The Beneficial Mechanical and Biological Outcomes of Thin Copper-Gallium Doped Silica-Rich Bio-Active Glass Implant-Type Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Source Cathode Target Materials
2.2. Deposition by RF-MS of Double-Layered Glass Coatings
2.3. Physico-Chemical Characterization
2.4. In Vitro Preliminary Biological Testing
2.4.1. Antibacterial Tests
2.4.2. Cytocompatibility Assays
2.5. Statistical Analysis
3. Results and Discussion
3.1. Physico-Chemical Analyses
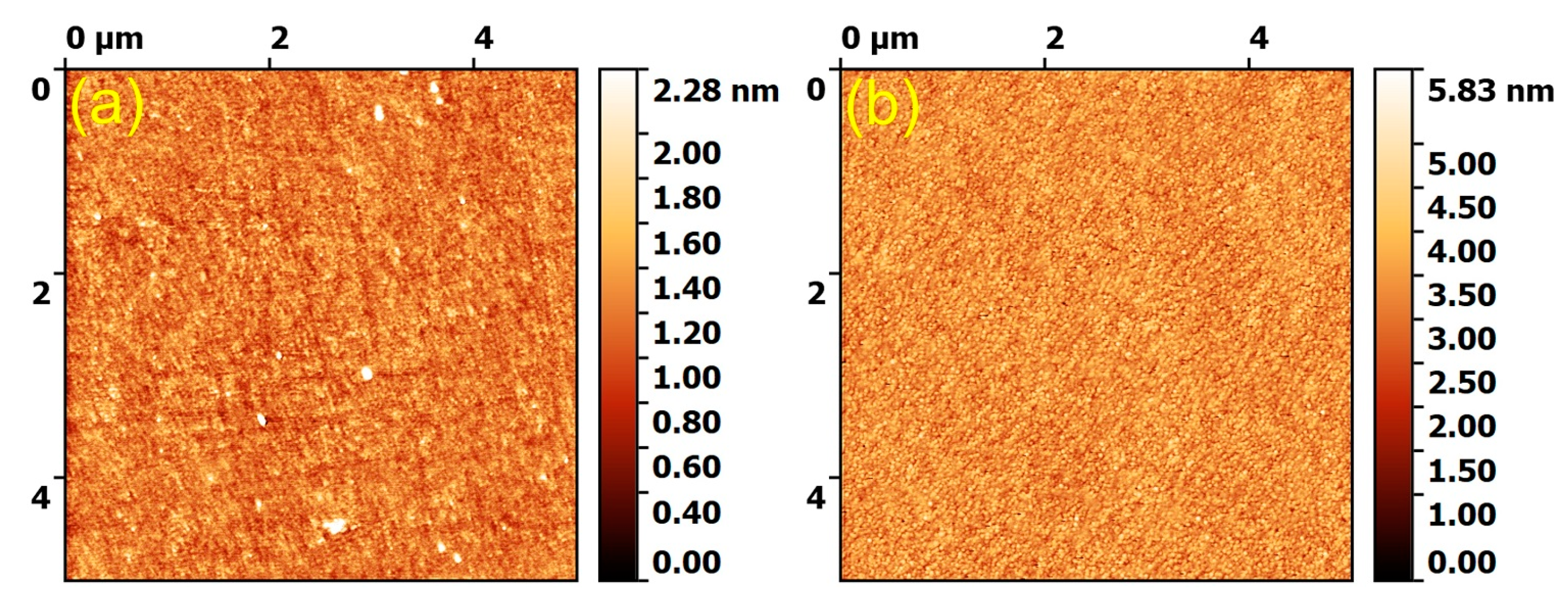
3.1.1. AFM Morphological Examination
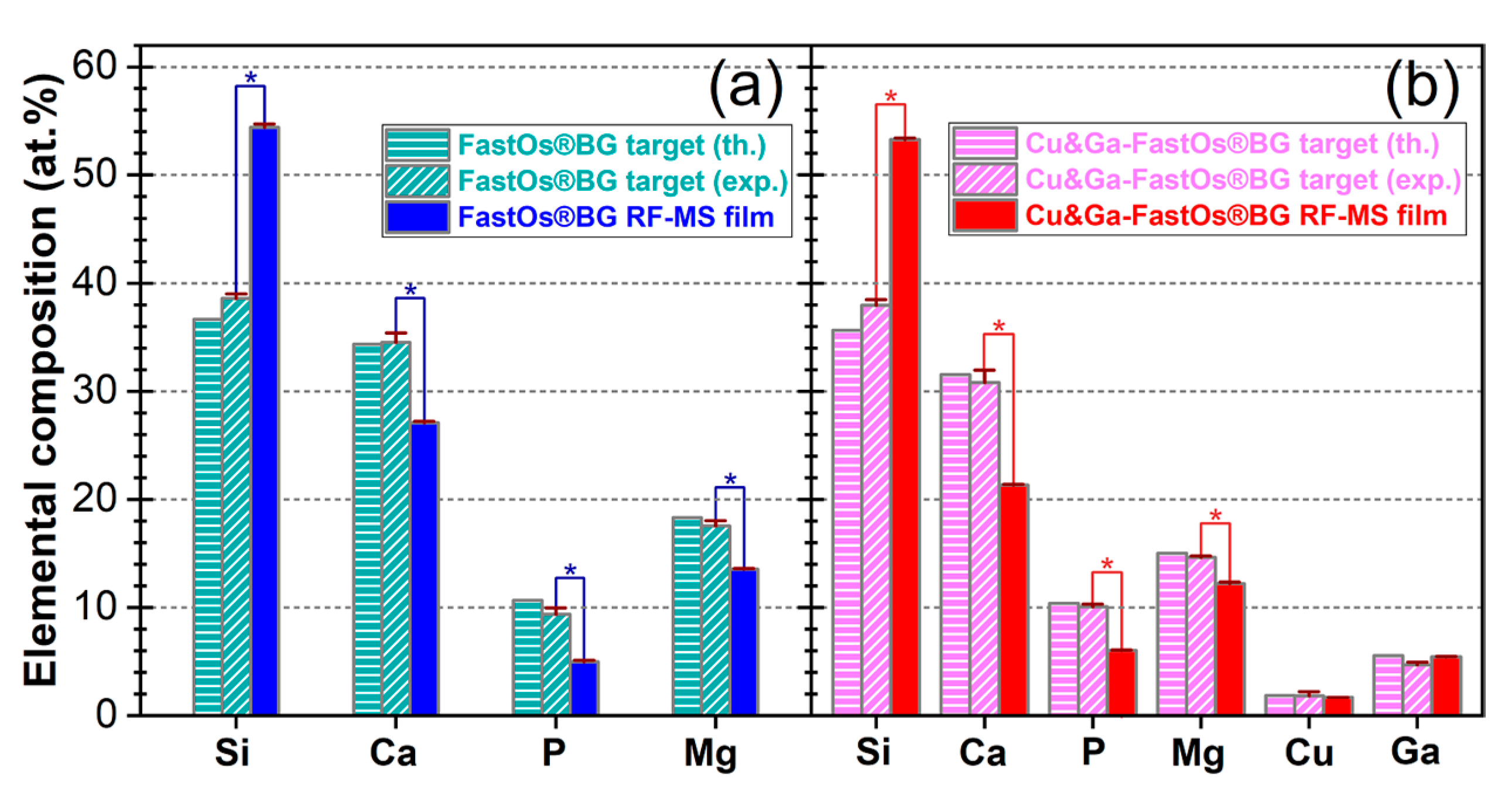
3.1.2. EDXS Compositional Analysis
3.1.3. XPS Chemical State Examination
3.1.4. FTIR Spectroscopy Structural Investigation
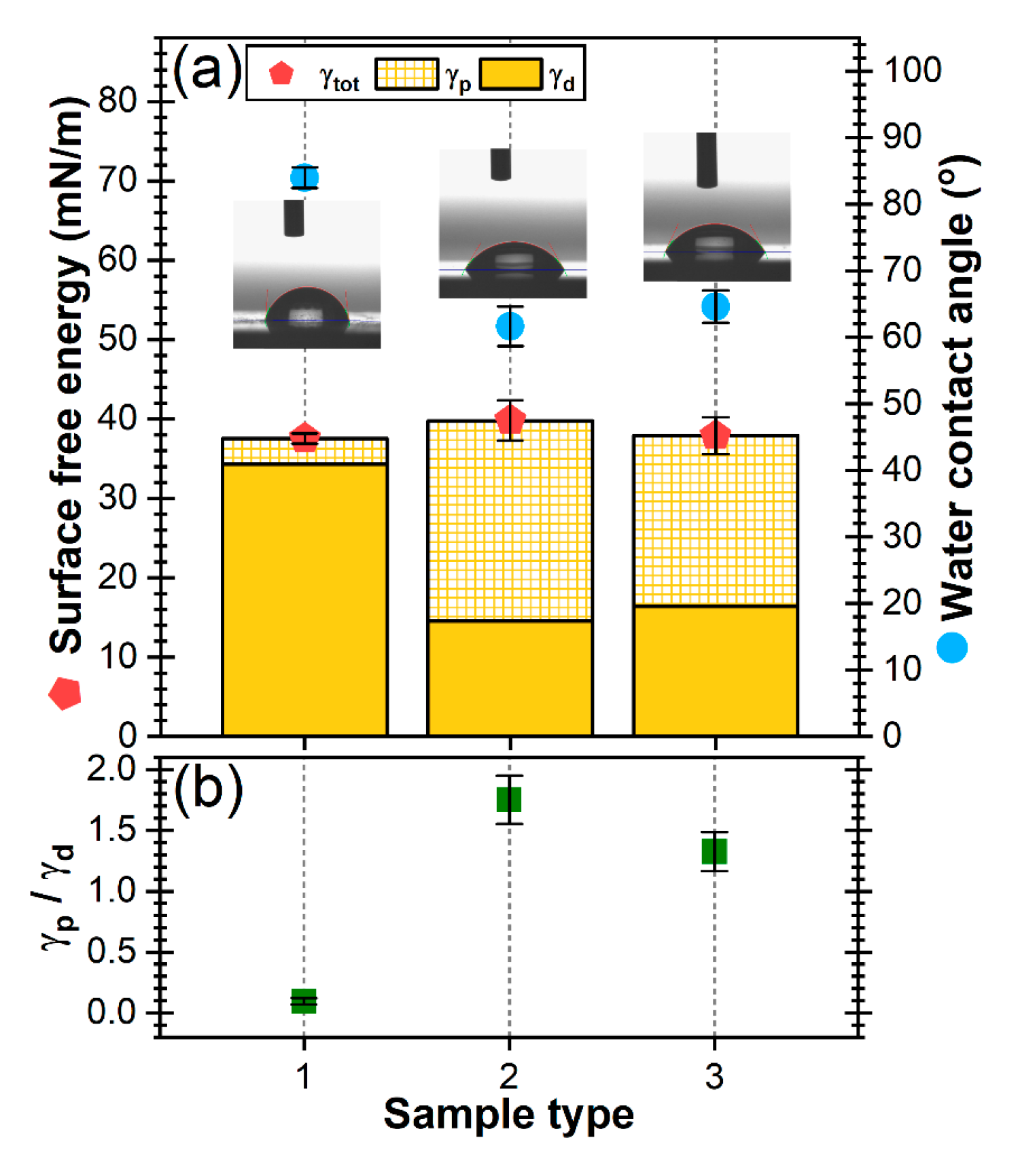
3.1.5. Surface Energy Measurements
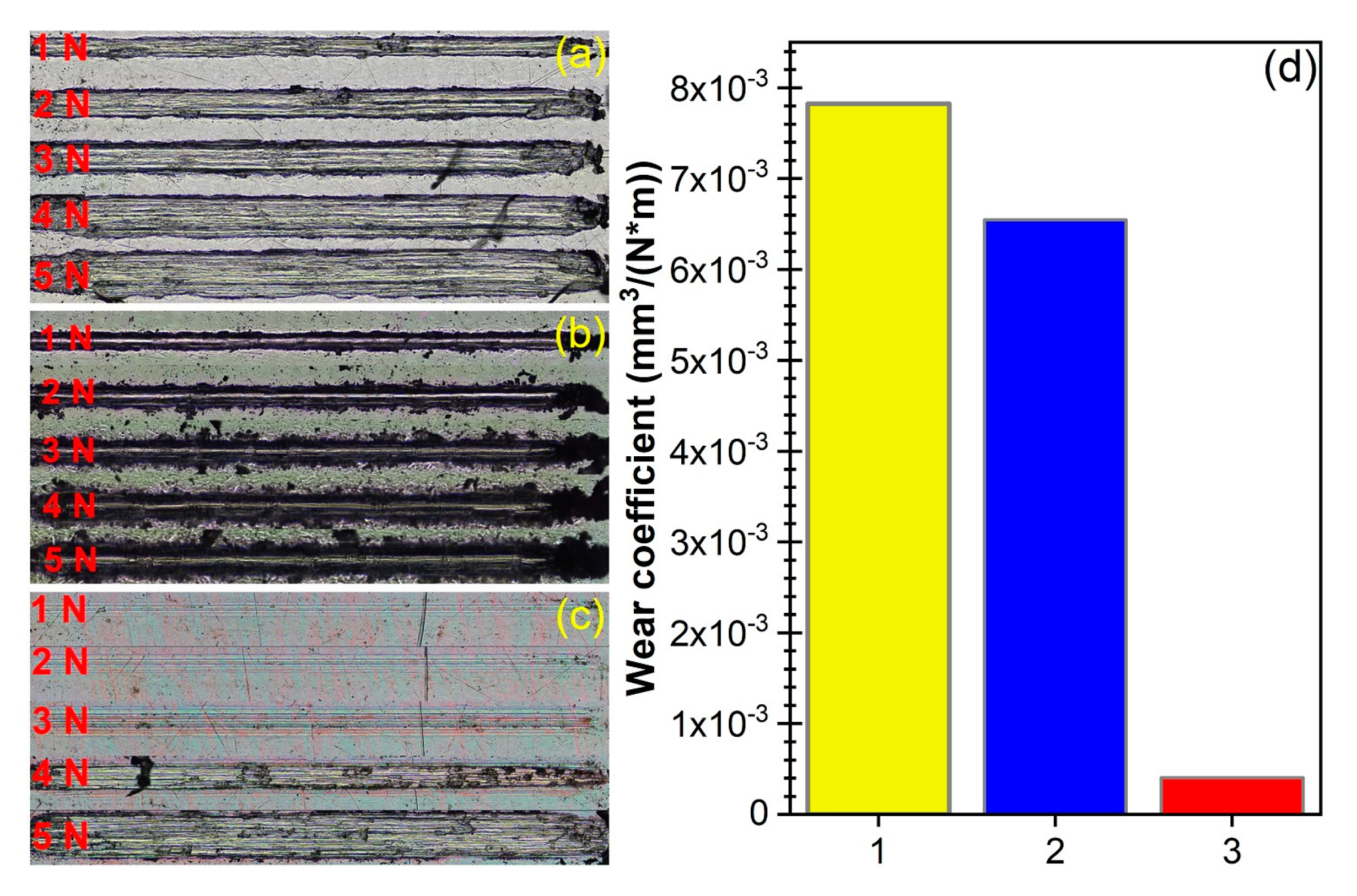
3.2. Mechanical Performance Characterization
3.3. In Vitro Preliminary Biological Evaluation
3.3.1. Antibacterial Efficacy at 24 h
3.3.2. Cytocompatibility Response at 24 h
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kawaguchi, K.; Iijima, M.; Endo, K.; Mizoguchi, I. Electrophoretic deposition as a new bioactive glass coating process for orthodontic stainless steel. Coatings 2017, 7, 199. [Google Scholar] [CrossRef] [Green Version]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive glasses: Where are we and where are we going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunello, G.; Elsayed, H.; Biasetto, L. Bioactive glass and silicate-based ceramic coatings on metallic implants: Open challenge or outdated topic? Materials 2019, 12, 2929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leite, Á.J.; Gonçalves, A.I.; Rodrigues, M.T.; Gomes, M.E.; Mano, J.F. Strontium-doped bioactive glass nanoparticles in osteogenic commitment. ACS Appl. Mater. Interfaces 2018, 10, 23311–23320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, H.R.; Gaddam, A.; Rebelo, A.; Brazete, D.; Stan, G.E.; Ferreira, J.M.F. Bioactive glasses and glass-ceramics for healthcare applications in bone regeneration and tissue engineering. Materials 2018, 11, 2530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prefac, G.A.; Milea, M.L.; Vadureanu, A.M.; Muraru, S.; Dobrin, D.I.; Isopencu, G.O.; Jinga, S.I.; Raileanu, M.; Bacalum, M.; Busuioc, C. CeO2 containing thin films as bioactive coatings for orthopaedic implants. Coatings 2020, 10, 642. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Cannillo, V. A comprehensive review of bioactive glass coatings: State of the art, challenges and future perspectives. Coatings 2020, 10, 757. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Popa, A.C.; Fernandes, H.R.; Necsulescu, M.; Luculescu, C.; Cioangher, M.; Dumitru, V.; Stuart, B.W.; Grant, D.M.; Ferreira, J.M.F.; Stan, G.E. Antibacterial efficiency of alkali-free bio-glasses incorporating ZnO and/or SrO as therapeutic agents. Ceram. Int. 2019, 45, 4368–4380. [Google Scholar] [CrossRef]
- Stan, G.E.; Popescu, A.C.; Mihailescu, I.N.; Marcov, D.A.; Mustata, R.C.; Sima, L.E.; Petrescu, S.M.; Ianculescu, A.; Trusca, R.; Morosanu, C.O. On the bioactivity of adherent bioglass thin films synthesized by magnetron sputtering techniques. Thin Solid Film. 2010, 518, 5955–5964. [Google Scholar] [CrossRef]
- Ciraldo, F.E.; Boccardi, E.; Melli, V.; Westhauser, F.; Boccaccini, A.R. Tackling bioactive glass excessive in vitro bioreactivity: Preconditioning approaches for cell culture tests. Acta Biomater. 2018, 75, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Bakry, A.S.; Tamura, Y.; Otsuki, M.; Kasugai, S.; Ohya, K.; Tagami, J. Cytotoxicity of 45S5 bioglass paste used for dentine hypersensitivity treatment. J. Dent. 2011, 39, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.M.F.; Rebelo, A. The key Features expected from a perfect bioactive glass—How Far we still are from an ideal composition? Biomed. J. Sci. Tech. Res. 2017, 1, 936–939. [Google Scholar] [CrossRef] [Green Version]
- Cacciotti, I. Bivalent cationic ions doped bioactive glasses: The influence of magnesium, zinc, strontium and copper on the physical and biological properties. J. Mater. Sci. 2017, 52, 8812–8831. [Google Scholar] [CrossRef]
- Kaya, S.; Cresswell, M.; Boccaccini, A.R. Mesoporous silica-based bioactive glasses for antibiotic-free antibacterial applications. Mater. Sci. Eng. C 2018, 83, 99–107. [Google Scholar] [CrossRef]
- Tite, T.; Popa, A.C.; Balescu, L.M.; Bogdan, I.M.; Pasuk, I.; Ferreira, J.M.F.; Stan, G.E. Cationic substitutions in hydroxyapatite: Current status of the derived biofunctional effects and their in vitro interrogation methods. Materials 2018, 11, 2081. [Google Scholar] [CrossRef] [Green Version]
- Drago, L.; Toscano, M.; Bottagisio, M. Recent evidence on bioactive glass antimicrobial and antibiofilm activity: A mini-review. Materials 2018, 11, 326. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef]
- Valappil, S.P.; Ready, D.; Abou Neel, E.A.; Pickup, D.M.; Chrzanowski, W.; O’Dell, L.A.; Newport, R.J.; Smith, M.E.; Wilson, M.; Knowles, J.C. Antimicrobial gallium-doped phosphate-based glasses. Adv. Funct. Mater. 2008, 18, 732–741. [Google Scholar] [CrossRef]
- Valappil, S.P.; Ready, D.; Abou Neel, E.A.; Pickup, D.M.; O’Dell, L.A.; Chrzanowski, W.; Pratten, J.; Newport, R.J.; Smith, M.E.; Wilson, M.; et al. Controlled delivery of antimicrobial gallium ions from phosphate-based glasses. Acta Biomater. 2009, 5, 1198–1210. [Google Scholar] [CrossRef] [Green Version]
- Gross, T.M.; Lahiri, J.; Golas, A.; Luo, J.; Verrier, F.; Kurzejewski, J.L.; Baker, D.E.; Wang, J.; Novak, P.F.; Snyder, M.J. Copper-containing glass ceramic with high antimicrobial efficacy. Nat. Commun. 2019, 10, 1979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Gargiulo, N.; Samuel, S.; Naveen, S.V.; Kamarul, T.; Towler, M.R. Gallium-containing mesoporous bioactive glass with potent hemostatic activity and antibacterial efficacy. J. Mater. Chem. B 2016, 4, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Salcedo, S.; Malavasi, G.; Salinas, A.J.; Lusvardi, G.; Rigamonti, L.; Menabue, L.; Vallet-Regi, M. Highly-bioreactive silica-based mesoporous bioactive glasses enriched with gallium(III). Materials 2018, 11, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Zhou, Y.; Xu, M.; Han, P.; Chen, L.; Chang, J.; Xiao, Y. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials 2013, 34, 422–433. [Google Scholar] [CrossRef]
- Bari, A.; Bloise, N.; Fiorilli, S.; Novajra, G.; Vallet-Regí, M.; Bruni, G.; Torres-Pardo, A.; González-Calbet, J.M.; Visai, L.; Vitale-Brovarone, C. Copper-containing mesoporous bioactive glass nanoparticles as multifunctional agent for bone regeneration. Acta Biomater. 2017, 55, 493–504. [Google Scholar] [CrossRef]
- Peszke, J.; Dulski, M.; Nowak, A.; Balin, K.; Zubko, M.; Sułowicz, S.; Nowak, B.; Piotrowska-Seget, Z.; Talik, E.; Wojtyniak, M.; et al. Unique properties of silver and copper silica-based nanocomposites as antimicrobial agents. RSC Adv. 2017, 7, 28092–28104. [Google Scholar] [CrossRef] [Green Version]
- Ryan, E.J.; Ryan, A.J.; González-Vázquez, A.; Philippart, A.; Ciraldo, F.E.; Hobbs, C.; Nicolosi, V.; Boccaccini, A.R.; Kearney, C.J.; O’Brien, F.J. Collagen scaffolds functionalised with copper-eluting bioactive glass reduce infection and enhance osteogenesis and angiogenesis both in vitro and in vivo. Biomaterials 2019, 197, 405–416. [Google Scholar] [CrossRef]
- Rau, J.V.; Curcio, M.; Raucci, M.G.; Barbaro, K.; Fasolino, I.; Teghil, R.; Ambrosio, L.; De Bonis, A.; Boccaccini, A.R. Cu-releasing bioactive glass coatings and their in vitro properties. ACS Appl. Mater. Interfaces 2019, 11, 5812–5820. [Google Scholar] [CrossRef]
- Miola, M.; Verné, E. Bioactive and antibacterial glass powders doped with copper by ion-exchange in aqueous solutions. Materials 2016, 9, 405. [Google Scholar] [CrossRef] [Green Version]
- Hoppe, A.; Meszaros, R.; Stähli, C.; Romeis, S.; Schmidt, J.; Peukert, W.; Marelli, B.; Nazhat, S.N.; Wondraczek, L.; Lao, J.; et al. In vitro reactivity of Cu doped 45S5 Bioglass® derived scaffolds for bone tissue engineering. J. Mater. Chem. B 2013, 1, 5659–5674. [Google Scholar] [CrossRef]
- Romero-Sánchez, L.B.; Marí-Beffa, M.; Carrillo, P.; Medina, M.Á.; Díaz-Cuenca, A. Copper-containing mesoporous bioactive glass promotes angiogenesis in an in vivo zebrafish model. Acta Biomater. 2018, 68, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wang, Y.; Zhang, Y.; Cui, D.; Gu, G.; Zhao, D. Gallium ions promote osteoinduction of human and mouse osteoblasts via the TRPM7/Akt signaling pathway. Mol. Med. Rep. 2020, 22, 2741–2752. [Google Scholar] [CrossRef] [PubMed]
- Wajda, A.; Goldmann, W.H.; Detsch, R.; Grünewald, A.; Boccaccini, A.R.; Sitarz, M. Structural characterization and evaluation of antibacterial and angiogenic potential of gallium-containing melt-derived and gel-derived glasses from CaO-SiO2 system. Ceram. Int. 2018, 44, 22698–22709. [Google Scholar] [CrossRef]
- Stan, G.E.; Morosanu, C.O.; Marcov, D.A.; Pasuk, I.; Miculescu, F.; Reumont, G. Effect of annealing upon the structure and adhesion properties of sputtered bio-glass/titanium coatings. Appl. Surf. Sci. 2009, 255, 9132–9138. [Google Scholar] [CrossRef]
- ISO—International Organization for Standardization. ISO 13779-2:2018—Implants for Surgery—Hydroxyapatite—Part 2: Thermally Sprayed Coatings of Hydroxyapatite; ISO—International Organization for Standardization: Geneva, Switzerland, 2018. [Google Scholar]
- Callahan, T.J.; Gatenberg, J.B.; Sands, B.E. STP1196—Calcium phosphate (Ca-P) coating draft guidance for preparation of food and drug administration (FDA) submissions for orthopedic and dental endosseous implants. In Characterization and Performance of Calcium Phosphate Coatings for Implants; ASTM International: West Conshohocken, PA, USA, 1994; pp. 185–197. [Google Scholar]
- Goel, A.; Kapoor, S.; Rajagopal, R.R.; Pascual, M.J.; Kim, H.-W.; Ferreira, J.M.F. Alkali-free bioactive glasses for bone tissue engineering: A preliminary investigation. Acta Biomater. 2012, 8, 361–372. [Google Scholar] [CrossRef]
- Kapoor, S.; Goel, A.; Tilocca, A.; Dhuna, V.; Bhatia, G.; Dhuna, K.; Ferreira, J.M.F. Role of glass structure in defining the chemical dissolution behavior, bioactivity and antioxidant properties of zinc and strontium co-doped alkali-free phosphosilicate glasses. Acta Biomater. 2014, 10, 3264–3278. [Google Scholar] [CrossRef]
- Popa, A.C.; Stan, G.E.; Besleaga, C.; Ion, L.; Maraloiu, V.A.; Tulyaganov, D.U.; Ferreira, J.M.F. Submicrometer hollow bioglass cones deposited by radio frequency magnetron sputtering: Formation mechanism, properties, and prospective biomedical applications. ACS Appl. Mater. Interfaces 2016, 8, 4357–4367. [Google Scholar] [CrossRef]
- Popa, A.C.; Stan, G.E.; Husanu, M.A.; Mercioniu, I.; Santos, L.F.; Fernandes, H.R.; Ferreira, J.M.F. Bioglass implant-coating interactions in synthetic physiological fluids with varying degrees of biomimicry. Int. J. Nanomed. 2017, 12, 683–707. [Google Scholar] [CrossRef] [Green Version]
- Cauchy, A.L. Sur la réfraction et la réflexion de la lumière. Bull. des Sci. Math. (Bull. Férussac) 1830, 14, 6–10. [Google Scholar]
- Galca, A.C.; Stan, G.E.; Trinca, L.M.; Negrila, C.C.; Nistor, L.C. Structural and optical properties of c-axis oriented aluminum nitride thin films prepared at low temperature by reactive radio-frequency magnetron sputtering. Thin Solid Film. 2012, 524, 328–333. [Google Scholar] [CrossRef]
- ISO—International Organization for Standardization. ISO 22309:2011—Microbeam Analysis—Quantitative Analysis Using Energy-Dispersive Spectrometry (EDS) for Elements with an Atomic Number of 11 (Na) or Above; ISO—International Organization for Standardization: Geneva, Switzerland, 2011. [Google Scholar]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Popa, A.C.; Stan, G.E.; Husanu, M.A.; Pasuk, I.; Popescu, I.D.; Popescu, A.C.; Mihailescu, I.N. Multi-layer haemocompatible diamond-like carbon coatings obtained by combined radio frequency plasma enhanced chemical vapor deposition and magnetron sputtering. J. Mater. Sci. Mater. Med. 2013, 24, 2695–2707. [Google Scholar] [CrossRef] [PubMed]
- Visan, A.; Grossin, D.; Stefan, N.; Duta, L.; Miroiu, F.M.; Stan, G.E.; Sopronyi, M.; Luculescu, C.; Freche, M.; Marsan, O.; et al. Biomimetic nanocrystalline apatite coatings synthesized by matrix assisted pulsed laser evaporation for medical applications. Mater. Sci. Eng. B 2014, 181, 56–63. [Google Scholar] [CrossRef] [Green Version]
- ISO—International Organization for Standardization. ISO 4624:2016—Paints and Varnishes—Pull-off Test for Adhesion; ISO—International Organization for Standardization: Geneva, Switzerland, 2016. [Google Scholar]
- ASTM International. ASTM D4541-17 Standard Test Method for Pull-Off Strength of Coatings Using Portable Adhesion; ASTM International: West Conshohocken, PA, USA, 2017; pp. 1–16. [Google Scholar]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Jacobs, R.; Meneve, J.; Dyson, G.; Teer, D.G.; Jennett, N.M.; Harris, P.; von Stebut, J.; Comte, C.; Feuchter, P.; Cavaleiro, A.; et al. A certified reference material for the scratch test. Surf. Coat. Technol. 2003, 174–175, 1008–1013. [Google Scholar] [CrossRef] [Green Version]
- Ingelbrecht, C.; Jennett, N.; Jacobs, R.; Meneve, J. The Certification of Critical Coating Failure Loads: A Reference Material for Scratch Testing According to ENV 1071-3; European Commission: Luxembourg, 1994; ISBN 928946870X. [Google Scholar]
- European Standards—CEN. EN1071-3/2005—Advanced Technical Ceramics—Methods of Test for Ceramic Coatings—Part 3: Determination of Adhesion and Other Mechanical Failure Modes by a Scratch Test; European Standards—CEN: Brussels, Belgium, 2005. [Google Scholar]
- Meneve, J.; Havermans, D.; von Stebut, J.; Jennett, N.; Banks, J.; Saunders, S.; Camino, D.; Teer, D.; Andersson, P.; Varjus, S. The Scratch Test: Atlas of Failure Modes. In World Tribology Congress: Abstracts of Papers; Institution of Mechanical Engineers: London, UK, 1997; p. 495. [Google Scholar]
- CDC. Available online: https://www.cdc.gov/infectioncontrol/pdf/guidelines/disinfection-guidelines-H.pdf (accessed on 3 November 2020).
- WHO. Available online: https://apps.who.int/phint/pdf/b/7.5.9.5.8-Methods-of-sterilization.pdf (accessed on 3 November 2020).
- ISO. ISO 22196:2011—Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces; ISO—International Organization for Standardization: Geneva, Switzerland, 2011. [Google Scholar]
- ISO. ISO 10993-5:2009—Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; ISO—International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Popa, A.C.; Marques, V.M.F.; Stan, G.E.; Husanu, M.A.; Galca, A.C.; Ghica, C.; Tulyaganov, D.U.; Lemos, A.F.; Ferreira, J.M.F. Nanomechanical characterization of bioglass films synthesized by magnetron sputtering. Thin Solid Film. 2014, 553, 166–172. [Google Scholar] [CrossRef]
- Besleaga, C.; Dumitru, V.; Trinca, L.M.; Popa, A.C.; Negrila, C.C.; Kołodziejczyk, Ł.; Luculescu, C.R.; Ionescu, G.C.; Ripeanu, R.G.; Vladescu, A.; et al. Mechanical, corrosion and biological properties of room-temperature sputtered aluminum nitride films with dissimilar nanostructure. Nanomaterials 2017, 7, 394. [Google Scholar] [CrossRef] [Green Version]
- Chemical Books Phosphorus Pentoxide. Available online: https://www.chemicalbook.com/ProductChemicalPropertiesCB9245033_EN.htm (accessed on 19 November 2020).
- Wagner, C.D.; Riggs, W.M.; Davis, L.E.; Moulder, J.F.; Muilenberg, G.E. (Eds.) Handbook of X-Ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics Division, Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992; ISBN 978-0-96-270262-4. [Google Scholar]
- Carli, R.; Bianchi, C.L. XPS analysis of gallium oxides. Appl. Surf. Sci. 1992, 74, 99–102. [Google Scholar] [CrossRef]
- Reed, T.B. Free Energy of Formation of Binary Compounds: An Atlas of Charts for High-Temperature Chemical Calculations; MIT Press: Cambridge, MA, USA, 1972; ISBN 978-0-26-218051-1. [Google Scholar]
- Serra, J.; Gonzalez, P.; Liste, S.; Serra, C.; Chiussi, S.; Leon, B.; Perez-Amor, M.; Ylanen, H.O.; Hupa, M. FTIR and XPS studies of bioactive silica based glasses. J. Non-Cryst. Solids 2003, 332, 20–27. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Bancroft, G.M.; Henderson, G.S.; Sawyer, R.; Secco, R.A. Direct and indirect evidence for free oxygen (O2–) in MO-silicate glasses and melts (M = Mg, Ca, Pb). Am. Mineral. 2015, 100, 2566–2578. [Google Scholar] [CrossRef]
- Ziemath, E.C.; Aegerter, M.A. Raman and Infrared investigations of glass and glass-ceramics with composition 2Na2O·1CaO·3SiO2. J. Mater. Res. 1994, 9, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Agathopoulos, S.; Tulyaganov, D.U.; Ventura, J.M.G.; Kannan, S.; Karakassides, M.A.; Ferreira, J.M.F. Formation of hydroxyapatite onto glasses of the CaO–MgO–SiO2 system with B2O3, Na2O, CaF2 and P2O5 additives. Biomaterials 2006, 27, 1832–1840. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, J.; Yao, L. A study of glass structure in Li2OSiO2, Li2OAl2O3SiO2 and LiAlSiON systems. J. Non-Cryst. Solids 1989, 112, 80–84. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, W.; Korpacz, A.N.; Dufour, C.R.; Weiland, Z.J.; Lambert, C.R.; Timko, M.T. Binary Liquid mixture contact-angle measurements for precise estimation of surface free energy. Langmuir 2019, 35, 12317–12325. [Google Scholar] [CrossRef]
- Lotfi, M.; Nejib, M.; Naceur, M. Cell Adhesion to Biomaterials: Concept of Biocompatibility. In Advances in Biomaterials Science and Biomedical Applications; Intech: Rijeka, Croatia, 2013; pp. 207–240. [Google Scholar] [CrossRef] [Green Version]
- Popescu, A.C.; Florian, P.E.; Stan, G.E.; Popescu-Pelin, G.; Zgura, I.; Enculescu, M.; Oktar, F.N.; Trusca, R.; Sima, L.E.; Roseanu, A.; et al. Physical-chemical characterization and biological assessment of simple and lithium-doped biological-derived hydroxyapatite thin films for a new generation of metallic implants. Appl. Surf. Sci. 2018, 439, 724–735. [Google Scholar] [CrossRef]
- Gentleman, M.M.; Gentleman, E. The role of surface free energy in osteoblast-biomaterial interactions. Int. Mater. Rev. 2014, 59, 417–429. [Google Scholar] [CrossRef]
- Pešáková, V.; Kubies, D.; Hulejová, H.; Himmlová, L. The influence of implant surface properties on cell adhesion and proliferation. J. Mater. Sci. Mater. Med. 2007, 18, 465–473. [Google Scholar] [CrossRef]
- Webb, K.; Hlady, V.; Tresco, P.A. Relative importance of surface wettability and charged functional groups on NIH 3T3 fibroblast attachment, spreading, and cytoskeletal organization. J. Biomed. Mater. Res. 1998, 41, 422–430. [Google Scholar] [CrossRef] [Green Version]
- Ng, C.H.; Rao, N.; Law, W.C.; Xu, G.; Cheung, T.L.; Cheng, F.T.; Wang, X.; Man, H.C. Enhancing the cell proliferation performance of NiTi substrate by laser diffusion nitriding. Surf. Coat. Technol. 2017, 309, 59–66. [Google Scholar] [CrossRef]
- Bellucci, D.; Bianchi, M.; Graziani, G.; Gambardella, A.; Berni, M.; Russo, A.; Cannillo, V. Pulsed electron deposition of nanostructured bioactive glass coatings for biomedical applications. Ceram. Int. 2017, 43, 15862–15867. [Google Scholar] [CrossRef]
- KYOCERA. Available online: https://kyocera-sgstool.co.uk/titanium-resources/titanium-information-everything-you-need-to-know/titaniumproperties/#:~:text=It’s%20Young’s%20modulus%20of%20elasticity,shear%20modulus%20of%2045%20GPA (accessed on 3 November 2020).





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stan, G.E.; Tite, T.; Popa, A.-C.; Chirica, I.M.; Negrila, C.C.; Besleaga, C.; Zgura, I.; Sergentu, A.C.; Popescu-Pelin, G.; Cristea, D.; et al. The Beneficial Mechanical and Biological Outcomes of Thin Copper-Gallium Doped Silica-Rich Bio-Active Glass Implant-Type Coatings. Coatings 2020, 10, 1119. https://doi.org/10.3390/coatings10111119
Stan GE, Tite T, Popa A-C, Chirica IM, Negrila CC, Besleaga C, Zgura I, Sergentu AC, Popescu-Pelin G, Cristea D, et al. The Beneficial Mechanical and Biological Outcomes of Thin Copper-Gallium Doped Silica-Rich Bio-Active Glass Implant-Type Coatings. Coatings. 2020; 10(11):1119. https://doi.org/10.3390/coatings10111119
Chicago/Turabian StyleStan, George E., Teddy Tite, Adrian-Claudiu Popa, Iuliana Maria Chirica, Catalin C. Negrila, Cristina Besleaga, Irina Zgura, Any Cristina Sergentu, Gianina Popescu-Pelin, Daniel Cristea, and et al. 2020. "The Beneficial Mechanical and Biological Outcomes of Thin Copper-Gallium Doped Silica-Rich Bio-Active Glass Implant-Type Coatings" Coatings 10, no. 11: 1119. https://doi.org/10.3390/coatings10111119
APA StyleStan, G. E., Tite, T., Popa, A.-C., Chirica, I. M., Negrila, C. C., Besleaga, C., Zgura, I., Sergentu, A. C., Popescu-Pelin, G., Cristea, D., Ionescu, L. E., Necsulescu, M., Fernandes, H. R., & Ferreira, J. M. F. (2020). The Beneficial Mechanical and Biological Outcomes of Thin Copper-Gallium Doped Silica-Rich Bio-Active Glass Implant-Type Coatings. Coatings, 10(11), 1119. https://doi.org/10.3390/coatings10111119









