Aluminum Oxide Ceramic Coatings on 316l Austenitic Steel Obtained by Plasma Electrolysis Oxidation Using a Pulsed Unipolar Power Supply
Abstract
:1. Introduction
- applying a preliminary treatment: the deposition on the steel surface of Al or other valve metal as Ti or Zr layer by a certain method (e.g., a porous oxidation barrier layer necessary to initiate the PEO process can be developed on carbon steel by spraying with an aluminum [28], or by aluminizing using dipping technique [29], and in the case of stainless steel, the specimens can be coated with a Ti film by magnetron sputtering [33], or aluminum/bimetallic stainless steel may be used [34], then it can be obtained the porous oxide film in the anodic oxidation stage of PEO process); the oxidation in autoclave of the stainless steel, which produces a thick layer of magnetite over a substrate [30];
- deposition of the porous oxide (Al2O3, SiO2) resulted from the micro-arc decomposition of aluminate or silicate electrolyte [35], in the case of carbon steel.
2. Materials and Methods
2.1. Preparation of PEO Coatings
2.2. Characterization of PEO Coatings
3. Results
3.1. X-ray Photoelectron Spectroscopy
3.2. X-ray Diffraction
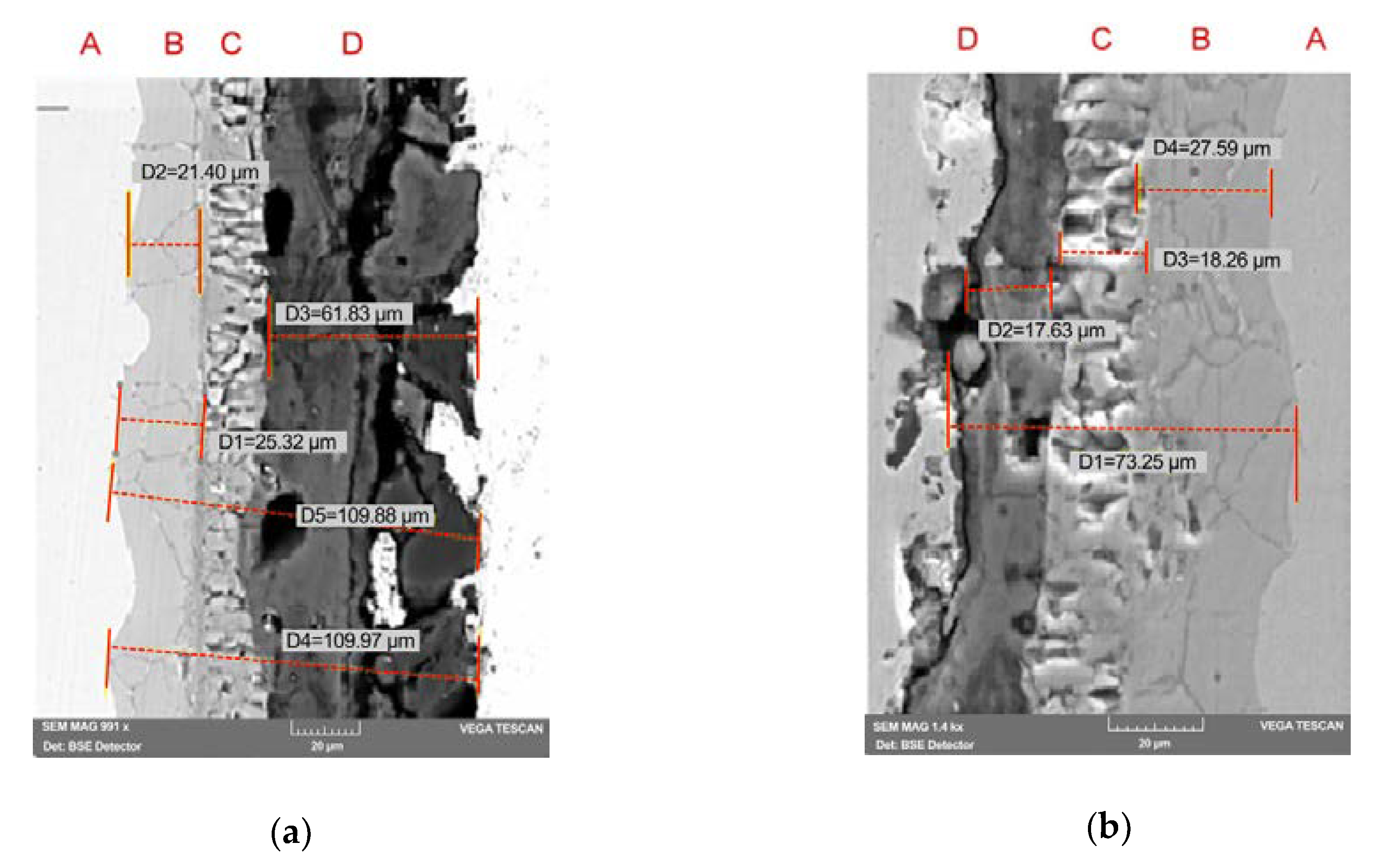
3.3. Morphology and Microstructure of PEO Coatings
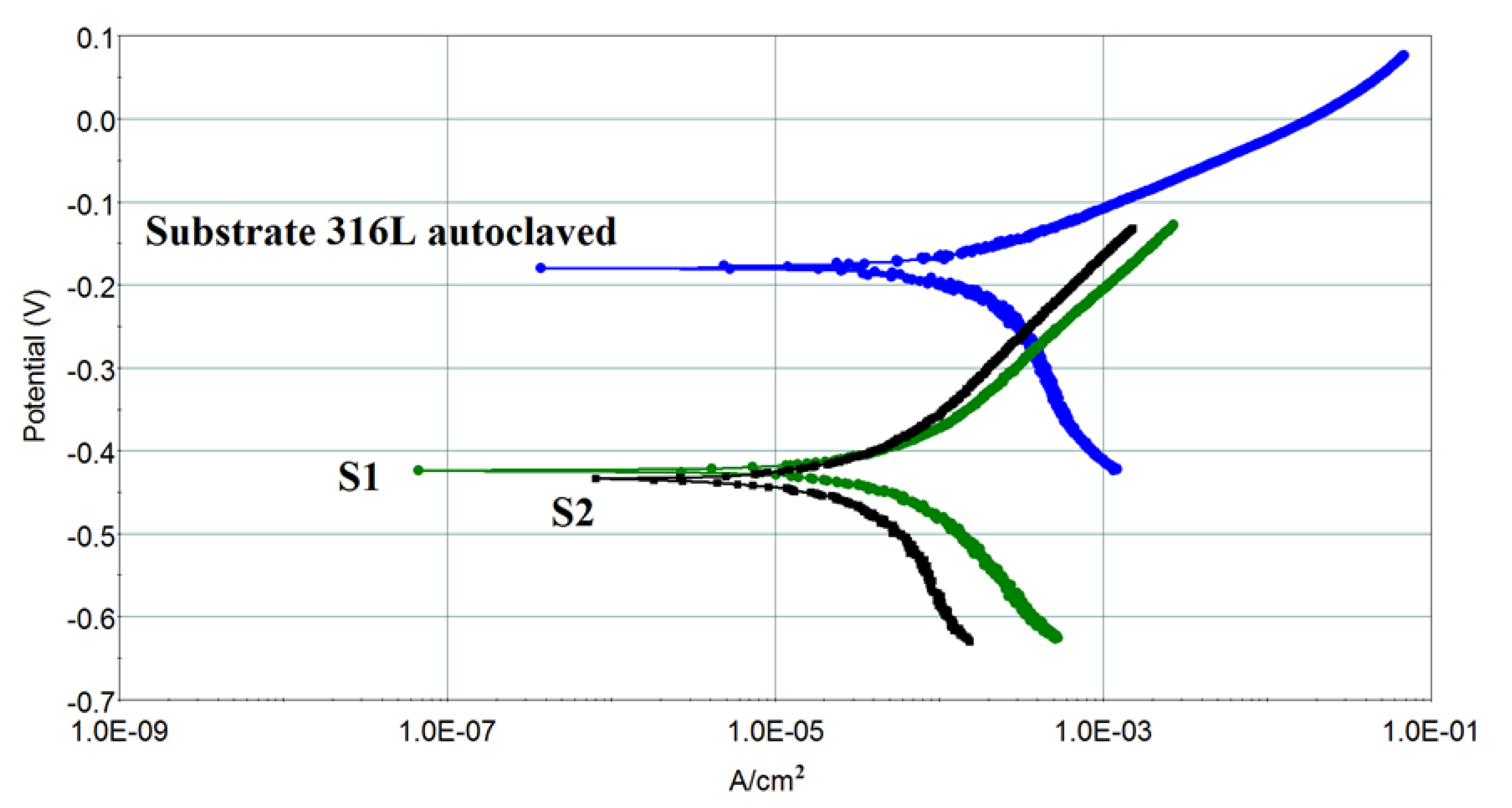
3.4. Corrosion Behavior
4. Discussion
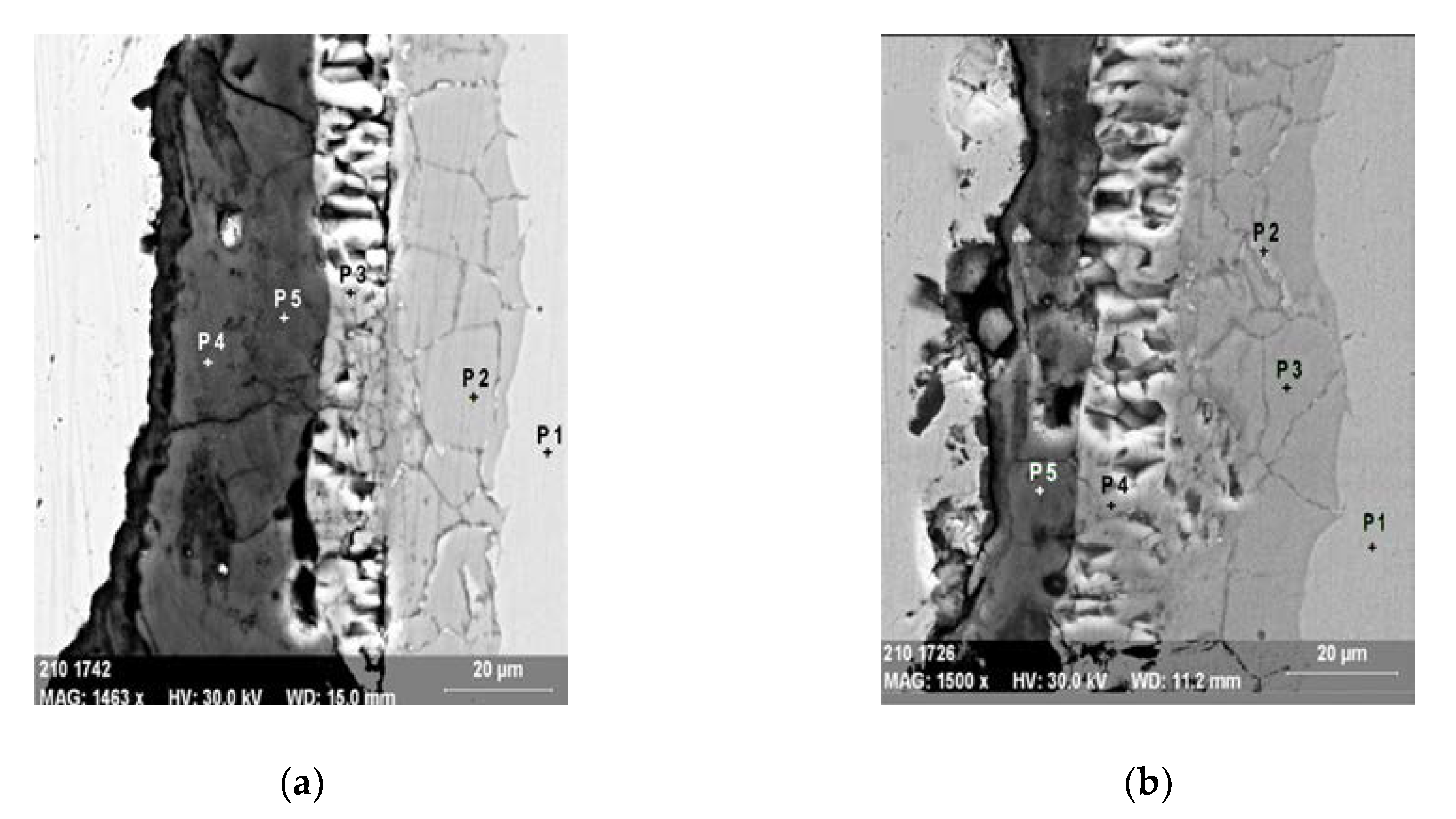
- an inner layer of oxide adjacent to the substrate of a layer, with fine granulation, non-porous, adherent (zone B, Figure 6); EDS analysis at points in the film section show that this oxide layer is rich in Cr (the analysis in point P2, Figure 7a, and points P2, P3, Figure 7b) show atomic concentrations of Cr in the 21- domain. 27% atom).
- the voltage in the direct current PEO mode (320 V in [30,32]) is applied permanently on the sample, while in PEO pulse mode, a time interval of one period is applied, after which it is zero. In our experiment, the pulse voltage applied is over 500 V, much higher than in DC, which allows for higher temperatures in electrical discharge in the microarc and the synthesis of a larger amount of Al2O3;
- between the two impulses, the oxidation layer material cools and crystallizes the molten portions of the layer, which makes it possible to obtain layers of oxide with much higher thickness than in the continuous PEO mode.
- development of easier pre-treatments for achieving the barrier layer necessary to start PEO processes, which will replace the autoclaving stage; we consider cathodic oxidation treatments in electrolysis plasma [44];
- the realization of the PEO treatments on the samples submitted prior to the pretreatment for the achievement of the barrier layer, following: the fulfillment of the conditions for the treatment of some large samples; clogging of the pores of the surface film developed by PEO by electroplating with different nanoparticles, as recommended in [45];
- testing the electrochemical behavior of the obtained structures, under test conditions that simulate the operating conditions (electrochemical autoclaves, circulation loops).
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, W.; Ding, M.-H.; Zhang, H.-S.; Zhang, B. Study on HfCxN1−x coatings deposited on biomedical AISI 316L by radio-frequency magnetron sputtering. J. Alloys Compd. 2018, 7305, 219–227. [Google Scholar] [CrossRef]
- Sabooria, A.; Aversa, A.; Bosio, F.; Bassini, E.; Librera, E.; De Chirico, M.; Biamino, S.; Ugues, D.; Fino, P.; Lombardi, M. An investigation on the effect of powder recycling on the microstructure and mechanical properties of AISI 316L produced by Directed Energy Deposition. Mat. Sci. Eng. A Struct. 2019, 766, 138360. [Google Scholar] [CrossRef]
- Sunil Kumar, P.; Acharyya, S.G. Controlling chloride induced stress corrosion cracking of AISI 316L stainless steel by application of buffing. Mater. Today Proc. 2019, 15, 138–144. [Google Scholar] [CrossRef]
- Weisenburger, A.; Schroer, C.; Jianu, A.; Heinzel, A.; Konys, J.; Steiner, H.; Müller, G.; Fazio, C.; Gessi, A.; Babayan, S.; et al. Long term corrosion on T91 and AISI 316L steel in flowing lead alloy and corrosion protection barrier developement: Experiments and models. J. Nucl. Mater. 2011, 415, 260–269. [Google Scholar] [CrossRef]
- Andrei, V.A.; Malinovschi, V.; Radulescu, C.; Ionita, I.; Torok, G.; Coaca, E.; Marin, A.H.; Bokuchava, G. Applications of plasma electrolytic saturation technique in the field of nuclear materials. J. Sci. Arts 2019, 1, 185–194. [Google Scholar]
- Sanchez-Tovar, R.; Montanes, M.T.; Garcia-Anton, J.; Guenbour, A. Influence of temperature and hydrodynamic conditions on the corrosion behavior of AISI 316L stainless steel in pure and polluted H3PO4: Application of the response surface methodology. Mater. Chem. Phys. 2012, 133, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, S.K.; Mishra, T.; Gunjan, M.K.; Bhattacharyy, A.S.; Singh, T.B.; Singh, R. Development and characterization of sol–gel silica–alumina composite coatings on AISI 316L for implant applications. Surf. Coat. Technol. 2007, 201, 7582–7588. [Google Scholar] [CrossRef]
- Bruschi, S.; Pezzato, L.; Ghiotti, A.; Dabala, M.; Bertolini, R. Effectiveness of using low-temperature coolants in machining to enhance durability of AISI 316L stainless steel for reusable biomedical devices. J. Manuf. Process. 2019, 39, 295–304. [Google Scholar] [CrossRef]
- Moreno Amado, M.; Alfonso, J.E.; Olaya Florez, J.J. Effect of Al and Ag dopants on the corrosion resistance of the AISI 316L-YSZ system. Ceram. Int. 2019, 45, 566–572. [Google Scholar] [CrossRef]
- Menezes, M.R.; Godoy, C.; Buono, V.T.L.; Schvartzman, M.M.M.; Avelar-Batista Wilson, J.C. Effect of shot peening and treatment temperature on wear and corrosion resistance of sequentially plasma treated AISI 316L steel. Surf. Coat. Technol. 2017, 30915, 651–662. [Google Scholar] [CrossRef]
- Aperador, W.; Bautista-Ruiz, J.; Delgado, E. Hot Corrosion Resistance of Al2O3 Coating Produced by Thermal Spray. Int. J. Electrochem. Sci. 2016, 11, 9424–9437. [Google Scholar] [CrossRef]
- Shen, Y.Z.; Guo, X.Z.; Lin, Y.B.; Tao, J. Al2O3 coatings fabricated on stainless steel/ aluminum composites by microarc oxidation. Surf. Eng. 2014, 30, 735–740. [Google Scholar] [CrossRef]
- Fedel, M.; Deflorian, F. Electrochemical characterization of atomic layer deposited Als2O3 coatings on AISI 316L stainless steel. Electrochim. Acta 2016, 203, 404–415. [Google Scholar] [CrossRef]
- Said, S.; Mikhail, S.; Riad, M. Recent processes for the production of alumina nano-particles. Mater. Sci. Energy Technol. 2020, 3, 344–363. [Google Scholar] [CrossRef]
- Akca, M.; Varisli, D. Performance of Co-Fe@Alumina catalysts in comparison to monometallic Co@Alumina and Fe@Alumina catalysts for microwave assisted COx-free hydrogen production. Mol. Catal. 2020, 485, 110823. [Google Scholar] [CrossRef]
- Song, X.; Zhang, K.; Song, Y.; Duan, Z.; Liu, Q.; Liu, Y. Morphology, microstructure and mechanical properties of electrospun alumina nanofibers prepared using different polymer templates: A comparative study. J. Alloys Compd. 2020, 829, 154502. [Google Scholar] [CrossRef]
- Abdo, S.; Saidani-Scott, H.; Benedi, J.; Tierney, M.J. Experimental study with analysis for a novel saturated activated alumina photovoltaic thermal system. Energy 2020, 197, 117210. [Google Scholar] [CrossRef]
- Kuang, W.; Wu, X.; Han, E.H. The oxidation behavior of 304 stainless steel in oxigenated high temperature water. Corros. Sci. 2010, 52, 4081–4087. [Google Scholar] [CrossRef]
- Shakhova, I.; Mironov, E.; Azarmi, F.; Safonov, A. Thermo-electrical properties of the alumina coatings deposited by different thermal spraying technologies. Ceram. Int. 2017, 43, 15392–15401. [Google Scholar] [CrossRef]
- Murray, J.W.; Rance, G.A.; Xu, F.; Hussain, T. Alumina-graphene nanocomposite coatings fabricated by suspension high velocity oxy-fuel thermal spraying for ultra-low-wear. J. Eur. Ceram. Soc. 2018, 38, 1819–1828. [Google Scholar] [CrossRef]
- Psyllaki, P.P.; Jeandin, M.; Pantelis, D.I. Microstructure and wear mechanisms of thermal-sprayed alumina coatings. Mater. Lett. 2001, 47, 77–82. [Google Scholar] [CrossRef]
- Sathish Sharma, G.; Sugavaneswaran, M.; Vijayalakshmi, U.; Prakash, R. Influence of γ-alumina coating on surface properties of direct metal laser sintered 316L stainless steel. Ceram. Int. 2019, 45, 13456–13463. [Google Scholar] [CrossRef]
- Arias, F.J.; Parks, G.T. An estimate of the order of magnitude of the explosion during a core meltdown-compaction accident for heavy liquid metal fast reactors: A disquieting result updating the Bethe-Tait model. Prog. Nucl. Energy 2015, 79, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Odaira, N.; Saito, S. Characterization of mechanical strain induced by lead-bismuth eutectic (LBE) freezing in stainless steel cup. Heliyon 2020, 6, e03429. [Google Scholar] [CrossRef] [PubMed]
- Engelko, V.; Mueller, G.; Rusanov, A.; Markov, V.; Tkachenko, K.; Weisenburger, A.; Kashtanov, A.; Chikiryaka, A.; Jianu, A. Surface modification/ alloying using intense pulsed electron beam as a tool for improving the corrosion resistance of steels exposed to heavy liquid metals. J. Nucl. Mater. 2011, 415, 270–275. [Google Scholar] [CrossRef]
- Bassini, S.; Antonelli, A.; Di Piazza, I.; Tarantino, M. Oxygen sensors for Heavy Liquid Metal coolants: Calibration and assessment of the minimum reading temperature. J. Nucl. Mater. 2017, 4861, 197–205. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Gu, W.; Shen, D.; Wang, Y.; Chen, G.; Feng, W.; Zhang, G.; Fan, S.; Liu, C.; Yang, S. Deposition of duplex Al2O3/aluminum coatings on steel using a combined technique of arc spraying and plasma electrolytic oxidation. Appl. Surf. Sci. 2006, 252, 2927–2932. [Google Scholar] [CrossRef]
- Wu, Z.; Xia, Y.; Li, G.; Xu, F. Structure and mechanical properties of ceramic coatings fabricated by plasma electrolytic oxidation on aluminized steel. Appl. Surf. Sci. 2007, 253, 8398–8403. [Google Scholar] [CrossRef]
- Huan, Z.; Fratila-Apachitei, L.E.; Apachitei, I.; Duszczyk, J. Characterization of Porous TiO2 Surfaces Formed on 316L Stainless Steel by Plasma Electrolytic Oxidation for Stent Applications. J. Funct. Biomater. 2012, 3, 349–360. [Google Scholar] [CrossRef] [Green Version]
- Malinovschi, V.; Marin, A.; Andrei, V.; Coaca, E.; Mihailescu, C.N.; Lungu, C.P.; Radulescu, C.; Dulama, I.D. Obtaining and characterization of PEO layers prepared on CP-Ti in sodium dihydrogen phosphate dihydrate acidic electrolyte solution. Surf. Coat. Technol. 2019, 375, 621–636. [Google Scholar] [CrossRef]
- Andrei, V.A.; Coaca, E.; Mihalache, M.; Malinovschi, V.; Patrascu-Minca, M. Study of ceramic-like aluminum oxide thin films developed using Plasma Electrolytic Oxidation applied on austenitic steels. Surf. Interface Anal. 2016, 48, 654–659. [Google Scholar] [CrossRef]
- Tian, S.; Sun, K.; Cui, H.; Xie, X.; Wang, X.; Wei, N.; Wang, H.; Wang, W.; Song, X.; Yang, K. Structural evolution and electrochemical corrosion behavior of Al–Ti–O amorphous-nanocrystalline composite films deposited by magnetron sputtering. Thin Solid Films 2019, 69231, 137640. [Google Scholar] [CrossRef]
- Mei, Y.; Ban, H. High strain rate behaviour of stainless-clad bimetallic steel. Eng. Struct. 2020, 207, 110219. [Google Scholar] [CrossRef]
- Xie, H.J.; Cheng, Y.L.; Li, S.X.; Cao, J.H.; Cao, L. Wear and corrosion resistant coatings on surface of cast A356 aluminum alloy by plasma electrolytic oxidation in moderately concentrated aluminate electrolytes. Trans. Nonferr. Metal. Soc. China 2017, 27, 336–351. [Google Scholar] [CrossRef]
- Karpushenkov, S.A.; Shchukin, G.L.; Belanovich, A.L.; Savenko, V.P.; Kulak, A.I. Plasma electrolytic ceramic-like aluminum oxide coatings on iron. J. Appl. Electrochem. 2010, 40, 365–374. [Google Scholar] [CrossRef]
- Malinovschi, V.; Marin, A.; Moga, S.; Negrea, D. Preparation and characterization of anticorrosive layers deposited by micro-arc oxidation on low carbon steel. Surf. Coat. Technol. 2014, 253, 194–198. [Google Scholar] [CrossRef]
- ASM Handbook Committee. Metals Handbook, Volume 8: Metallography, Structures and Phase Diagrams, 8th ed.; American Society for Metals: Metals Park, OH, USA, 1973. [Google Scholar]
- Watts, J.F.; Wolstenholme, J. An Introduction to Surface Analysis by XPS and AES; John Wiley & Sons Ltd.: West Sussex, UK, 2003; p. 132. [Google Scholar]
- Brabers, V.A.M. Progress in spinel ferrite research. In Handbook of Magnetic Materials; Buschow, K.H.J., Ed.; North Holland Publishing: Amsterdam, The Netherlands, 1995; Volume 8, pp. 189–324. [Google Scholar]
- Chanda, D.; Hnat, J.; Paidar, M.; Bouzek, K. Evolution of physicochemical and electrocatalytic properties of NiCo2O4(AB2O4) spinel oxide with the effect of Fe substitution at the A site leading to efficient anodic O2 evolution in an alkaline environment. Int. J. Hydrogen Energy 2014, 39, 5713–5722. [Google Scholar] [CrossRef]
- Cisse, S.; Laffont, L.; Tanguy, B.; Laffont, M.C.; Andrieu, E. Effect of surface preparation on the corrosion of austenitic stainless steel 304L in high temperature steam and simulated PWR primary water. Corros. Sci. 2012, 56, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Rocchini, G. The determination of Tafel slopes by an integral method. Corros. Sci. 1994, 36, 113–125. [Google Scholar] [CrossRef]
- Jin, X.; Wang, B.; Xue, W.; Du, J.; Wu, X.; Wu, J. Characterization of wear-resistant coatings on 304 stainless steel fabricated by cathodic plasma electrolytic oxidation. Surf. Coat. Technol. 2013, 236, 22–28. [Google Scholar] [CrossRef]
- Aliofkhazraei, A.; Rouhaghdam, A.S. Fabrication of Nanostructures by Fabrication of Nanostructures by Plasma Electrolysis; WILEY-VCH: Weinheim, Germany, 2010; pp. 211–236. [Google Scholar]
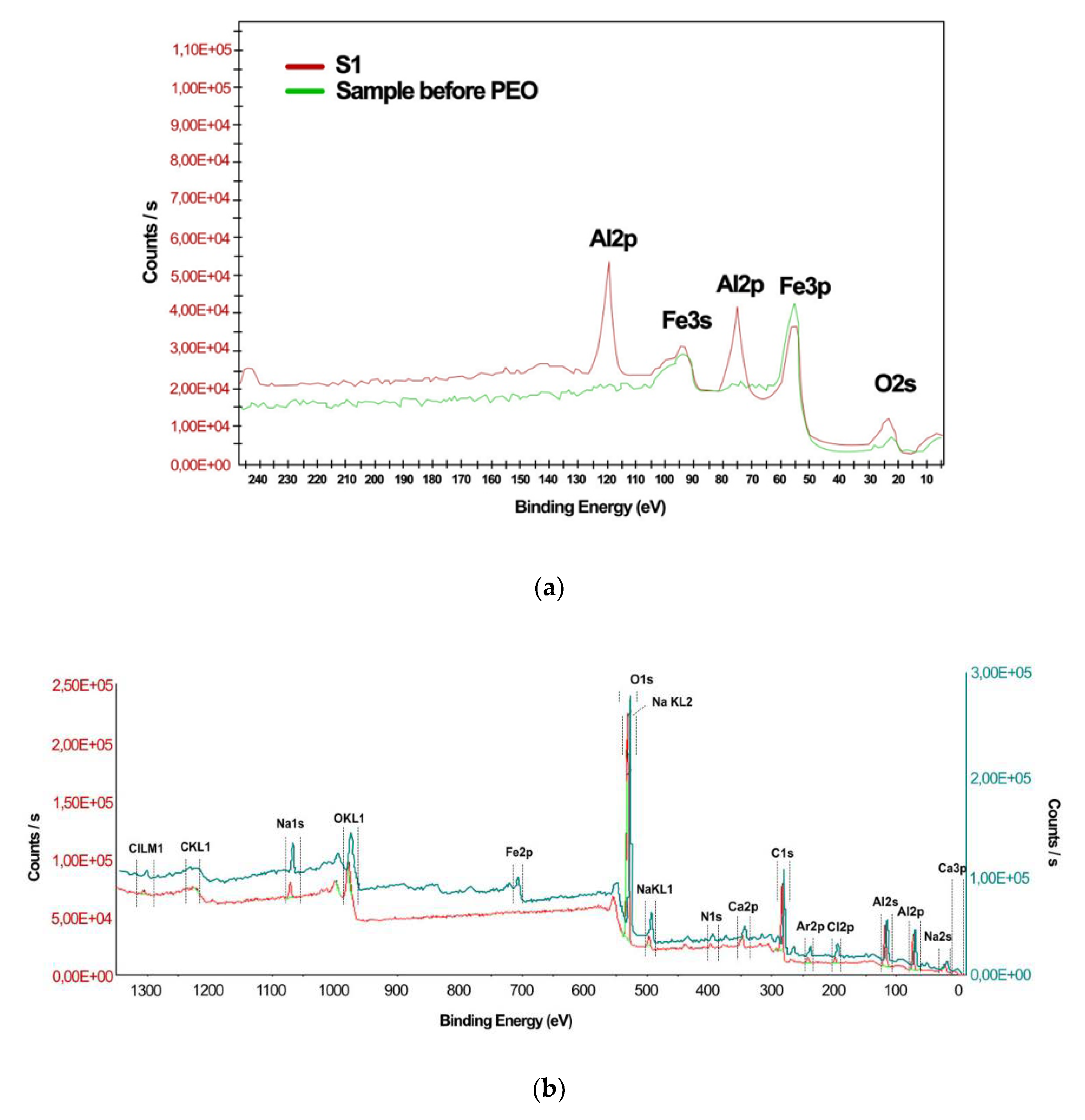
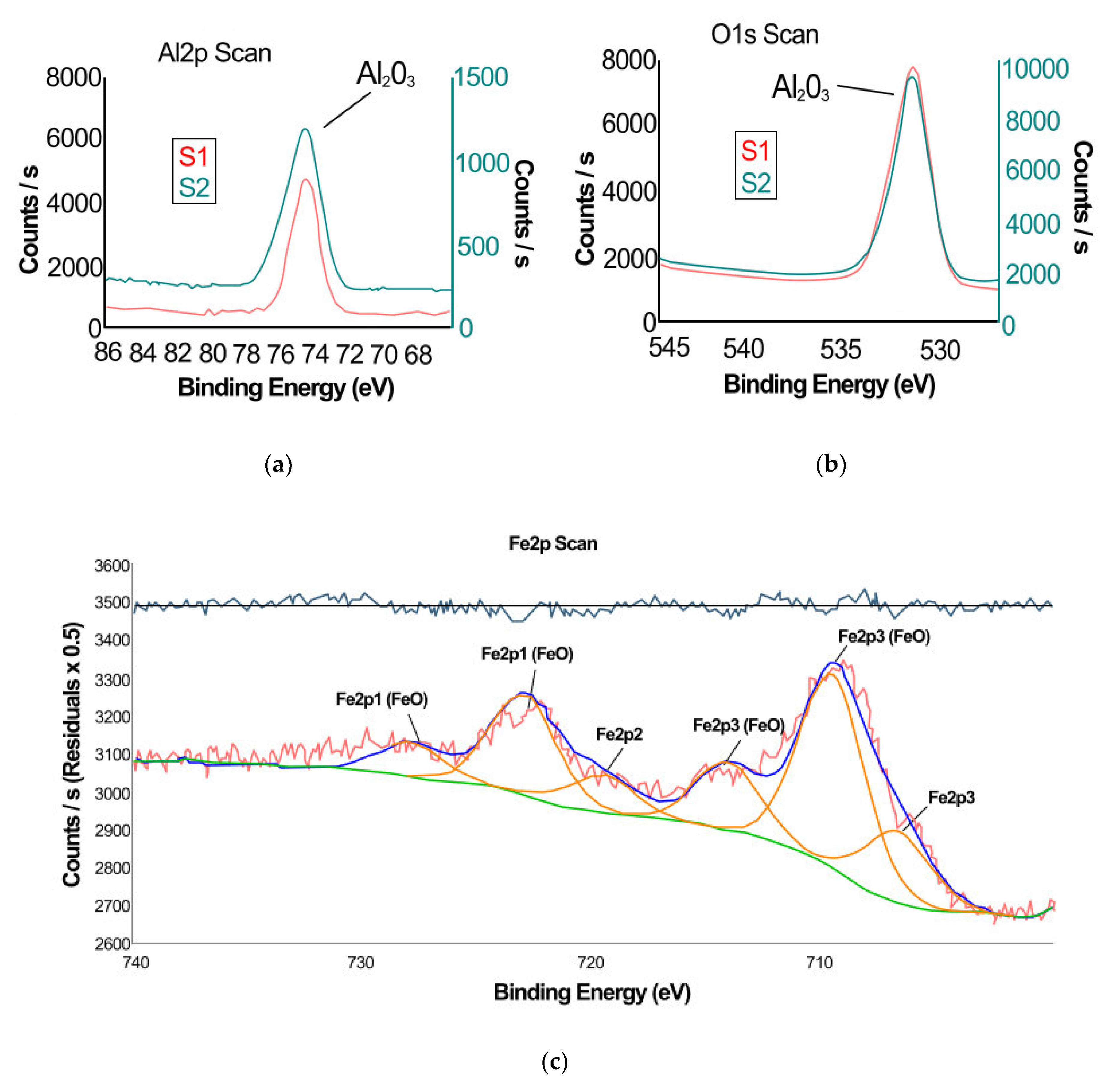
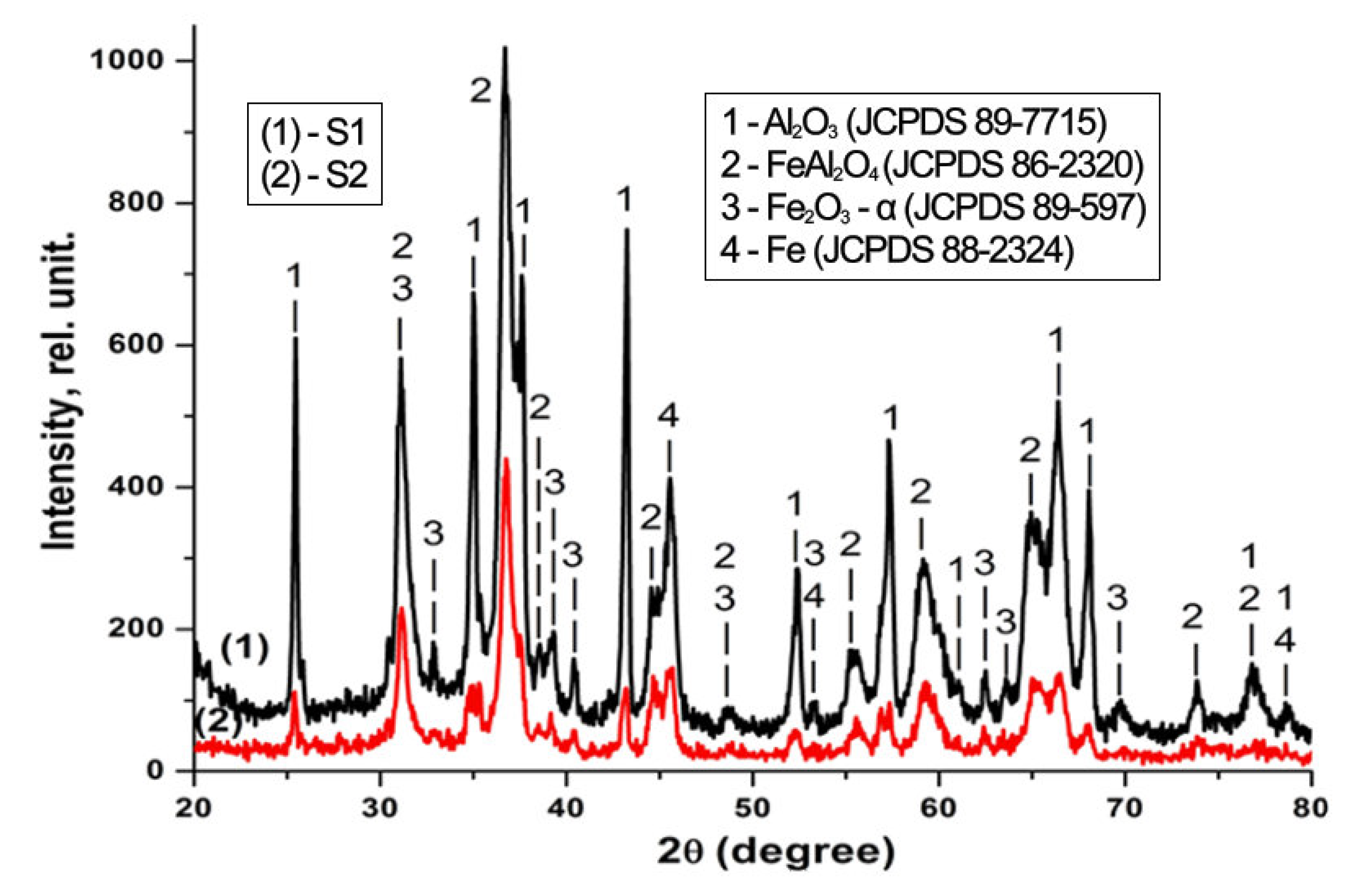

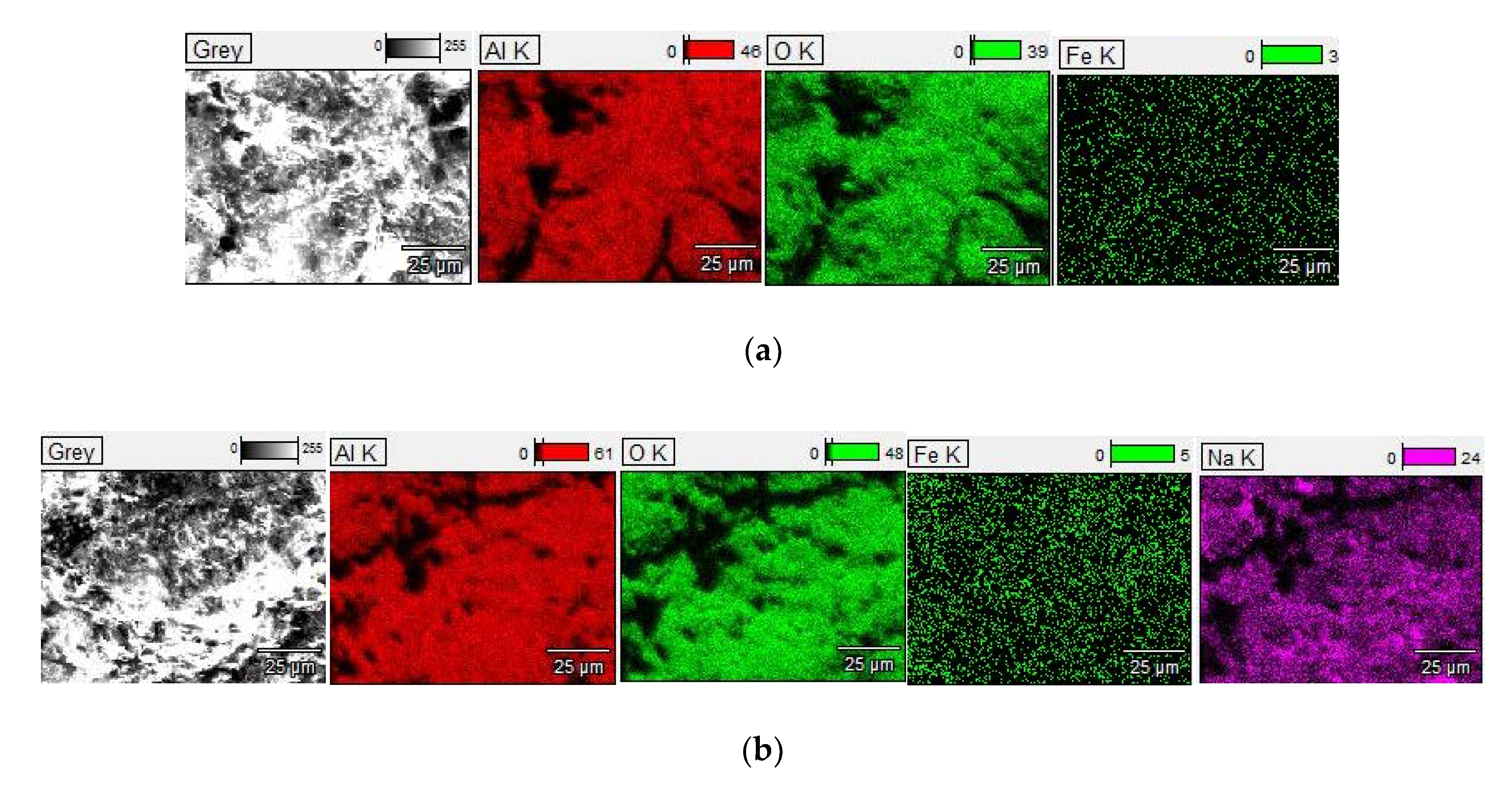
| Sample Code | Usd[1] [V] | U[2] [V] | Uamp[3] [V] | η[4] [%] | τ[5] [min] |
|---|---|---|---|---|---|
| S1 | 120 | 260 | 580 | 40 | 5 |
| S2 | 120 | 220 | 540 | 37 | 5 |
| Sample Code | Name | Peak BE (Binding Energy) | Atomic [%] |
|---|---|---|---|
| S1 | Al2p | 74.6 | 35.8 |
| O1s | 531.1 | 64.2 | |
| S2 | Al2p | 74.6 | 35.7 |
| Fe2p | 709.3 | 3.0 | |
| O1s | 531.1 | 61.3 |
| Element | S1 | S2 |
|---|---|---|
| Content [atom.%±S.D.%] | ||
| Al | 25.0 ± 0.09 | 30.0 ± 0.11 |
| O | 49.0 ± 1.42 | 57.0 ± 0.27 |
| Fe | 1.4 ± 0.07 | 0.7 ± 0.05 |
| Na | nd | 2.3 ± 0.02 |
| C | 24.6 ± 0.12 | 10.0 ± 0.15 |
| Sample | Point | Elements [atom.%] | ||||||
|---|---|---|---|---|---|---|---|---|
| O | Al | Cr | Mn | Fe | Ni | Mo | ||
| S1 | P1 | - | - | 18.2 | 1.6 | 68.1 | 8.6 | 3.5 |
| P2 | 9.4 | - | 26.4 | - | 47.0 | 13.8 | 3.4 | |
| P3 | 19.3 | - | 0.7 | 0.4 | 79.6 | - | - | |
| P4 | 30.5 | 36.9 | - | - | 32.6 | - | - | |
| P5 | 31.7 | 45.9 | - | - | 22.4 | - | - | |
| S2 | P1 | - | - | 18.0 | 1.3 | 70.1 | 8.2 | 2.4 |
| P2 | 6.5 | - | 21.2 | - | 57.7 | 14.6 | - | |
| P3 | 4.9 | - | 25.4 | - | 52.7 | 17.0 | - | |
| P4 | 17.5 | - | - | 1.3 | 81.2- | - | - | |
| P5 | 34.4 | 36.9 | - | - | 28.7 | - | ||
| Parameter [Measurement Unit] | 316L | S1 | S2 |
|---|---|---|---|
| Ecorr1 [mV] | −180 | −424 | −434 |
| icorr2 [µA/cm2] | 129.6 | 50.06 | 112.6 |
| Vcorr3 [mmpy] | 0.38 | 0.147 | 0.331 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrei, V.A.; Radulescu, C.; Malinovschi, V.; Marin, A.; Coaca, E.; Mihalache, M.; Mihailescu, C.N.; Dulama, I.D.; Teodorescu, S.; Bucurica, I.A. Aluminum Oxide Ceramic Coatings on 316l Austenitic Steel Obtained by Plasma Electrolysis Oxidation Using a Pulsed Unipolar Power Supply. Coatings 2020, 10, 318. https://doi.org/10.3390/coatings10040318
Andrei VA, Radulescu C, Malinovschi V, Marin A, Coaca E, Mihalache M, Mihailescu CN, Dulama ID, Teodorescu S, Bucurica IA. Aluminum Oxide Ceramic Coatings on 316l Austenitic Steel Obtained by Plasma Electrolysis Oxidation Using a Pulsed Unipolar Power Supply. Coatings. 2020; 10(4):318. https://doi.org/10.3390/coatings10040318
Chicago/Turabian StyleAndrei, Victor Aurel, Cristiana Radulescu, Viorel Malinovschi, Alexandru Marin, Elisabeta Coaca, Maria Mihalache, Cristian Nicolae Mihailescu, Ioana Daniela Dulama, Sofia Teodorescu, and Ioan Alin Bucurica. 2020. "Aluminum Oxide Ceramic Coatings on 316l Austenitic Steel Obtained by Plasma Electrolysis Oxidation Using a Pulsed Unipolar Power Supply" Coatings 10, no. 4: 318. https://doi.org/10.3390/coatings10040318
APA StyleAndrei, V. A., Radulescu, C., Malinovschi, V., Marin, A., Coaca, E., Mihalache, M., Mihailescu, C. N., Dulama, I. D., Teodorescu, S., & Bucurica, I. A. (2020). Aluminum Oxide Ceramic Coatings on 316l Austenitic Steel Obtained by Plasma Electrolysis Oxidation Using a Pulsed Unipolar Power Supply. Coatings, 10(4), 318. https://doi.org/10.3390/coatings10040318








