñoz, P. Food applications of active packaging EVOH films containing cyclodextrins for the preferential scavenging of undesirable compounds. J. Food Eng. 2011, 104, 380–386. [Google Scholar] [CrossRef] [Green Version]
- López-de-Dicastillo, C.; Jordá, M.; Catalá, R.; Gavara, R.; Hernández-Muñoz, P. Development of active polyvinyl alcohol/β-cyclodextrin composites to scavenge undesirable food components. J. Agric. Food Chem. 2011, 59, 11026–11033. [Google Scholar] [CrossRef]
- Martinez, A.M.; Abenojar, J.; Lopez de Armentia, S. Environmentally friendly plasma activation of acrylonitrile–butadiene–styrene and polydimethylsiloxane surfaces to improve paint adhesion. Coatings 2018, 8, 428. [Google Scholar] [CrossRef] [Green Version]
- Carrino, L.; Polini, W.; Sorrentino, L. Ageing time of wettability on polypropylene surfaces processed by cold plasma. J. Mater. Process. Technol. 2004, 153–154, 519–525. [Google Scholar] [CrossRef]
- Morra, M.; Occhiello, E.; Garbassi, F. Contact angle hysteresis on oxygen plasma treated polypropylene surfaces. J. Colloid Interface Sci. 1989, 132, 504–508. [Google Scholar] [CrossRef]
- Jokinen, V.; Suvanto, P.; Franssila, S. Oxygen and nitrogen plasma hydrophilization and hydrophobic recovery of polymers. Biomicrofluidics 2012, 6, 16501–1650110. [Google Scholar] [CrossRef] [Green Version]
- Yun, Y.I.; Kim, K.S.; Uhm, S.-J.; Khatua, B.B.; Cho, K.; Kim, J.K.; Park, C.E. Aging behavior of oxygen plasma-treated polypropylene with different crystallinities. J. Adhes. Sci. Technol. 2004, 18, 1279–1291. [Google Scholar] [CrossRef]

| Plasma Duration (Min) | Contact Angle (°) | Atomic % Carbon | Atomic % Nitrogen | Atomic % Oxygen |
|---|---|---|---|---|
| 0 | 126.8 ± 4.8 † | 98.27 ± 0.35 † | 0.29 ± 0.19 | 1.45 ± 0.24 † |
| 1 | 95.0 ± 7.4 | 88.47 ± 0.70 | 0.34 ± 0.19 | 11.19 ± 0.51 |
| 2.5 | 87.1 ± 10.4 | 88.18 ± 0.75 | 0.45 ± 0.40 | 11.37 ± 0.63 |
| 5 | 70.1 ± 9.5 | 85.79 ± 0.29 | 0.53 ± 0.17 | 13.68 ± 0.28 |
| 10 | 65.8 ± 9.9 | 82.18 ± 0.89 | 0.71 ± 0.07 * | 17.11 ± 0.85 |
| 20 | 57.9 ± 9.7 | 79.93 ± 1.28 | 0.72 ± 0.15 * | 19.36 ± 1.20 |
| Plasma Duration (Min) | V50 (µL) |
|---|---|
| 0 | 246.67 |
| 1 | 163.75 |
| 2.5 | 163.75 |
| 5 | 162.34 |
| 10 | 159.65 |
| 20 | 166.81 |
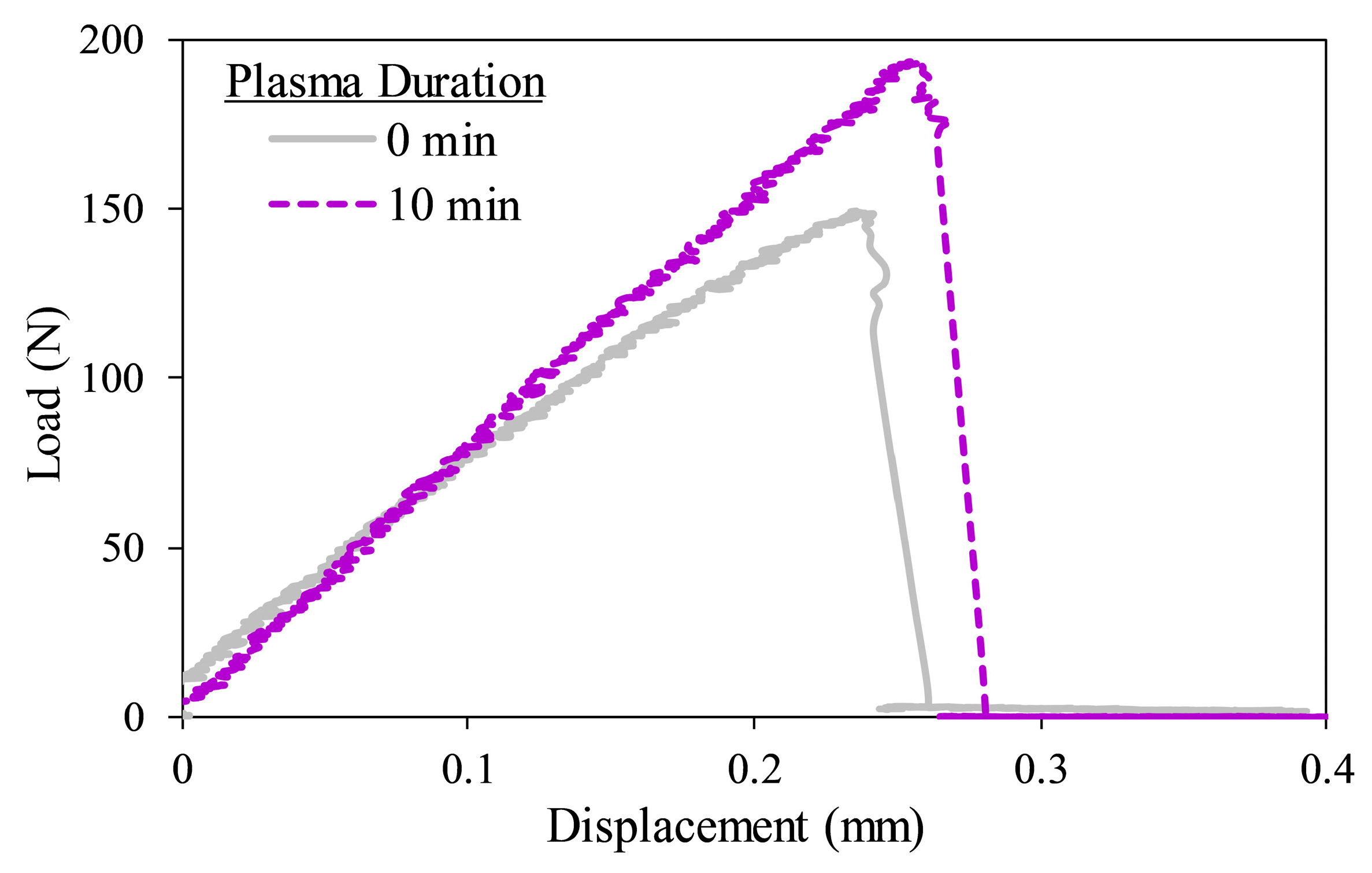
| Plasma Duration (Min) | Ultimate Lap-Shear Strength (kPa) | Lap-Shear Toughness (J/m2) |
|---|---|---|
| 0 | 222.3 ± 31.7 | 32.9 ± 12.3 |
| 10 | 318.7 ± 40.0 * | 66.4 ± 22.5 * |
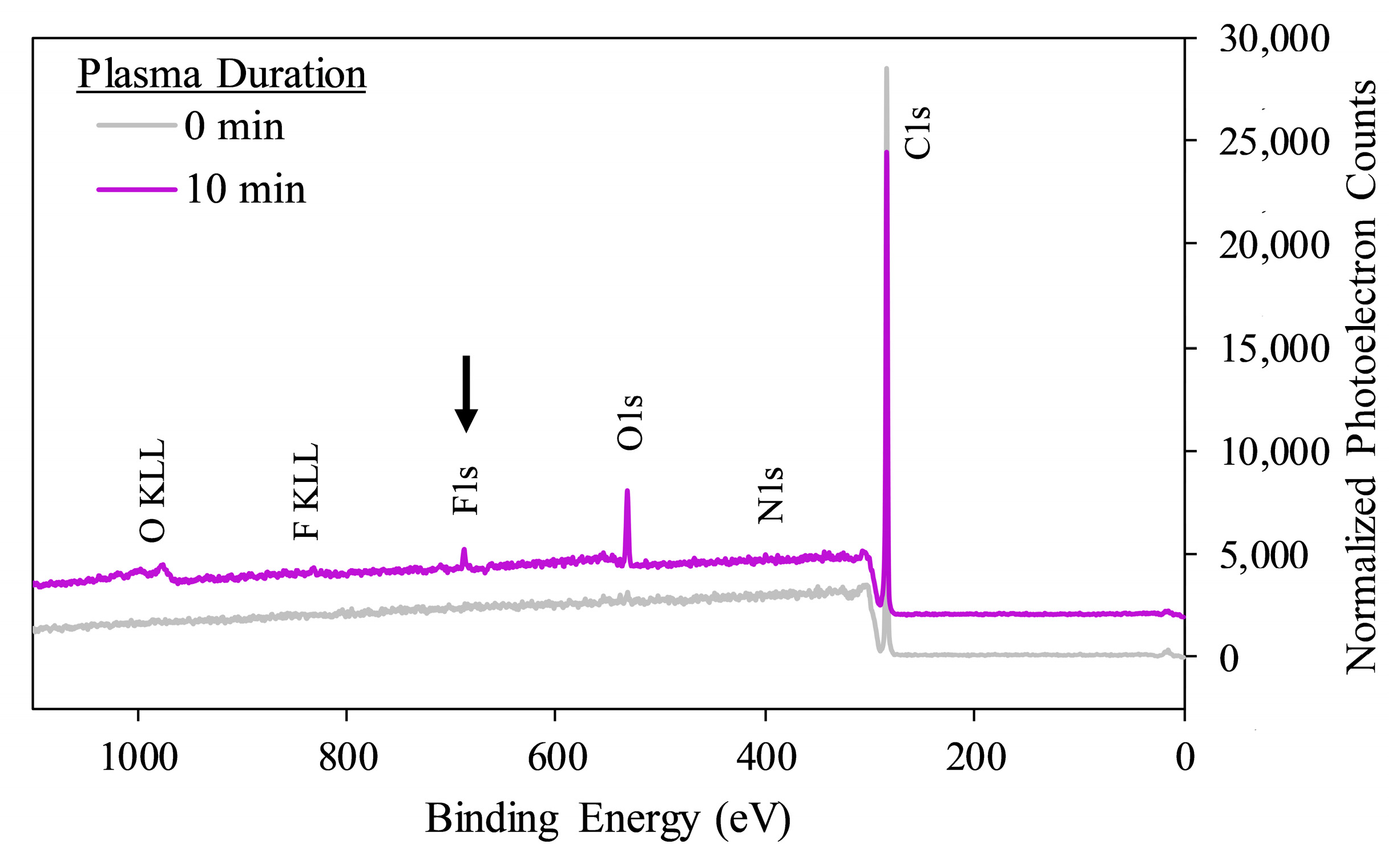
| Plasma Duration (Min) | 2-TPI Exposure | Atomic % Carbon | Atomic % Nitrogen | Atomic % Oxygen | Atomic % Fluorine |
|---|---|---|---|---|---|
| 0 | − | 99.34 ± 0.37 | 0.42 ± 0.02 | 0.25 ± 0.35 | 0.00 ± 0.00 |
| 0 | + | 99.55 ± 0.64 | 0.00 ± 0.00 | 0.15 ± 0.21 | 0.31 ± 0.43 |
| 10 | − | 87.09 ± 0.11 | 0.89 ± 0.06 | 11.97 ± 0.26 | 0.07 ± 0.09 |
| 10 | + | 90.41 ± 0.49 | 0.72 ± 0.32 | 7.48 ± 0.53 | 1.39 ± 0.08 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Learn, G.D.; Lai, E.J.; von Recum, H.A. Nonthermal Plasma Treatment Improves Uniformity and Adherence of Cyclodextrin-Based Coatings on Hydrophobic Polymer Substrates. Coatings 2020, 10, 1056. https://doi.org/10.3390/coatings10111056
Learn GD, Lai EJ, von Recum HA. Nonthermal Plasma Treatment Improves Uniformity and Adherence of Cyclodextrin-Based Coatings on Hydrophobic Polymer Substrates. Coatings. 2020; 10(11):1056. https://doi.org/10.3390/coatings10111056
Chicago/Turabian StyleLearn, Greg D., Emerson J. Lai, and Horst A. von Recum. 2020. "Nonthermal Plasma Treatment Improves Uniformity and Adherence of Cyclodextrin-Based Coatings on Hydrophobic Polymer Substrates" Coatings 10, no. 11: 1056. https://doi.org/10.3390/coatings10111056
APA StyleLearn, G. D., Lai, E. J., & von Recum, H. A. (2020). Nonthermal Plasma Treatment Improves Uniformity and Adherence of Cyclodextrin-Based Coatings on Hydrophobic Polymer Substrates. Coatings, 10(11), 1056. https://doi.org/10.3390/coatings10111056





