Protein–TiO2: A Functional Hybrid Composite with Diversified Applications
Abstract
:1. Introduction
2. Proteins: Applications and Limitations
3. Possible Structural Interaction between R-Groups Amino Acid with TiO2 Nanoparticles
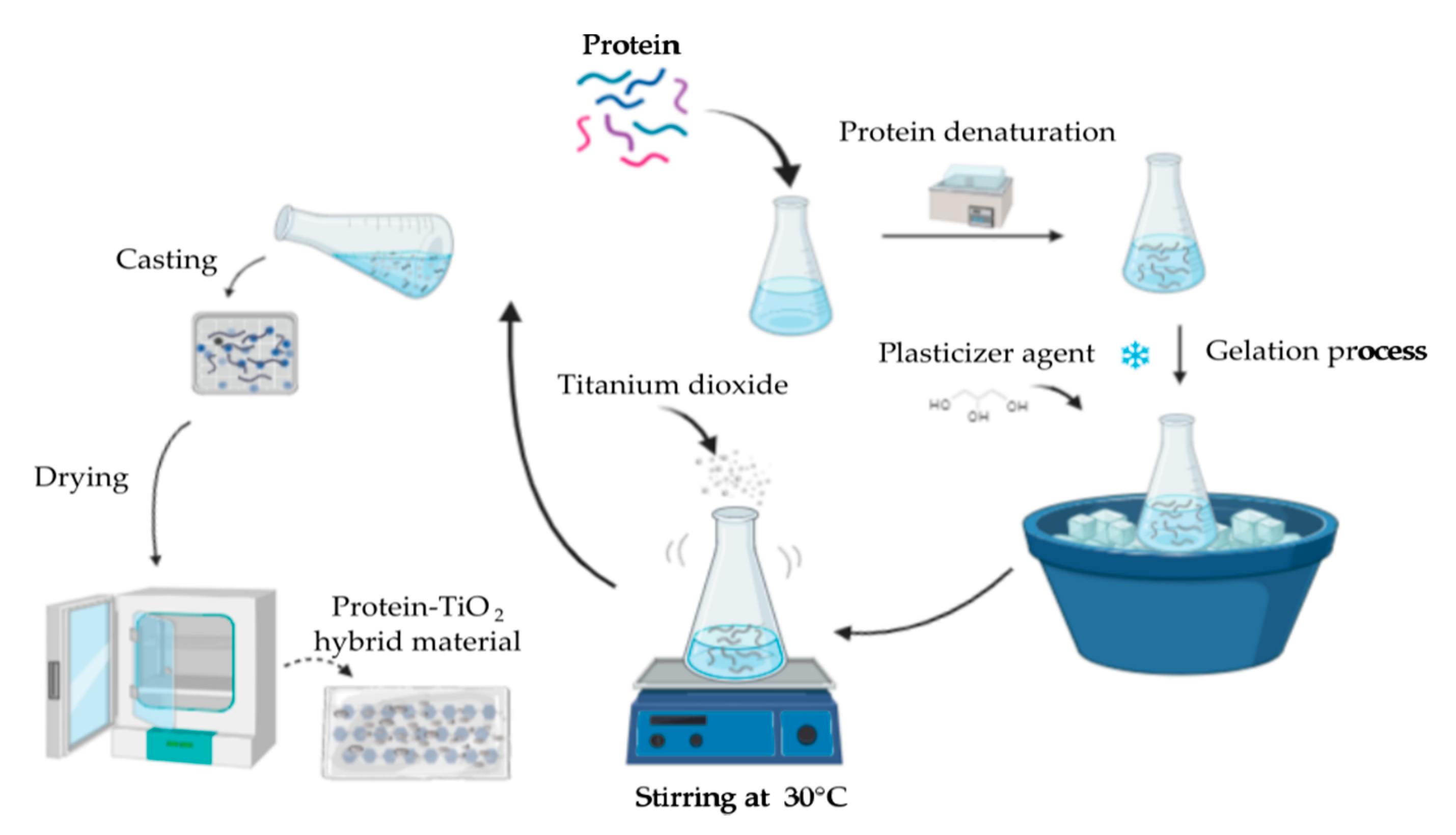
4. Preparation of Functionalized Protein–TiO2 Materials
4.1. Evaporative Casting Method
4.2. Dip Coating Method
4.3. Layer-by-Layer Deposition Method
4.4. Freeze-Drying Method
4.5. Electospinning Method
4.6. Electrochemical Method
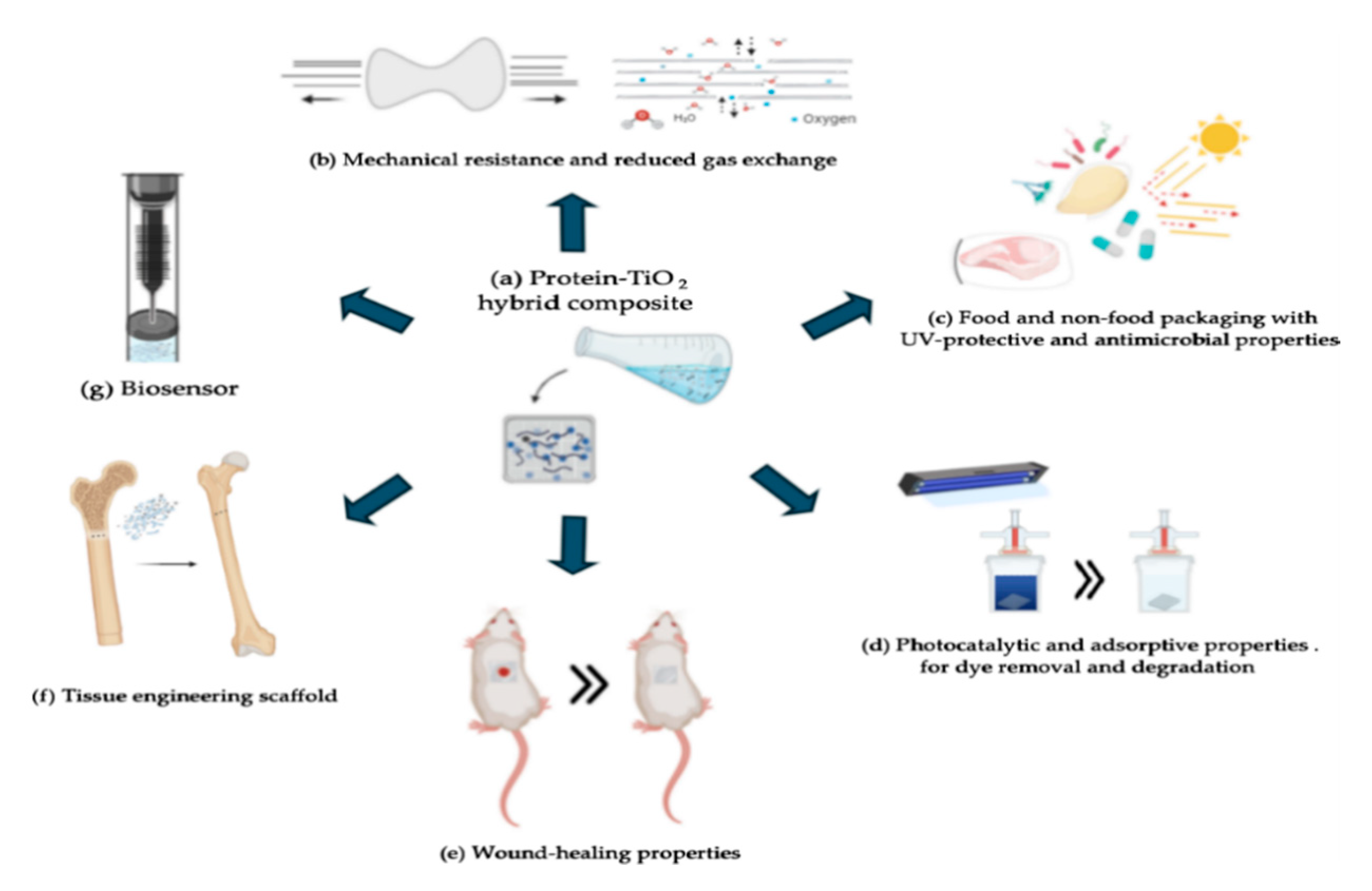
5. Applications of Protein–TiO2 Hybrid Composites
5.1. Gelatin–TiO2 Hybrid Composite
5.1.1. Food and Non-Food Packaging Applications of Gelatin–TiO2 Hybrid Composite
5.1.2. Biomedical Applications of Gelatin–TiO2 Hybrid Composite
5.1.3. Other Applications of Gelatin–TiO2 Hybrid Composite
5.2. Whey Protein–TiO2 Hybrid Composite
5.2.1. Food and Non-Food Packaging Applications of Whey Protein–TiO2 Hybrid Composite
5.2.2. Other Applications of Whey Protein–TiO2 Hybrid Composite
5.3. Collagen–TiO2 Hybrid Composite
5.3.1. Biomedical Applications of Collagen–TiO2 Hybrid Composite
5.3.2. Other Applications of Collagen–TiO2 Hybrid Composite
5.4. Soy Protein–TiO2 Hybrid Composite
5.4.1. Food and Non-Food Packaging Applications of Collagen–TiO2 Hybrid Composite
5.4.2. Other Applications of Soy Protein Isolate–TiO2 Hybrid Composite
5.5. Other Proteins Functionalized with TiO2
5.5.1. Packaging Applications of Non-Conventional Proteins Functionalized with TiO2
5.5.2. Environmental Applications of Non-Conventional Proteins Functionalized with TiO2
5.5.3. Other Applications of Non-Conventional Proteins Functionalized with TiO2
6. Disadvantages of Protein–TiO2 Hybrid Composites and Perspectives
7. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Riahi, Z.; Priyadarshi, R.; Rhim, J.; Bagheri, R. Gelatin-based functional films integrated with grapefruit seed extract and TiO2 for active food packaging applications. Food Hydrocoll. 2021, 112, 106314. [Google Scholar] [CrossRef]
- Avramescu, S.M.; Butean, C.; Popa, C.V.; Ortan, A.; Moraru, I.; Temocico, G. Edible and functionalized films/coatings-performances and perspectives. Coatings 2020, 10, 687. [Google Scholar] [CrossRef]
- Lin, D.; Yang, Y.; Wang, J.; Yan, W.; Wu, Z.; Chen, H.; Zhang, Q.; Wu, D.; Qin, W.; Tu, Z. Preparation and characterization of TiO2–Ag loaded fish gelatin-chitosan antibacterial composite film for food packaging. Int. J. Biol. Macromol. 2020, 154, 123–133. [Google Scholar] [CrossRef] [PubMed]
- El-wakil, N.A.; Hassan, E.A.; Abou-zeid, R.E.; Dufresne, A. Development of wheat gluten/nanocellulose/titanium dioxide nanocomposites for active food packaging. Carbohydr. Polym. 2015, 124, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Park, I.; Neupane, M.P.; Bae, T.; Lee, M. Effects of a carbon nanotube-collagen coating on a titanium surface on osteoblast growth. Appl. Surf. Sci. 2014, 292, 828–836. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Kamil, M.P.; Fatimah, S.; Nisa, N.; Ko, Y.G. Recent advances in hybrid organic-inorganic materials with spatial architecture for state-of-the-art applications. Prog. Mater. Sci. 2020, 112, 100663. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Zhang, M.; He, J.; Zheng, B.; Liu, F.; Zhao, Z.; Liu, Y. Use of silver nanoparticle–gelatin/alginate scaffold. Coatings 2020, 10, 948. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Villagrán-de la Mora, Z.; Ruvalcaba-Gómez, J.M.; Romero-Toledo, R.; Sandoval-Contreras, T.; Aguilera-Aguirre, S.; Montalvo-González, E.; Pérez-Larios, A. Use of titanium dioxide (TiO2) nanoparticles as reinforcement agent of polysaccharide-based materials. Processes 2020, 8, 1395. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Ruvalcaba-Gómez, J.M.; Maytorena-Verdugo, C.I.; González-Silva, N.; Romero-Toledo, R.; Aguilera-Aguirre, S.; Pérez-Larios, A.; Montalvo-González, E. Chitosan–TiO2: A versatile hybrid composite. Materials 2020, 13, 811. [Google Scholar] [CrossRef] [Green Version]
- Omar, N.; Selvami, S.; Kaisho, M.; Yamada, M.; Yasui, T.; Fukumoto, M. Deposition of titanium dioxide coating by the cold-spray process on annealed stainless steel substrate. Coatings 2020, 10, 991. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Rhim, J.W.; Azizi-Lalabadi, M.; Hemmati-Dinarvand, M.; Ehsani, A. Preparation and characterization of functional sodium caseinate/guar gum/TiO2/cumin essential oil composite film. Int. J. Biol. Macromol. 2020, 145, 835–844. [Google Scholar] [CrossRef] [PubMed]
- de Fonseca, J.M.; Valencia, G.A.; Soares, L.S.; Dotto, M.E.R.; Campos, C.E.M.; de Moreira, R.F.P.M.; Fritz, A.R.M. Hydroxypropyl methylcellulose–TiO2 and gelatin–TiO2 nanocomposite films: Physicochemical and structural properties. Int. J. Biol. Macromol. 2020, 151, 944–956. [Google Scholar] [CrossRef] [PubMed]
- Anaya-Esparza, L.M.; Montalvo-González, E.; González-Silva, N.; Méndez-Robles, M.D.; Romero-Toledo, R.; Yahia, E.M.; Pérez-Larios, A. Synthesis and characterization of TiO2–ZnO–MgO mixed oxide and their antibacterial activity. Materials 2019, 12, 698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anaya-Esparza, L.M.; González-Silva, N.; Yahia, E.M.; González-Vargas, O.A.; Montalvo-González, E.; Pérez-Larios, A. Effect of TiO2–ZnO–MgO mixed oxide on microbial growth and toxicity against Artemia salina. Nanomaterials 2019, 9, 992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortelli, S.; Costa, A.L. Insulating thermal and water-resistant hybrid coating for fabrics. Coatings 2020, 10, 72. [Google Scholar] [CrossRef] [Green Version]
- Fathi, N.; Almasi, H.; Pirouzifard, M.K. Sesame protein isolate based bionanocomposite films incorporated with TiO2 nanoparticles: Study on morphological, physical and photocatalytic properties. Polym. Test. 2019, 77, 105919. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Y.; Liu, F.; Ren, F.; Zhao, G.; Leng, X. Fabrication and characterization of TiO2/whey protein isolate nanocomposite film. Food Hydrocoll. 2011, 25, 1098–1104. [Google Scholar] [CrossRef]
- Farshchi, E.; Pirsa, S.; Roufegarinejad, L.; Alizadeh, M.; Rezazad, M. Photocatalytic/biodegradable film based on carboxymethyl cellulose, modified by gelatin and TiO2–Ag nanoparticles. Carbohydr. Polym. 2019, 216, 189–196. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, N.; Wang, H.Y.; Sui, S.Y.; Sun, X.X.; Ma, Z.S. The effects of ultrasonic/microwave assisted treatment on the properties of soy protein isolate/titanium dioxide films. LWT-Food Sci. Technol. 2014, 57, 548–555. [Google Scholar] [CrossRef]
- Boughriba, S.; Souissi, N.; Jridi, M.; Li, S.; Nasri, M. Thermal, mechanical and microstructural characterization and antioxidant potential of Rhinobatos cemiculus gelatin films supplemented by titanium dioxide doped silver nanoparticles. Food Hydrocoll. 2020, 103, 105695. [Google Scholar] [CrossRef]
- Vejdan, A.; Ojagh, S.M.; Adeli, A.; Abdollahi, M. Effect of TiO2 nanoparticles on the physico-mechanical and ultraviolet light barrier properties of fish gelatin/agar bilayer film. LWT Food Sci. Technol. 2016, 71, 88–95. [Google Scholar] [CrossRef]
- Montazer, M.; Pakdel, E.; Moghadam, M.B. Nano titanium dioxide on wool keratin as UV absorber stabilized by butane tetra carboxylic acid (BTCA): A statistical prospect. Fibers Polym. 2010, 11, 967–975. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zhu, B.B.; Li, D.Z.; Fu, X.Z.; Shi, L. Preparation and characterization of TiO2/SPI composite film. Mater. Lett. 2012, 83, 42–45. [Google Scholar] [CrossRef]
- Kadam, D.M.; Thunga, M.; Srinivasan, G.; Wang, S.; Kessler, M.R.; Grewell, D.; Yu, C.; Lamsal, B. Effect of TiO2 nanoparticles on thermo-mechanical properties of cast zein protein films. Food Packag. Shelf Life 2017, 13, 35–43. [Google Scholar] [CrossRef] [Green Version]
- He, Q.; Huang, Y.; Lin, B.; Wang, S. A nanocomposite film fabricated with simultaneously extracted protein-polysaccharide from a marine alga and TiO2 nanoparticles. J. Appl. Phycol. 2017, 29, 1541–1552. [Google Scholar] [CrossRef]
- He, Q.; Zhang, Y.; Cai, X.; Wang, S. Fabrication of gelatin–TiO2 nanocomposite film and its structural, antibacterial and physical properties. Int. J. Biol. Macromol. 2016, 84, 153–160. [Google Scholar] [CrossRef]
- Fan, X.; Chen, K.; He, X.; Li, N.; Huang, J.; Tang, K.; Li, Y.; Wang, F. Nano-TiO2/collagen-chitosan porous scaffold for wound repairing. Int. J. Biol. Macromol. 2016, 91, 15–22. [Google Scholar] [CrossRef]
- Alizadeh Sani, M.; Ehsani, A.; Hashemi, M. Whey protein isolate/cellulose nanofibre/TiO2 nanoparticle/rosemary essential oil nanocomposite film: Its effect on microbial and sensory quality of lamb meat and growth of common foodborne pathogenic bacteria during refrigeration. Int. J. Food Microbiol. 2017, 251, 8–14. [Google Scholar] [CrossRef]
- Feng, Z.; Li, L.; Wang, Q.; Wu, G.; Liu, C.; Jiang, B.; Xu, J. Effect of antioxidant and antimicrobial coating based on whey protein nanofibrils with TiO2 nanotubes on the quality and shelf life of chilled meat. Int. J. Mol. Sci. 2019, 20, 1184. [Google Scholar] [CrossRef] [Green Version]
- Montes-de-Oca-Ávalos, J.M.; Altamura, D.; Jorge, R. Relationship between nano/microstructure and physical properties of TiO2–sodium caseinate composite films. Food Res. Int. 2018, 105, 129–139. [Google Scholar] [CrossRef]
- Zolfi, M.; Khodaiyan, F.; Mousavi, M.; Hashemi, M. The improvement of characteristics of biodegradable films made from kefiran-whey protein by nanoparticle incorporation. Carbohydr. Polym. 2014, 109, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Zink, J.; Wyrobnik, T.; Prinz, T.; Schmid, M. Physical, chemical and biochemical modifications of protein-based films and coatings: An extensive review. Int. J. Mol. Sci. 2016, 17, 1376. [Google Scholar] [CrossRef] [PubMed]
- Böhmer-Maas, B.W.; Martins, L.; Murowaniecki, D.; Zavareze, R.; Carlos, R. Photocatalytic zein–TiO2 nanofibers as ethylene absorbers for storage of cherry tomatoes. Food Packag. Shelf Life 2020, 24, 100508. [Google Scholar] [CrossRef]
- Li, N.; Fan, X.; Tang, K.; Zheng, X.; Liu, J.; Wang, B. Nanocomposite scaffold with enhanced stability by hydrogen bonds between collagen, polyvinyl pyrrolidone and titanium dioxide. Coll. Surf. B 2016, 140, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Pop, O.L.; Pop, C.R.; Dufrechou, M.; Vodnar, D.C.; Socaci, S.A.; Dulf, F.V.; Minervini, F.; Suharoschi, R. Edible films and coatings functionalization by probiotic incorporation: A review. Polymers 2020, 12, 12. [Google Scholar] [CrossRef] [Green Version]
- Acquah, C.; Zhang, Y.; Dubé, M.A.; Udenigwe, C.C. Formation and characterization of protein-based films from yellow pea (Pisum sativum) protein isolate and concentrate for edible applications. Curr. Res. Food Sci. 2020, 2, 61–69. [Google Scholar] [CrossRef]
- Agudelo-Cuartas, C.; Granda-restrepo, D.; Sobral, P.J.A.; Hernandez, H. Characterization of whey protein-based fi lms incorporated with natamycin and nanoemulsion of α-tocopherol. Heliyon 2020, 6, e03809. [Google Scholar] [CrossRef]
- Guo, X.; Ren, C.; Zhang, Y.; Cui, H.; Shi, C. Stability of zein-based films and their mechanism of change during storage at different temperatures and relative humidity. J. Food Process. Preserv. 2020, 44, 1–10. [Google Scholar] [CrossRef]
- Su, J.; Huang, Z.; Liu, K.; Fu, L.; Liu, H. Mechanical properties, biodegradation and water vapor permeability of blend films of soy protein isolate and poly(vinyl alcohol) compatibilized by glycerol. Polym. Bull. 2007, 921, 913–921. [Google Scholar] [CrossRef]
- Wang, N.; Saleh, A.S.M.; Xiao, Z. Effect of protein aggregates on properties and structure of rice bran protein-based film at different pH. J. Food Sci. Technol. 2019, 56, 5116–5127. [Google Scholar] [CrossRef]
- Mangavel, C.; Rossignol, N.; Perronnet, A.; Barbot, A.; Gueguen, J. Properties and microstructure of thermo-pressed Wheat gluten films: A comparison with cast films. Biomacromolecules 2004, 5, 1596–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexandre, E.M.C.; Lourenço, R.V.; Bittante, A.M.Q.B.; Moraes, I.C.F.; do Amaral Sobral, P.J. Gelatin-based films reinforced with montmorillonite and activated with nanoemulsion of ginger essential oil for food packaging applications. Food Packag. Shelf Life 2016, 10, 87–96. [Google Scholar] [CrossRef]
- Dias, J.R.; Baptista-silva, S.; De Oliveira, C.M.T.; Sousa, A.; Oliveira, A.L. In situ crosslinked electrospun gelatin nanofibers for skin regeneration. Eur. Polym. J. 2017, 95, 161–173. [Google Scholar] [CrossRef] [Green Version]
- Rouse, J.G.; Van Dyke, M.E. A review of keratin-based biomaterials for biomedical applications. Materials 2010, 3, 999–1014. [Google Scholar] [CrossRef] [Green Version]
- Hauzoukim; Swain, S.; Mohanty, B.; Hauzoukim, S.S.; Mohanty, B. Functionality of protein-based edible coating—Review. J. Entomol. Zool. Stud. 2020, 8, 1432–1440. [Google Scholar]
- Kanmani, P.; Rhim, J.W. Physicochemical properties of gelatin/silver nanoparticle antimicrobial composite films. Food Chem. 2014, 148, 162–169. [Google Scholar] [CrossRef]
- Bang, Y.J.; Shankar, S.; Rhim, J.W. In situ synthesis of multi-functional gelatin/resorcinol/silver nanoparticles composite films. Food Packag. Shelf Life 2019, 22, 100399. [Google Scholar] [CrossRef]
- Cano, A.; Andres, M.; Chiralt, A.; González-Martinez, C. Use of tannins to enhance the functional properties of protein based films. Food Hydrocoll. 2020, 100. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Moghaddas Kia, E.; Ghasempour, Z.; Ehsani, A. Preparation of active nanocomposite film consisting of sodium caseinate, ZnO nanoparticles and rosemary essential oil for food packaging applications. J. Polym. Environ. 2020. [Google Scholar] [CrossRef]
- Azevedo, V.M.; Dias, M.V.; de Siqueira Elias, H.H.; Fukushima, K.L.; Silva, E.K.; de Deus Souza Carneiro, J.; de Fátima Ferreira Soares, N.; Borges, S.V. Effect of whey protein isolate films incorporated with montmorillonite and citric acid on the preservation of fresh-cut apples. Food Res. Int. 2018, 107, 306–313. [Google Scholar] [CrossRef]
- Pintado, C.M.B.S.; Ferreira, M.A.S.S.; Sousa, I. Properties of whey protein-based films containing organic acids and nisin to control Listeria Monocytogenes. J. Food Prot. 2009, 72, 1891–1896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pluta-Kubica, A.; Jamróz, E.; Kawecka, A.; Juszczak, L.; Krzyściak, P. Active edible furcellaran/whey protein films with yerba mate and white tea extracts: Preparation, characterization and its application to fresh soft rennet-curd cheese. Int. J. Biol. Macromol. 2020, 155, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Arfat, Y.A.; Benjakul, S.; Prodpran, T.; Sumpavapol, P.; Songtipya, P. Physico-mechanical characterization and antimicrobial properties of fish protein isolate/fish skin gelatin-zinc oxide (ZnO) nanocomposite films. Food Bioprocess Technol. 2016, 9, 101–112. [Google Scholar] [CrossRef]
- Salama, A. Soy protein acid hydrolysate/silica hybrid material as novel adsorbent for methylene blue. Compos. Commun. 2019, 12, 101–105. [Google Scholar] [CrossRef]
- Asadzadeh, F.; Pirsa, S. Specific removal of nitrite from Lake Urmia sediments by biohydrogel based on isolated soy protein/tragacanth/mesoporous silica nanoparticles/lycopene. Glob. Chall. 2020, 2000061, 1–12. [Google Scholar] [CrossRef]
- Hou, C.; Xu, Z.; Qiu, W.; Wu, R.; Wang, Y.; Xu, Q.; Liu, X.Y. A Biodegradable and stretchable protein-based sensor as artificial electronic skin for human motion detection. Small 2019, 1805084, 1–8. [Google Scholar] [CrossRef]
- You, R.; Zhang, J.; Gu, S.; Zhou, Y.; Li, X.; Ye, D.; Xu, W. Regenerated egg white/silk fibroin composite films for biomedical applications. Mater. Sci. Eng. C 2017, 79, 430–435. [Google Scholar] [CrossRef]
- Topoglidis, E.; Cass, A.E.G.; Gilardi, G.; Sadeghi, S.; Beaumont, N.; Durrant, J.R. Protein adsorption on nanocrystalline TiO2 films: An immobilization strategy for bioanalytical devices. Anal. Chem. 1998, 70, 5111–5113. [Google Scholar] [CrossRef]
- Ranjan, S.; Dasgupta, N.; Sudandiradoss, C.; Ramalingam, C.; Kumar, A. Titanium dioxide nanoparticle-protein interaction explained by docking approach. Int. J. Nanomed. 2018, 13, 47–50. [Google Scholar] [CrossRef] [Green Version]
- Hashim, J.; Looney, L.; Hashmi, M.S.J. Metal matrix composites: Production by the stir casting method. J. Mater. Process. Technol. 1999, 92, 1–7. [Google Scholar] [CrossRef]
- Asri, R.I.M.; Harun, W.S.W.; Hassan, M.A.; Ghani, S.A.C.; Buyong, Z. A review of hydroxyapatite-based coating techniques: Sol-gel and electrochemical depositions on biocompatible metals. J. Mech. Behav. Biomed. Mater. 2016, 57, 95–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jilani, A.; Abdel-wahab, M.S.; Hammad, A.H. Advance deposition techniques for thin film and coating. In Modern Technologies for Creating the Thin-Film Systems and Coatings; BoD–Books on Demand: Norderstedt, Germany, 2017; pp. 137–149. [Google Scholar]
- Tang, X.; Yan, X. Dip-coating for fibrous materials: Mechanism, methods and applications. J. Sol-Gel Sci. Technol. 2017, 81, 378–404. [Google Scholar] [CrossRef]
- Andres, C.M.; Kotov, N.A. Inkjet deposition of layer-by-layer assembled films. J. Am. Chem. Soc. 2010, 132, 14496–14502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, J.J.; Björnmalm, M.; Caruso, F. Technology-driven layer-by-layer assembly of nanofilms. Science 2015, 348, 411–422. [Google Scholar] [CrossRef] [Green Version]
- Baudron, V.; Gurikov, P.; Smirnova, I.; Whitehouse, S. Porous starch materials via supercritical-and freeze-drying. Gels 2019, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Liew, K.B.; Odeniyi, M.A.; Peh, K.K. Application of freeze-drying technology in manufacturing orally disintegrating films. Pharm. Dev. Technol. 2016, 21, 346–353. [Google Scholar] [CrossRef]
- Matysiak, W.; Tanski, T.; Smok, W. Electrospinning as a versatile method of composite thin films fabrication for selected applications. Solid State Phenom. 2019, 293, 35–49. [Google Scholar] [CrossRef]
- Amrollahi, P.; Krasinki, J.S.; Vaidyanathan, R.; Tayebi, L.; Vashaee, D. Electrophoretic deposition (EPD): Fundamentals and applications from nano- to micro-scale structures. Handb. Nanoelectrochemistry 2015. [Google Scholar] [CrossRef]
- Emregul, E.; Kocabay, O.; Derkus, B.; Yumak, T.; Emregul, K.C.; Sinag, A.; Polat, K. A novel carboxymethylcellulose-gelatin-titanium dioxide-superoxide dismutase biosensor; electrochemical properties of carboxymethylcellulose-gelatin-titanium dioxide-superoxide dismutase. Bioelectrochemistry 2013, 90, 8–17. [Google Scholar] [CrossRef]
- Ferreira, R.; Padilla, R.; Urkasemsin, G.; Yoon, K.; Goeckner, K.; Hu, W.S.; Ko, C.C. Titanium-enriched hydroxyapatite-gelatin scaffolds with osteogenically differentiated progenitor cell aggregates for calvaria bone regeneration. Tissue Eng. Part A 2013, 19, 1803–1816. [Google Scholar] [CrossRef] [Green Version]
- Gautam, R.K.; Kakatkar, A.S.; Karani, M.N. Development of protein-based biodegradable films from fish processing waste. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 878–888. [Google Scholar] [CrossRef]
- Nikpasand, A.; Parvizi, M.R. Evaluation of the Effect of titatnium dioxide nanoparticles/gelatin composite on infected skin wound healing; An animal model study. Bull. Emerg. Trauma 2019, 7, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Azizi-Lalabadi, M.; Alizadeh-Sani, M.; Divband, B.; Ehsani, A.; Julian, D. Nanocomposite films consisting of functional nanoparticles (TiO2 and ZnO) embedded in 4A-Zeolite and mixed polymer matrices (gelatin and polyvinyl alcohol). Food Res. Int. 2020, 137, 109716. [Google Scholar] [CrossRef] [PubMed]
- Azizi-Lalabadi, M.; Ehsani, A.; Ghanbarzadeh, B.; Divband, B. Polyvinyl alcohol/gelatin nanocomposite containing ZnO, TiO2 or ZnO/TiO2 nanoparticles doped on 4A-zeolite: Microbial and sensory qualities of packaged white shrimp during refrigeration. Int. J. Food Microbiol. 2020, 312, 108375. [Google Scholar] [CrossRef]
- Nassiri, R.; Mohammady Nafchi, A. Antimicrobial and barrier properties of bovine gelatin films reinforced by nano TiO2. J. Chem. Health Risks 2013, 3, 21–28. [Google Scholar]
- Pirsa, S.; Farshchi, E.; Roufegarinejad, L. Antioxidant/antimicrobial film based on carboxymethyl cellulose/gelatin/TiO2–Ag nanocomposite. J. Polym. Environ. 2020. [Google Scholar] [CrossRef]
- Vejdan, A.; Ojagh, S.M.; Abdollahi, M. Effect of gelatin/agar bilayer film incorporated with TiO2 nanoparticles as a UV absorbent on fish oil photooxidation. Int. J. Food Sci. Technol. 2017, 52, 1862–1868. [Google Scholar] [CrossRef]
- Urruela-Barrios, R.; Ramírez-Cedillo, E.; de León, A.D.; Alvarez, A.J.; Ortega-Lara, W. Alginate/gelatin hydrogels reinforced with TiO2 and β-TCP fabricated by microextrusion-based printing for tissue regeneration. Polymers 2019, 11, 457. [Google Scholar] [CrossRef] [Green Version]
- Lai, M.; Jin, Z.; Qiao, W. Surface immobilization of gelatin onto TiO2 nanotubes to modulate osteoblast behavior. Coll. Surf. B 2017, 159, 743–749. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, X.; Mao, H.; Huang, Y.; Ding, Q.; Pang, X. Hydroxyapatite/gelatin functionalized graphene oxide composite coatings deposited on TiO2 nanotube by electrochemical deposition for biomedical applications. Appl. Surf. Sci. 2015, 329, 76–82. [Google Scholar] [CrossRef]
- Hosokawa, A.; Kato, Y.; Terada, K. Imprinting on empty hard gelatin capsule shells containing titanium dioxide by application of the UV laser printing technique. Drug Dev. Ind. Pharm. 2014, 9045, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Hayajneh, M.T.; Almomani, M.; Al-daraghmeh, M. Enhancement the corrosion resistance of AISI-304 stainless steel by nanocomposite gelatin-titanium dioxide coatings. Manuf. Technol. 2019, 19, 759–766. [Google Scholar] [CrossRef]
- Liu, B.; Xiao, J.; Xu, L.; Yao, Y.; Costa, B.F.O.; Domingos, V.F.; Ribeiro, E.S.; Shi, F.N.; Zhou, K.; Su, J.; et al. Gelatin-assisted sol-gel derived TiO2 microspheres for hydrogen storage. Int. J. Hydrog. Energy 2015, 40, 4945–4950. [Google Scholar] [CrossRef]
- Jao, D.; Xue, Y.; Medina, J.; Hu, X. Protein-based drug-delivery materials. Materials 2017, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Sani, M.; Khezerlou, A.; Ehsani, A. Fabrication and characterization of the bionanocomposite film based on whey protein biopolymer loaded with TiO2 nanoparticles, cellulose nanofibers and rosemary essential oil. Ind. Crops Prod. 2018, 124, 300–315. [Google Scholar] [CrossRef]
- Zhou, J.J.; Wang, S.Y.; Gunasekaran, S. Preparation and characterization of whey protein film incorporated with TiO2 nanoparticles. J. Food Sci. 2009, 74. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Mohammadian, E.; Julian, D. Eco-friendly active packaging consisting of nanostructured biopolymer matrix reinforced with TiO2 and essential oil: Application for preservation of refrigerated meat. Food Chem. 2020, 322, 126782. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.; Chen, Y.; Xia, W.; Xiong, Y.L.; Wang, H. Enhanced physicochemical properties of chitosan/whey protein isolate composite film by sodium laurate-modified TiO2 nanoparticles. Carbohydr. Polym. 2016, 138, 59–65. [Google Scholar] [CrossRef]
- Zolfi, M.; Khodaiyan, F.; Mousavi, M.; Hashemi, M. Development and characterization of the kefiran-whey protein isolate-TiO2 nanocomposite films. Int. J. Biol. Macromol. 2014, 65, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Gohargani, M.; Lashkari, H.; Shirazinejad, A. Study on biodegradable chitosan-whey protein-based film containing bionanocomposite TiO2 and Zataria multiflora essential oil. J. Food Qual. 2020, 8844167. [Google Scholar] [CrossRef]
- Ortelli, S.; Malucelli, G.; Cuttica, F.; Blosi, M.; Zanoni, I.; Luisa, A. Coatings made of proteins adsorbed on TiO2 nanoparticles: A new flame retardant approach for cotton fabrics. Cellulose 2018, 25, 2739–2749. [Google Scholar] [CrossRef]
- Córdoba, L.C.; Hélary, C.; Montemor, F.; Coradin, T. Bi-layered silane–TiO2/collagen coating to control biodegradation and biointegration of Mg alloys. Mater. Sci. Eng. C 2019, 94, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Truc, N.T.; Minh, H.H.; Khanh, L.L.; Thuy, V.M.; Van Toi, V.; Van Man, T.; Nam, H.C.N.; Quyen, T.N.; Hiep, N.T. Modification of type I collagen on TiO2 surface using electrochemical deposition. Surf. Coat. Technol. 2018, 344, 664–672. [Google Scholar] [CrossRef]
- Nojiri, T.; Chen, C.Y.; Kim, D.M.; Da Silva, J.; Lee, C.; Maeno, M.; Mcclelland, A.A.; Tse, B.; Nagai, S.I.; Hatakeyama, W.; et al. Establishment of perpendicular protrusion of type I collagen on TiO2 nanotube surface as a priming site of peri-implant connective fibers. J. Nanobiotechnol. 2019, 17, 34. [Google Scholar] [CrossRef] [Green Version]
- Bishal, A.K.; Sukotjo, C.; Jokisaari, J.R.; Klie, R.F.; Takoudis, C.G. Enhanced bioactivity of collagen fiber functionalized with room temperature atomic layer deposited titania. ACS Appl. Mater. Interfaces 2018, 10, 34443–34454. [Google Scholar] [CrossRef]
- Vedhanayagam, M.; Anandasadagopan, S.; Nair, B.U.; Sreeram, K.J. Polymethyl methacrylate (PMMA) grafted collagen scaffold reinforced by PdO–TiO2 nanocomposites. Mater. Sci. Eng. C 2019, 110378. [Google Scholar] [CrossRef]
- Erciyes, A.; Ocak, B. Physico-mechanical, thermal, and ultraviolet light barrier properties of collagen hydrolysate films from leather solid wastes incorporated with nano TiO2. Polym. Compos. 2019, 40, 4716–4725. [Google Scholar] [CrossRef]
- Luo, T.; Wan, X.J.; Jiang, S.X.; Zhang, L.Y.; Hong, Z.Q.; Liu, J. Preparation and photocatalytic performance of fibrous Tb3+-doped TiO2 using collagen fiber as template. Appl. Phys. A Mater. Sci. Process. 2018, 124, 304. [Google Scholar] [CrossRef]
- Chen, H.; Liu, H.; Guo, Y.; Wang, B.; Wei, Y.; Zhang, Y.; Wu, H. Hierarchically ordered mesoporous TiO2 nanofiber bundles derived from natural collagen fibers for lithium and sodium storage. J. Alloys Compd. 2017, 731, 844–852. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.; Li, R.; Zhang, H.; Cao, W.; Li, T.; Zhang, Y. Preparation and characterization of soy protein isolate films incorporating modified nano-TiO2. Int. J. Food Eng. 2019, 15, 1–13. [Google Scholar] [CrossRef]
- Malathi, A.N.; Kumar, N.; Nidoni, U.; Hiregoudar, S. Development of soy protein isolate films reinforced with titanium dioxide nanoparticles. Int. J. Agric. Environ. Biotechnol. 2017, 10, 141. [Google Scholar] [CrossRef]
- Burgos, N.; Valdés, A.; Jiménez, A. Valorization of agricultural wastes for the production of protein-based biopolymers. J. Renew. Mater. 2016, 4, 165–177. [Google Scholar] [CrossRef] [Green Version]
- Calva-Estrada, S.J.; Jiménez-Fernández, M.; Lugo-Cervantes, E. Protein-based films: Advances in the development of biomaterials applicable to food packaging. Food Eng. Rev. 2019, 11, 78–92. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, S.; Lin, B.; Wang, W.; Wang, S. Studies on fresh-keeping strawberry using TiCVSPI composite film. J. Chin. Inst. Food Sci. Technol. 2015, 15, 120–125. [Google Scholar] [CrossRef]
- Hoseiniyan, F.; Amiri, S.; Rezazadeh Bari, M.; Rezazad Bari, L.; Dodangeh, S. Effect of soy protein isolate and TiO2 edible coating on quality and shelf-life of grapes varieties Hosseini and Ghezel Ozom. FSCT 2020, 17, 29–41. [Google Scholar]
- Vasconcellos, F.C.S.; Woiciechowski, A.L.; Soccol, V.T.; Mantovani, D.; Soccol, C.R. Antimicrobial and antioxidant properties of β-conglycinin and glycinin from soy protein isolate. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 144–157. [Google Scholar]
- Calza, P.; Zacchigna, D.; Laurenti, E. Degradation of orange dyes and carbamazepine by soybean peroxidase immobilized on silica monoliths and titanium dioxide. Environ. Sci. Pollut. Res. 2016, 23, 23742–23749. [Google Scholar] [CrossRef]
- Amjadi, S.; Almasi, H.; Ghorbani, M.; Ramazani, S. Preparation and characterization of TiO2NPs and betanin loaded zein/sodium alginate nanofibers. Food Packag. Shelf Life 2020, 24, 100504. [Google Scholar] [CrossRef]
- Wu, S.; Chen, X.; Yi, M.; Ge, J.; Yin, G.; Li, X. Improving thermal, mechanical, and barrier properties of feather keratin/polyvinyl alcohol/Tris (hydroxymethyl) aminomethane nanocomposite films by incorporating sodium montmorillonite and TiO2. Nanomaterials 2019, 9, 298. [Google Scholar] [CrossRef] [Green Version]
- Villanueva, M.E.; Puca, M.; Pérez Bravo, J.; Bafico, J.; Campo Dall Orto, V.; Copello, G.J. Dual adsorbent-photocatalytic keratin-TiO2 nanocomposite for trimethoprim removal from wastewater. New J. Chem. 2020, 44, 10964–10972. [Google Scholar] [CrossRef]
- Siriorn, I.N.A.; Jatuphorn, W. Investigation of morphology and photocatalytic activities of electrospun chicken feather keratin/PLA/TiO2/clay nanofibers. E3S Web Conf. 2020, 141, 01003. [Google Scholar] [CrossRef]
- Babitha, S.; Korrapati, P.S. TiO2 immobilized zein microspheres: A biocompatible adsorbent for effective dye decolourisation. RSC Adv. 2015, 5, 26475–26481. [Google Scholar] [CrossRef]
- Johari, N.; Hosseini, H.R.M.; Samadikuchaksaraei, A. Novel fluoridated silk fibroin/TiO2 nanocomposite scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2017, 82, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Mehrabani, M.G.; Karimian, R.; Rakhshaei, R.; Pakdel, F.; Eslami, H.; Fakhrzadeh, V.; Rahimi, M.; Salehi, R.; Kafil, H.S. Chitin/silk fibroin/TiO2 bio-nanocomposite as a biocompatible wound dressing bandage with strong antimicrobial activity. Int. J. Biol. Macromol. 2018, 116, 966–976. [Google Scholar] [CrossRef]
- Kazek-Kesik, A.; Peitryga, K.; Basiaga, M.; Blacha-Grzechnik, A.; Dercz, G.; Kalemba-Rec, I.; Pamula, E.; Simka, W. Lactoferrin and collagen type I as components of composite formed. Surf. Coat. Technol. 2017, 328, 1–12. [Google Scholar] [CrossRef]
- Feng, X.; Guo, Y.; Chen, J.; Zhang, J. Nano-TiO2 induced secondary structural transition of silk fibroin studied by two-dimensional Fourier-transform infrared correlation spectroscopy and Raman spectroscopy. J. Biomater. Sci. 2012, 18, 1443–1456. [Google Scholar] [CrossRef]
- Hu, M.; Lin, D.; Shang, Y.; Hu, Y.; Lu, W.; Huang, X.; Ning, K.; Chen, Y.; Wang, Y. CO2-induced pH reduction increases physiological toxicity of nano-TiO2 in the mussel Mytilus coruscus. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Han, S.; Zheng, P.; Zhou, D.; Zhou, S.; Jia, G. Effect of oral exposure to titanium dioxide nanoparticles on lipid metabolism in Sprague-Dawley rats. Nanoscale 2020, 12, 5973–5986. [Google Scholar] [CrossRef]
- Runa, S.; Khanal, D.; Kemp, M.L.; Payne, C.K. TiO2 nanoparticles alter the expression of peroxiredoxin antioxidant genes. J. Phys. Chem. C 2016, 120, 20736–20742. [Google Scholar] [CrossRef]
- Tucci, P.; Porta, G.; Agostini, M.; Dinsdale, D.; Iavicoli, I.; Cain, K.; Finazzi-Agró, A.; Melino, G.; Willis, A. Metabolic effects of TiO2 nanoparticles, a common component of sunscreens and cosmetics, on human keratinocytes. Cell Death Dis. 2013, 4, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gheshlaghi, Z.N.; Riazi, G.H.; Ahmadian, S.; Ghafari, M.; Mahinpour, R. Toxicity and interaction of titanium dioxide nanoparticles with microtubule protein. Acta Biochim. Biophys. Sin. 2008, 40, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Grassian, V.H. Bovine serum albumin adsorption on TiO2 nanoparticle surfaces: Effects of pH and co-adsorption of phosphate on protein-surface interactions and protein structure. J. Phys. Chem. C 2017, 121, 21763–21771. [Google Scholar] [CrossRef]
- Sharif, H.A.; Rasha, A.A.E.; Ramia, Z.A.B. Titanium dioxide content in foodstuffs from the Jordanian market: Spectrophotometric evaluation of TiO2 nanoparticles. Int. Food Res. J. 2015, 22, 1024–1029. [Google Scholar]
- Wang, Y.Q.; Zhang, H.M.; Wang, R.H. Investigation of the interaction between colloidal TiO2 and bovine hemoglobin using spectral methods. Colloids Surfaces B Biointerfaces. 2008, 65, 190–196. [Google Scholar] [CrossRef] [PubMed]
| Protein Source | Application | Ref. |
|---|---|---|
| Yellow pea protein isolate | Food and non-food packaging | [36] |
| Whey protein | Food and non-food packaging | [37] |
| Corn zein | Food and non-food packaging | [38] |
| Soy protein isolate | Food and non-food packaging | [39] |
| Rice bran | Food and non-food packaging | [40] |
| Wheat gluten | Food and non-food packaging | [41] |
| Gelatin | Food and non-food packaging | [42] |
| Gelatin | Biomedical | [43] |
| Keratin | Biomedical | [44] |
| Protein Source | Functional Agent | Application | Ref. |
|---|---|---|---|
| Gelatin | Silver-NPs | Active food packaging. | [46] |
| Gelatin | Resorcinol and silver-NPs | Active food packaging. | [47] |
| Caseinate/gelatin | Tannins | Active food packaging. | [48] |
| Sodium caseinate | ZnO-NPs and REO | Active food packaging. | [49] |
| Whey protein | Montmorillonite and citric acid | Active food packaging. | [50] |
| Whey protein | Organic acids and nisin | Active food packaging. | [51] |
| Furcellaran/whey protein | Yerba mate extracts | Active food packaging. | [52] |
| Yellow pea protein isolate | Whey protein isolate | Active food packaging. | [36] |
| Fish protein isolate | Gelatin and ZnO-NPs | Active food packaging. | [53] |
| Soy protein hydrolysate | Silica | Environmental remediation. | [54] |
| Soy protein isolate | Tragacanth, silica, and lycopene | Environmental remediation. | [55] |
| Silk fibroin | Ag NPs | Biomedical. | [56] |
| Egg white protein | Silk fibroin | Biomedical. | [57] |
| Immunological Protein | Abbreviation | Binding Energy | Intermolecular Energy |
|---|---|---|---|
| Intercellular adhesion molecule 1 | ICAM−I | −11.63 | −12.73 |
| Mitogen−activated protein kinases | P−38 | −11.73 | −12.83 |
| The nuclear factor−kB | NF−kB | −8.29 | −9.39 |
| Cyclooxygenase 2 | COX−2 | NR | NR |
| Interleukin 8 | IL−8 | −4.04 | −5.14 |
| Placental growth factor | PIGF | −9.36 | −10.36 |
| C–X–C motif chemokine ligand 1 | CXCL−I | 1.67 | 0.57 |
| C–X–C motif chemokine ligand 3 | CXCL−3 | NR | NR |
| C–X–C motif chemokine ligand 5 | CXCL−5 | 576.34 | 575.34 |
| C–X–C motif chemokine ligand 20 | CCL−20 | −8.25 | −9.34 |
| The cluster of differentiation 35 | CD 35 | 5420 | 5420 |
| The cluster of differentiation 66b | CD 66b | NR | NR |
| Matrix metallopeptidase 9 | MMP−9 | −9.01 | −10.11 |
| Application | Method/Presentation | * Composition | TiO2 Specifications | Relevant Results | Ref. |
|---|---|---|---|---|---|
| Food and non-food packaging | Evaporative casting/Film | Gelatin (4 g 100 mL−1), glycerol (30% w/w) | Commercial SM: Hydrothermal (TiO2): 0.5% w/w Size: 25 nm CP: Anatase | TiO2 enhanced the physicochemical and antimicrobial properties of gelatin film. | [1] |
| Food and non-food packaging | Evaporative casting/Film | Fish gelatin (2.3% w/v), chitosan (1% w/v), glycerol (1% w/v) | (TiO2:Ag): 0.4% w/w | Hybrid films showed improved physicochemical and antimicrobial properties. | [3] |
| Food and non-food packaging | Evaporative casting/Film | Gelatin (4 g 100 mL−1), glycerol (25% w/w) | Commercial (TiO2): 1% w/w Size: <10 nm CP: Anatase | TiO2 improved UV-barrier, thermal, mechanical, and water-related properties of gelatin film. | [12] |
| Food and non-food packaging | Evaporative casting/Film | CMC (1 g 100 mL−1), gelatin (1 g 100 mL−1) | Commercial (TiO2:Ag): 0.4% w/w Size: 20 nm | TiO2 improved the technological and photocatalytic properties of gelatin film. | [18] |
| Food and non-food packaging | Evaporative casting/Film | Gelatin (4 g 100 mL−1), glycerol (15% w/w) | SM: Sol–gel (TiO2:Ag): 1% w/w Size: 10–20 nm CP: Anatase Body-centered tetragonal crystal structure | TiO2 improved the technological properties of the gelatin film. | [20] |
| Food and non-food packaging | Evaporative casting/Film | Agar (1.5 g 100 mL−1), gelatin (4 g 100 mL−1), glycerol (35% w/v) | Commercial (TiO2): 0.5% w/w Size: 10–20 nm CP: Anatase-Rutile | The hybrid film showed a marked UV-protective effect and improved water resistance. | [21] |
| Food and non-food packaging | Evaporative casting/Film | Gelatin (15 mg·mL−1) | (TiO2): 0.5% w/w Size: 12.2 nm CP: Anatase Crystal structure | The film exhibits antibacterial activity. | [26] |
| Food and non-food packaging | Evaporative casting/Film | Gelatin (3 g 80 mL−1), PVA (3 g 80 mL−1), glycerol (30% w/w) | Commercial (TiO2:4A zeolite): 1% w/w | Functionalization improved the physicochemical and antimicrobial properties of the gelatin–PVA film. | [74] |
| Food and non-food packaging | Evaporative casting/Coating | Gelatin (3 g 80 mL−1), PVA (3 g 80 mL−1), glycerol (30% w/w) | Commercial (TiO2:4A zeolite): 1% w/w | The hybrid film effectively extended the shelf life of white shrimp. | [75] |
| Food and non-food packaging | Evaporative casting/Film | Gelatin (8% w/w), sorbitol: glycerol ratio 3:1 (40% w/w) | Commercial (TiO2): 1% w/w Size: 10–15 nm CP: Anatase-Rutile | The hybrid film showed antimicrobial properties. | [76] |
| Food and non-food packaging | Evaporative casting/Film | Gelatin (1 g 100 mL−1), CMC (1 g 100 mL−1) | Commercial (TiO2:Ag): 0.4% w/w Size: 21 nm CP: Anatase | Hybrid films showed improved physicochemical and antimicrobial properties. | [77] |
| Food and non-food packaging | Evaporative casting/Film | Gelatin (4% w/w), agar (1.5% w/v), glycerol (35% w/w) | Commercial (TiO2): 2% w/w | Gelatin–TiO2 effectively delayed fish oil oxidation. | [78] |
| Biomedical | Freeze-drying/Hydrogel | Sodium alginate (2% w/v), gelatin (0.5% w/v), β-tP (1% w/v) | SM: Electrochemical anodization (TiO2): 0.1% w/v Size: 110 nm CP: Anatase Nanotubes | Hybrid hydrogel had adequate porosity and mechanical resistance. | [79] |
| Biomedical | NI/Scaffold | NI | SM: Biometic (TiO2): NI Size: 30–35 nm CP: Anatase | Hybrid scaffold promoted osteointegration and enhanced bone regeneration. | [71] |
| Biomedical | NI/NI | Gelatin (2 mg·mL−1) | Electrochemical anodization (TiO2): NI Size: 100 nm CP: Rutile Nanotubes (20 nm × 350 nm) Low crystal structure | Hybrid material could potentially be used for orthopedic and dental applications. | [80] |
| Biomedical | Electrochemical deposition/coating | Hap (NI), Gelatin (100 mg 100 mL−1), GO (2 mg mL−1) | Hydrothermal (TiO2): NI Crystal structure | The hybrid coating showed excellent biocompatibility with MC3T3-E1 cells. | [81] |
| Biomedical | Freeze-drying/Hydrogel | Gelatin (2 g 100 mL−1) | (TiO2): 0.5% w/v | The hybrid composite had better wound-healing properties than gelatin film. | [73] |
| Biomedical | Polymer blend/Biosensor | CMC:Gelatin (3.75 mg), solution of superoxide dismutase (4733 U, 1 mg), glutaraldehyde (0.005 M) | SM: Hydrothermal (TiO2): 0.1% w/w Size: 50 nm CP: Anatase | The biosensor exhibited high analytical performance, high sensitivity, and fast response time for superoxide radical detection. | [70] |
| Pharmaceutical | NI/Capsule | Gelatin (NI) | Commercial (TiO2): 3.5% w/w Size: 177.2 nm CP: Anatase Crystalline structure | The capsules could be printed gray by UV-laser. | [82] |
| Metal corrosion resistance | NI/Coating | Gelatin (8 wt.% in 20 wt.% acetic acid) | Commercial (TiO2): 3% w/w Size: 10–25 nm CP: Anatase Purity: >99% Density: 3.9 g·cm−3 | Gelatin–TiO2 composite improved the corrosion resistance of steel material. | [83] |
| Hydrogen production | NI/microspheres | Gelatin (5 g 100 mL−1) | SM: Sol–gel Titania precursor (10 mL of tetra-n-butyl titanate in 50 mL of ethyl alcohol) Size: 50–100 nm CP: Anatase High crystallinity and purity | Gelatin improved the adsorptive properties of TiO2. | [84] |
| Application | Method/Presentation | * Composition | TiO2 Specifications | Relevant Results | Ref. |
|---|---|---|---|---|---|
| Food and non-food packaging | Evaporative casting/Film | WPI (10% w/w) | Commercial (TiO2): 0.25% w/w Size: 50–100 nm CP: Anatase Purity: >98.5% | TiO2 improved the physicochemical properties of whey protein film. | [17] |
| Food and non-food packaging | Evaporative casting/Film | WPI (10% w/v), cellulose (1% w/v), glycerol (6% w/v), REO (2% w/v) | Commercial (TiO2): 1% w/v Size: 10–25 nm CP: Anatase | Coated meat exhibited microbial stability during cold storage. | [28] |
| Food and non-food packaging | Evaporative casting/Film | WPI nanofibers (5% w/v), glycerol (4% w/v), | Commercial (TiO2): 1% w/w Size: 20 nm Nanotubes Purity: >99% | The hybrid film effectively extends the shelf life of chilled meat. | [29] |
| Food and non-food packaging | Evaporative casting/Film | WPI (5% w/v), kefiran (5% w/v), glycerol (35% w/w) | Commercial (MMT-TiO2): 1% w/w CP: Anatase | TiO2 improved the physicochemical properties of kefiran–whey protein film. | [31] |
| Food and non-food packaging | Evaporative casting/Film | WPI (10% w/v), cellulose (1% w/v), glycerol (6% w/v), REO (2% w/v) | Commercial (TiO2): 1% w/v Size: 10–25 nm CP: Anatase Purity: >99% | The hybrid film exhibited antimicrobial and antioxidant properties. | [86] |
| Food and non-food packaging | Evaporative casting/Film | WPI (5% w/v), TiO2 (1% w/w), glycerol (5% w/v) | Commercial (TiO2): 1% w/w Size: <20 nm CP: Anatase | TiO2 improved the physicochemical properties of whey protein film. | [87] |
| Food and non-food packaging | Evaporative casting/Film | WPI (10% w/v), cellulose (1% w/v), glycerol (6% w/v), REO (2% w/v) | Commercial (TiO2): 1% w/v Size: 10–25 nm CP: Anatase Purity: >99% | Meat treated with the hybrid film showed reduced lipid peroxidation during cold storage. | [88] |
| Food and non-food packaging | Evaporative casting/Film | Chitosan (1.5 g 50 mL−1 of acetic acid), WPI (0.5 g 50 mL−1 of water) | Commercial (TiO2): 0.01 g CP: Anatase Crystalline structure | The hybrid film exhibited improved physicochemical properties. | [89] |
| Food and non-food packaging | Evaporative casting/Film | WPI (5% w/v), kefiran (5% w/v), glycerol (35% w/w) | Commercial (MMT-TiO2): 1% w/w Size: 20 nm CP: Anatase | The hybrid film exhibited improved physicochemical properties. | [90] |
| Food and non-food packaging | Evaporative casting/Film | WPI (3% w/v), chitosan (10 g/L), ZMEO (1% v/v), glycerol (30% w/w) | Commercial (TiO2): 2% w/w CP: Anatase-Rutile | The hybrid film exhibited antimicrobial activity. | [91] |
| Textile | Dip-pad-dry-cure process/Coating | WPI (3% w/v), cotton fabrics (200 g/m2) | Commercial (TiO2): 6% w/w | The hybrid coating exhibited improved antimicrobial activity. | [92] |
| Application | Method/Presentation | * Composition | TiO2 Specifications | Relevant Results | Ref. |
|---|---|---|---|---|---|
| Biomedical | Dip coating/Composite | Collagen-MWCNTs composite coated Ti incorporated with 20 µg/cm2 of MWCNTs | Commercial (TiO2): NI | The high roughness of hybrid materials improved cell proliferation. | [5] |
| Biomedical | Dip coating/NI | Volume ratio 1:1.5 GPTMS-TiO2 solutions into a Collagen solution (3 mg·mL−1) to cover the Mg alloys | SM: Sol–gel (TiO2): NI TiO2 with an amorphous structure | Protect alloy from corrosion, promote fibroblast proliferation. | [93] |
| Biomedical | NI/Film | Collagen (0.5 mg·m−1) | SM: Electrochemical deposition (TiO2): NI TiO2 with a crystalline structure | The hybrid film showed rapid cell adhesion and proliferation. | [94] |
| Biomedical | NI/Composite | Collagen (NI) | Commercial SM: Anodization (TiO2): 0.3% w/w Size: 67 nm | Hybrid composite facilitated epithelial cell stretching and sheet formation. | [95] |
| Biomedical | Atomic layer deposition/Membrane | Collagen membrane (25 mm × 15 mm) | Commercial (TiO2): NI | The hybrid membrane exhibited the proliferation of osteoblast. | [96] |
| Biomedical | NI/Composite | Mol ratio 1:1 of PdO–TiO2 incorporated to g-PMMA–Collagen | SM: Sol–gel (TiO2): NI Size: 8 nm CP: Anatase | TiO2 incorporation improved thermal stability, mechanical strength, and enhancement of collagen. | [97] |
| Biomedical | Freeze-drying process/Aerogel | Collagen–PVP–TiO2 1:20:0.5 mass ratio | SM: Sol–gel (TiO2): NI Size: 24.4 nm CP: Anatase–Rutile | PVP improves the thermal stability and coercivity of the nanocomposite scaffold. | [34] |
| Biomedical | Freeze-drying process | Collagen–chitosan–TiO2 1:1:0.1 mass ratio | SM: Sol–gel (TiO2): NI Size: 20–30 nm CP: Anatase | TiO2 improves mechanical properties, resistance to degradation, and antibacterial ability, and wound repair. | [27] |
| Non-food packing | NI/NI | Collagen (4 g 100 mL−1) | SM: Sol–gel (TiO2): 2% w/w Size: 30 nm CP: Anatase | TiO2 increases the thermal stability of collagen film improves and reduces UV light penetration, and solubility. | [98] |
| Environmental remediation | Dip coating/NI | Collagen (template) | (TiO2: Tb3+): 2% w/w Size: 9.6 nm CP: Anatase | Collagen structure was preserved and photocatalytic performance of TiO2 increased. | [99] |
| Electrochemical studies | Chemical reactions/NI | NI | SM: Template (TiO2): NI Size: 10–20 nm CP: Anatase | Hybrid material showed excellent electrochemical lithium and sodium storage properties. | [100] |
| Application | Method/Presentation | * Composition | TiO2 Specifications | Relevant Results | Ref. |
|---|---|---|---|---|---|
| Food packaging | Evaporative casting/Films | Soy protein isolate (5 g 100 mL−1) glycerol (0.4 g) | Commercial (TiO2): 1.5% w/v CP: Anatase | TiO2 improved the physicochemical and antimicrobial properties of the soy protein isolate film. | [101] |
| Food packaging | Evaporative casting/Films | Soy protein isolate (5 g 100 mL−1), sorbitol (20%), glycerol (10%) | Commercial (TiO2): 2% w/w | The hybrid film exhibited improved mechanical properties. | [102] |
| Food packaging | Evaporative casting/ Films | Soy protein isolate (5%), glycerol (2%), | Commercial (TiO2): 0.5% w/w Size: 15–30 nm CP: Anatase | TiO2 improved the physicochemical and antimicrobial properties of the soy protein isolate film. | [19] |
| Food packaging | Evaporative casting/Films | Soy protein isolate (4.5 g 150 mL−1), glycerol (3.75 g 150 mL−1) | (TiO2): 1.33% w/w | Hybrid composite effectively extended the shelf life of strawberries and antimicrobial activity. | [105] |
| Food packaging | Evaporative casting/ Films | Soy protein isolate (NI) | (TiO2): NI | Grapes treated with hybrid films showed higher quality parameters than uncoated fruits. | [106] |
| Food and non-food packaging | Evaporative casting/Films | Soy protein isolate (4.5 g 150 mL−1), glycerol (2%) | Commercial (TiO2): 1.33% w/w TiO2 with crystalline structure | The hybrid composite showed antimicrobial activity. | [23] |
| Application | Method/Presentation | * Composition | TiO2 Specifications | Relevant Results | Ref. |
|---|---|---|---|---|---|
| Food and non-food packaging | Evaporative casting/Film | Zein (13.5% w/v), glycerol:PEG 600 (3.3% w/w) | Commercial SM: Hydrothermal (TiO2:SiO2): 1.5% w/v Size: 100–180 nm | TiO2 improved the mechanical, thermal, and water-related properties of zein film. | [24] |
| Food packaging | Evaporative casting/Nanofibers | Zein (3 g 10 mL−1 of 70% aqueous ethanol) | Commercial (TiO2:SiO2): 5% w/w Size: <25 nm CP: Anatase Purity: 99.7% | Coated fruits extend their shelf life. | [33] |
| Food and non-food packaging | Evaporative casting/Film | Zein: sodium alginate (90:10), betanin (1%) | Commercial (TiO2): 0.5% w/w Size: 10–25 nm | The hybrid film exhibited antimicrobial activity. | [109] |
| Food and non-food packaging | Evaporative casting/Film | Sodium caseinate (8 g 100 mL−1), guar gum (0.3% w/w), CEO (2% w/w) | Commercial (TiO2): 1% w/w Size: 10–25 nm CP: Anatase Purity: >99% | The hybrid film exhibited antimicrobial activity. | [11] |
| Food and non-food packaging | Evaporative casting/Film | Sodium caseinate (2.5% w/w), glycerol (2% w/w) | Commercial (P25) (TiO2): 0.5% w/w | TiO2 improved the mechanical, thermal, and water-related properties of the film. | [30] |
| Food and non-food Packaging | Evaporative casting/Films | Feather keratin (1.2 g), PVA (13.33 g) | Commercial (P25) (TiO2): 3% w/w Size: 60 nm CP: Anatase Purity: 99.8% | The hybrid material exhibited improved physicochemical properties. | [110] |
| Food and non-food Packaging | Catalyst curing/Composite | Raw wool keratin (350 g/m2), BTCA (12.6%) | Commercial (P25) (TiO2): 0.6 g·L−1 Size: 21 nm CP: Anatase-Rutile Crystalline structure | The hybrid material showed an improved UV-protective effect. | [22] |
| Environmental remediation | Evaporative casting/Film | Sesame protein (3 g 100 mL−1), glycerol (30% in total solid content) | Commercial (P25) (TiO2): 3% w/w Size: 21 nm CP: Anatase-Rutile Crystalline structure | The hybrid film exhibited photocatalytic activity against methylene blue. | [16] |
| Environmental remediation | Hydrogel synthesis/Hydrogel | Keratin (1% w/v) | Commercial (P25) (TiO2): 10 w/w CP: Anatase-Rutile | Hybrid hydrogel effectively removes trimethoprim from wastewater. | [111] |
| Environmental remediation | Electrospinning/Nanofibers | Keratin–PLA–TiO2 mass ratio of 33:33:33 | Commercial (P25) CP: Anatase | The hybrid nanofibers effectively remove methylene blue dye from the aqueous solution. | [112] |
| Environmental remediation | Biometic/Microspheres | NI | Anatase | The hybrid composite showed good photocatalytic properties again or dye yellow and blue acid dyes. | [113] |
| Biomedical | Freeze dried/Scaffolds | Silk fibroin (2% w/v), F (2% v/v) | Commercial (P25) (TiO2): 15 w/w | SF–TiO2:F exhibited biocompatibility and improved mechanical properties. | [114] |
| Biomedical | Freeze-dried/Scaffolds | Silk fibroin (2.5% w/v), chitin (2.5% w/v), glutaraldehyde (0.25% v/v) | Commercial (P25) (TiO2): 1.5% w/w Size: 10–15 nm CP: Anatase Purity: >99% | Hybrid material exhibited antimicrobial activity, also it is biocompatible and biodegradable. | [115] |
| Biomedical | Dip-coating/Coating | Lactoferrin (0.2 mg·mL−1), collagen (0.2 mg·mL−1) | SM: Sol-gel (TiO2): NI Size: 200 nm CP: Anatase Crystalline structure | The hybrid coating showed enhanced biocompatibility with MG-6e cells. | [116] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anaya-Esparza, L.M.; Villagrán-de la Mora, Z.; Rodríguez-Barajas, N.; Sandoval-Contreras, T.; Nuño, K.; López-de la Mora, D.A.; Pérez-Larios, A.; Montalvo-González, E. Protein–TiO2: A Functional Hybrid Composite with Diversified Applications. Coatings 2020, 10, 1194. https://doi.org/10.3390/coatings10121194
Anaya-Esparza LM, Villagrán-de la Mora Z, Rodríguez-Barajas N, Sandoval-Contreras T, Nuño K, López-de la Mora DA, Pérez-Larios A, Montalvo-González E. Protein–TiO2: A Functional Hybrid Composite with Diversified Applications. Coatings. 2020; 10(12):1194. https://doi.org/10.3390/coatings10121194
Chicago/Turabian StyleAnaya-Esparza, Luis Miguel, Zuamí Villagrán-de la Mora, Noé Rodríguez-Barajas, Teresa Sandoval-Contreras, Karla Nuño, David A. López-de la Mora, Alejandro Pérez-Larios, and Efigenia Montalvo-González. 2020. "Protein–TiO2: A Functional Hybrid Composite with Diversified Applications" Coatings 10, no. 12: 1194. https://doi.org/10.3390/coatings10121194
APA StyleAnaya-Esparza, L. M., Villagrán-de la Mora, Z., Rodríguez-Barajas, N., Sandoval-Contreras, T., Nuño, K., López-de la Mora, D. A., Pérez-Larios, A., & Montalvo-González, E. (2020). Protein–TiO2: A Functional Hybrid Composite with Diversified Applications. Coatings, 10(12), 1194. https://doi.org/10.3390/coatings10121194











