Raman Study of Nanocrystalline-Doped Ceria Oxide Thin Films
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xia, Y.; Liu, X.; Bai, Y.; Li, H.; Deng, X.; Niu, X.; Wu, X.; Zhou, D.; Wang, Z.; Meng, J. Electrical properties optimization of calcium Co-doping system: CeO2–Sm2O3. Int. J. Hydrog. Energy 2012, 37, 11934–11940. [Google Scholar] [CrossRef]
- Sun, C.; Li, H.; Chen, L. Nanostructured ceria-based materials: Synthesis, properties, and applications. Energy Environ. Sci. 2012, 5, 8475. [Google Scholar] [CrossRef]
- Wachsman, E.D.; Lee, K.T. Lowering the temperature of solid oxide fuel cells. Science 2011, 334, 935–939. [Google Scholar] [CrossRef]
- Aboud, A.A.; Al-Kelesh, H.; El Rouby, W.M.; Farghali, A.A.; Hamdedein, A.; Khedr, M.H. CO2 responses based on pure and doped CeO2 nano-pellets. J. Mater. Res. Technol. 2018, 7, 14–20. [Google Scholar] [CrossRef]
- Florea, M.; Postole, G.; Urdă, A.; Neaţu, F.; Massin, L.; Gelin, P.; Matei-Rutkovska, F. Influence of Gd and Pr doping on the properties of ceria: Texture, structure, redox behaviour and reactivity in CH4/H2O reactions in the presence of H2S. Catal. Sci. Technol. 2018, 8, 1333–1348. [Google Scholar] [CrossRef]
- He, D.; Hao, H.; Chen, D.; Liu, J.; Yu, J.; Lu, J.; Liu, F.; Wan, G.; He, S.; Luo, Y. Synthesis and application of rare-earth elements (Gd, Sm, and Nd) doped ceria-based solid solutions for methyl mercaptan catalytic decomposition. Catal. Today 2017, 281, 559–565. [Google Scholar] [CrossRef]
- Anjaneya, K.; Manjanna, J.; Nayaka, G.; Kumar, V.A.; Govindaraj, G.; Ganesha, K. Citrate-complexation synthesized Ce0.85Gd0.15O2−δ (GDC15) as solid electrolyte for intermediate temperature SOFC. Phys. B Condens. Matter 2014, 447, 51–55. [Google Scholar] [CrossRef]
- Arai, H.; Kunisaki, T.; Shimizu, Y.; Seiyama, T. Electrical properties of calcia-doped ceria with oxygen ion conduction. Solid State Ion. 1986, 20, 241–248. [Google Scholar] [CrossRef]
- Pergolesi, D.; Gilardi, E.; Fabbri, E.; Roddatis, V.; Harrington, G.F.; Lippert, T.K.; Kilner, J.A.; Traversa, E. Interface effects on the ionic conductivity of doped ceria–yttria-stabilized zirconia heterostructures. ACS Appl. Mater. Interfaces 2018, 10, 14160–14169. [Google Scholar] [CrossRef]
- Tuller, H. Ionic conduction in nanocrystalline materials. Solid State Ion. 2000, 131, 143–157. [Google Scholar] [CrossRef] [Green Version]
- Anwar, M.; Kumar, S.; Arshi, N.; Ahmed, F.; Seo, Y.; Lee, C.; Koo, B.H. Structural and optical study of samarium doped cerium oxide thin films prepared by electron beam evaporation. J. Alloys Compd. 2011, 509, 4525–4529. [Google Scholar] [CrossRef]
- Vinodkumar, T.; Rao, B.G.; Reddy, B.M. Influence of isovalent and aliovalent dopants on the reactivity of cerium oxide for catalytic applications. Catal. Today 2015, 253, 57–64. [Google Scholar] [CrossRef]
- Kosacki, I.; Suzuki, T.; Anderson, H.U.; Colomban, P. Raman scattering and lattice defects in nanocrystalline CeO2 thin films. Solid State Ion. 2002, 149, 99–105. [Google Scholar] [CrossRef]
- Sal’Nikov, V.V.; Pikalova, E. Raman and impedance spectroscopic studies of the specific features of the transport properties of electrolytes based on CeO2. Phys. Solid State 2015, 57, 1944–1952. [Google Scholar] [CrossRef]
- Pederson, L.; Singh, P.; Zhou, X.-D. Application of vacuum deposition methods to solid oxide fuel cells. Vacuum 2006, 80, 1066–1083. [Google Scholar] [CrossRef]
- Arunkumar, P.; Ramaseshan, R.; Dash, S.; Basu, J.; Ravindran, T.R.; Balakumar, S.; Babu, K.S.; Pandiyan, A. Texturing of pure and doped CeO2 thin films by EBPVD through target engineering. RSC Adv. 2014, 4, 33338. [Google Scholar] [CrossRef]
- Scagliotti, M. Plasma-sprayed zirconia electrolytes. Solid State Ion. 1988, 28, 1766–1769. [Google Scholar] [CrossRef]
- Song, H.; Xia, C.; Meng, G.; Peng, D. Preparation of Gd2O3 doped CeO2 thin films by oxy-acetylene combustion assisted aerosol-chemical vapor deposition technique on various substrates and zone model for microstructure. Thin Solid Film. 2003, 434, 244–249. [Google Scholar] [CrossRef]
- Zarkov, A.; Stanulis, A.; Mikoliunaite, L.; Katelnikovas, A.; Ramanauskas, R.; Tautkus, S.; Jasulaitiene, V.; Kareiva, A. Chemical solution deposition of pure and Gd-doped ceria thin films: Structural, morphological and optical properties. Ceram. Int. 2017, 43, 4280–4287. [Google Scholar] [CrossRef]
- Hong, Y.; Kim, S.; Kim, W.; Yoon, H.H. Fabrication and characterization GDC electrolyte thin films by e-beam technique for IT-SOFC. Curr. Appl. Phys. 2011, 11, S163–S168. [Google Scholar] [CrossRef]
- Petrov, I.; Barna, P.B.; Hultman, L.; Greene, J. Microstructural evolution during film growth. J. Vac. Sci. Technol. A 2003, 21, S117–S128. [Google Scholar] [CrossRef]
- Barna, P.; Adamik, M. Fundamental structure forming phenomena of polycrystalline films and the structure zone models. Thin Solid Film. 1998, 317, 27–33. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Takai, O.; Saito, N. Effect of growth temperature on structural and morphological evolution of epitaxial SrTiO3 thin films grown on LaAlO3 (001) substrates by ion beam sputter deposition. Vacuum 2014, 109, 175–179. [Google Scholar] [CrossRef]
- Kaiser, N. Review of the fundamentals of thin-film growth. Appl. Opt. 2002, 41, 3053–3060. [Google Scholar] [CrossRef]
- Guenther, K.H. Revisiting structure-zone models for thin-film growth. In Proceedings of the 34th Annual International Technical Symposium on Optical and Optoelectronic Applied Science and Engineering, San Diego, CA, USA, 8–13 July 1990. [Google Scholar]
- Watanabe, K.; Sunahara, K.; Fujima, T. Method for Vapor Depositing A Cerium Oxide Film. U.S. Patent 4,242,373, 30 December 1980. [Google Scholar]
- Wang, S.; Tian, E.; Lung, C. Surface energy of arbitrary crystal plane of bcc and fcc metals. J. Phys. Chem. Solids 2000, 61, 1295–1300. [Google Scholar] [CrossRef]
- Souza, E.C.C.; Brito, H.; Muccillo, E. Optical and electrical characterization of samaria-doped ceria. J. Alloys Compd. 2010, 491, 460–464. [Google Scholar] [CrossRef]
- Acharya, S.A.; Gaikwad, V.; D’Souza, S.; Barman, S.R. Gd/Sm dopant-modified oxidation state and defect generation in nano-ceria. Solid State Ion. 2014, 260, 21–29. [Google Scholar] [CrossRef]
- Kuo, Y.-L.; Su, Y.-M.; Chang, J.-Y. A facile method for the deposition of Gd2O3-doped ceria films by atmospheric pressure plasma jet. Thin Solid Film. 2014, 570, 215–220. [Google Scholar] [CrossRef]
- Wiktorczyk, T.; Bieganski, P.; Zielony, E. Preparation and optical characterization of e-beam deposited cerium oxide films. Opt. Mater. 2012, 34, 2101–2107. [Google Scholar] [CrossRef]
- Graham, G. Empirical method for determining CeO2-particle size in catalysts by raman spectroscopy. J. Catal. 1991, 130, 310–313. [Google Scholar] [CrossRef]
- Weber, W.H.; Hass, K.C.; McBride, J.R. Raman study of CeO2: Second-order scattering, lattice dynamics, and particle-size effects. Phys. Rev. B 1993, 48, 178–185. [Google Scholar] [CrossRef]
- Sato, T.; Tateyama, S. Temperature dependence of the linewidth of the first-order Raman spectrum for crystalline CeO2. Phys. Rev. B 1982, 26, 2257–2260. [Google Scholar] [CrossRef]
- Spanier, J.E.; Robinson, R.D.; Zhang, F.; Chan, S.-W.; Herman, I.P. Size-dependent properties of CeO2−y nanoparticles as studied by Raman scattering. Phys. Rev. B 2001, 64, 245407. [Google Scholar] [CrossRef] [Green Version]
- Anjaneya, K.; Nayaka, G.; Manjanna, J.; Govindaraj, G.; Ganesha, K. Studies on structural, morphological and electrical properties of Ce0.8Ln0.2O2−δ (Ln = Y3+, Gd3+, Sm3+, Nd3+ and La3+) solid solutions prepared by citrate complexation method. J. Alloys Compd. 2014, 585, 594–601. [Google Scholar] [CrossRef]
- Anjaneya, K.; Nayaka, G.; Manjannaa, J.; Govindaraj, G.; Ganesha, K. Preparation and characterization of Ce1−xGdxO2−δ (x = 0.1–0.3) as solid electrolyte for intermediate temperature SOFC. J. Alloys Compd. 2013, 578, 53–59. [Google Scholar] [CrossRef]
- Mishra, M.; Kuppusami, P.; Reddy, V.R.; Ghosh, C.; Divakar, R.; Ramaseshan, R.; Singh, A.; Thirumurugesan, R.; Mohandas, E. Influence of CeO2 layer thickness on the properties of CeO2/Gd2O3 multilayers prepared by pulsed laser deposition. Vacuum 2015, 113, 64–74. [Google Scholar] [CrossRef]
- Tsunekawa, S.; Sivamohan, R.; Ito, S.; Kasuya, A.; Fukuda, T. Structural study on monosize CeO2-x nano-particles. Nanostruct. Mater. 1999, 11, 141–147. [Google Scholar] [CrossRef]
- Lair, V.; Živković, L.; Lupan, O.; Ringuedé, A. Synthesis and characterization of electrodeposited samaria and samaria-doped ceria thin films. Electrochim. Acta 2011, 56, 4638–4644. [Google Scholar] [CrossRef]
- Chaubey, N.; Wani, B.N.; Bharadwaj, S.; Chattopadhyaya, M. Physicochemical properties of rare earth doped ceria Ce0.9Ln0.1O1.95 (Ln = Nd, Sm, Gd) as an electrolyte material for IT-SOFC/SOEC. Solid State Sci. 2013, 20, 135–141. [Google Scholar] [CrossRef]

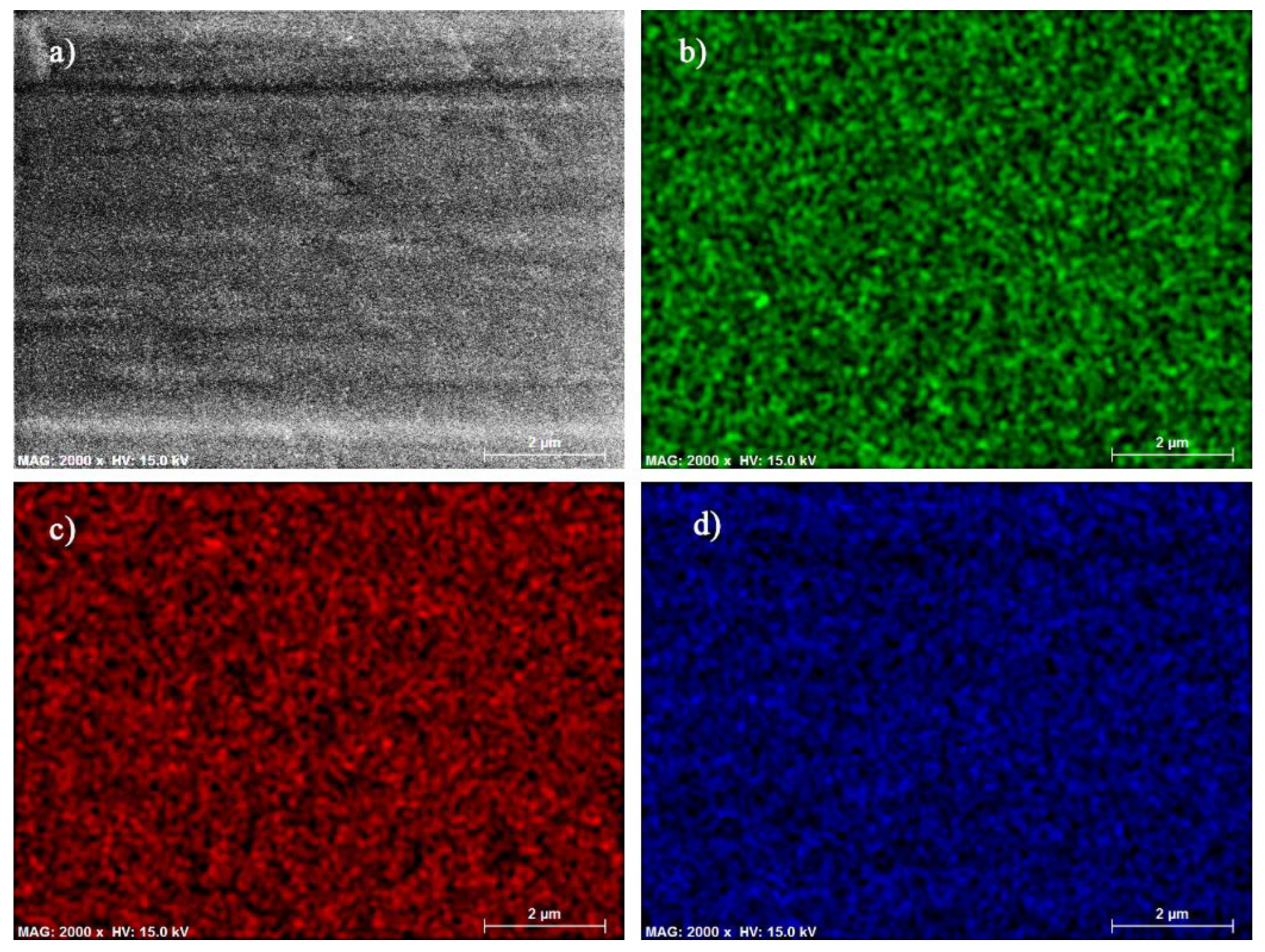


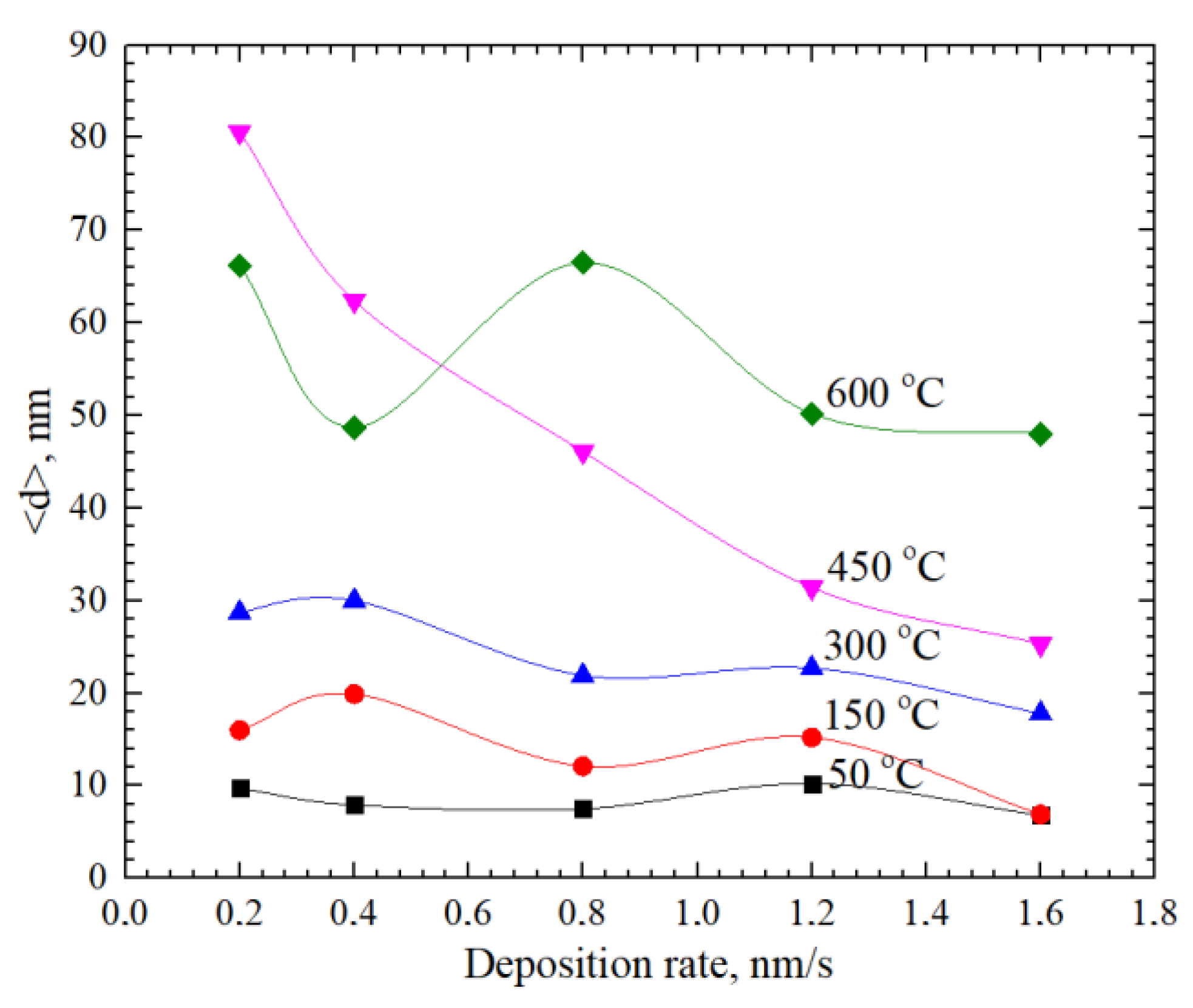
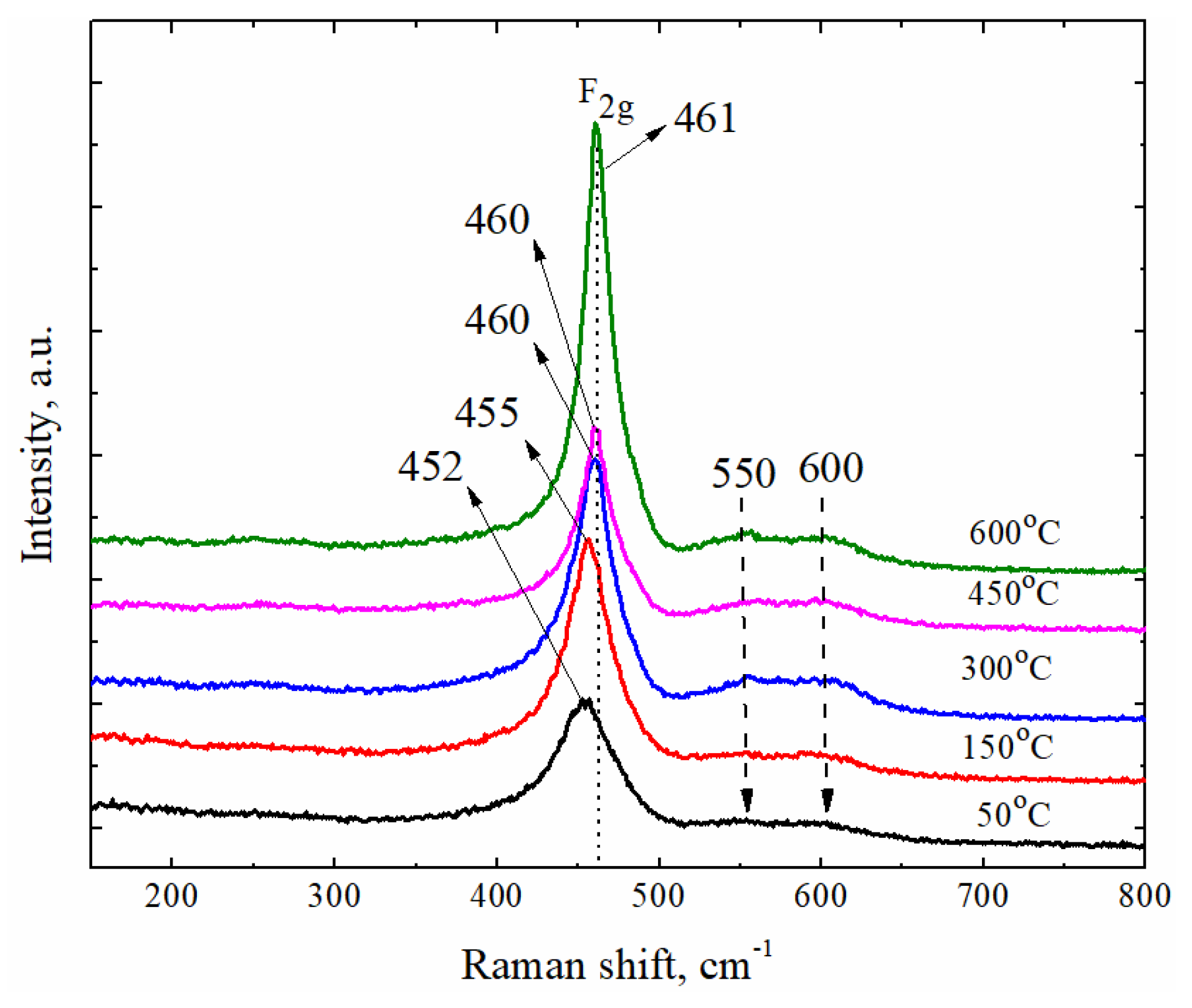
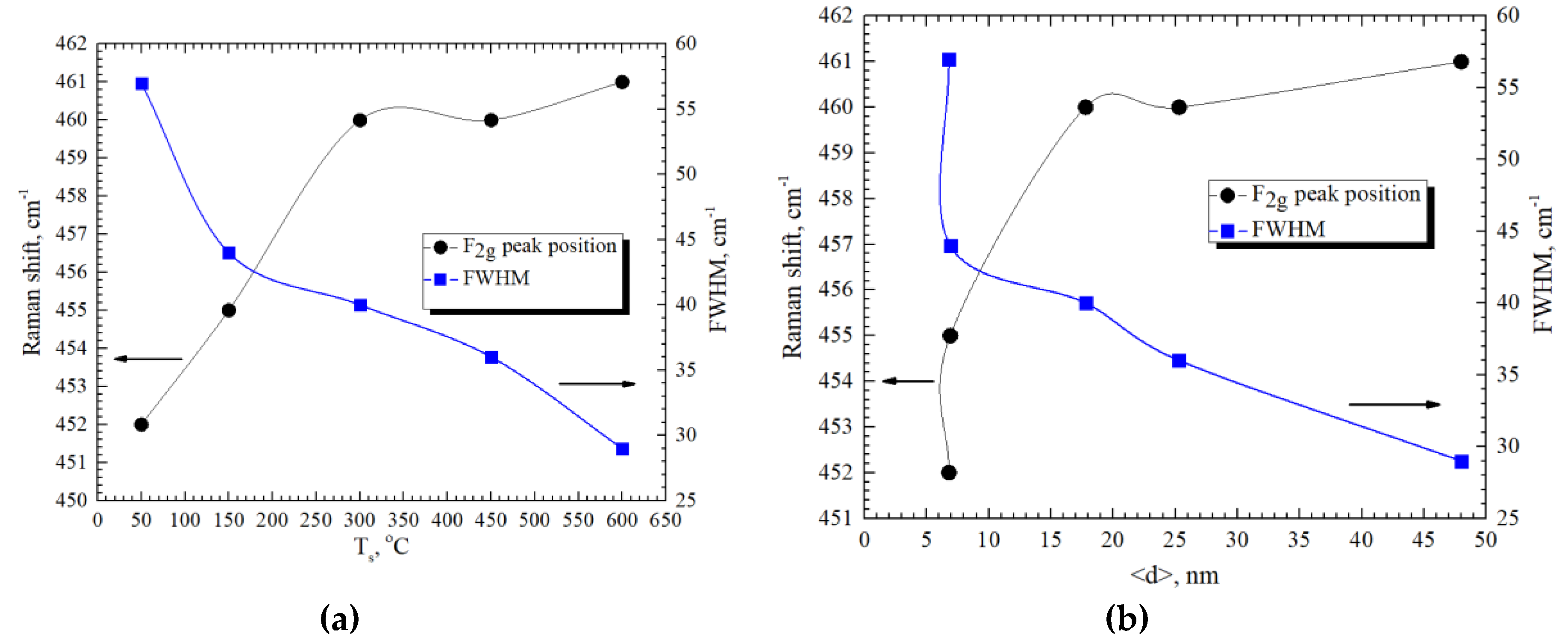


| GDC10-TC (BET 6.44 m2/g), mol % | ||||||
| Element\deposition rate | 0.2 nm/s | 0.4 nm/s | 0.8 nm/s | 1.2 nm/s | 1.6 nm/s | Initial powder |
| O2 | 58.4 | 57.5 | 59.4 | 58.8 | 60.2 | 48.7 |
| Ce | 34.3 | 35.4 | 35.1 | 36.7 | 35.7 | 43.3 |
| Gd | 7.3 | 7.1 | 5.5 | 4.5 | 4.1 | 8.0 |
| SDC15-TC (BET 8.0 m2/g), mol % | ||||||
| Element\deposition rate | 0.2 nm/s | 0.4 nm/s | 0.8 nm/s | 1.2 nm/s | 1.6 nm/s | Initial powder |
| O2 | 59.4 | 56.6 | 59.4 | 59.7 | 61.4 | 57.8 |
| Ce | 34.1 | 36.0 | 33.7 | 32.3 | 33.0 | 35.1 |
| Sm | 6.5 | 7.3 | 6.9 | 8.0 | 5.6 | 7.1 |
| GDC20-TC (BET 5.8 m2/g), mol % | ||||||
| Element\deposition rate | 0.2 nm/s | 0.4 nm/s | 0.8 nm/s | 1.2 nm/s | 1.6 nm/s | Initial powder |
| O2 | 58.3 | 57.0 | 57.2 | 59.2 | 58.0 | 53.7 |
| Ce | 35.1 | 35.5 | 33.2 | 35.0 | 34.5 | 35.6 |
| Gd | 6.6 | 7.5 | 9.6 | 5.8 | 7.5 | 10.7 |
| SDC20-TC (BET 6.2 m2/g), mol % | ||||||
| Element\deposition rate | 0.2 nm/s | 0.4 nm/s | 0.8 nm/s | 1.2 nm/s | 1.6 nm/s | Initial powder |
| O2 | 71.0 | 72.2 | 71.9 | 71.8 | 72.7 | 66.8 |
| Ce | 25.6 | 23.7 | 24.0 | 21.5 | 23.5 | 26.5 |
| Sm | 3.4 | 4.1 | 4.1 | 5.9 | 3.8 | 6.7 |
| Temperature, °C | GDC10 | GDC20 | SDC15 | SDC20 |
|---|---|---|---|---|
| 50 °C | 459 cm−1 | 458 cm−1 | 460 cm−1 | 452 cm−1 |
| 150 °C | 461 cm−1 | 460 cm−1 | 462 cm−1 | 455 cm−1 |
| 300 °C | 460 cm−1 | 461 cm−1 | 461 cm−1 | 460 cm−1 |
| 450 °C | 462 cm−1 | 461 cm−1 | 462 cm−1 | 460 cm−1 |
| 600 °C | 462 cm−1 | 461 cm−1 | 463 cm−1 | 464 cm−1 |
| Parameters | GDC | SDC | ||
|---|---|---|---|---|
| c, mol% | 10 | 20 | 15 | 20 |
| a, Å | 5.42 | 5.44 | 5.43 | 5.44 |
| Raman shift, cm−1 | 462 | 459 | 460 | 457 |
| FWHM, cm−1 | 31 | 36 | 43 | 46 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kainbayev, N.; Sriubas, M.; Virbukas, D.; Rutkuniene, Z.; Bockute, K.; Bolegenova, S.; Laukaitis, G. Raman Study of Nanocrystalline-Doped Ceria Oxide Thin Films. Coatings 2020, 10, 432. https://doi.org/10.3390/coatings10050432
Kainbayev N, Sriubas M, Virbukas D, Rutkuniene Z, Bockute K, Bolegenova S, Laukaitis G. Raman Study of Nanocrystalline-Doped Ceria Oxide Thin Films. Coatings. 2020; 10(5):432. https://doi.org/10.3390/coatings10050432
Chicago/Turabian StyleKainbayev, Nursultan, Mantas Sriubas, Darius Virbukas, Zivile Rutkuniene, Kristina Bockute, Saltanat Bolegenova, and Giedrius Laukaitis. 2020. "Raman Study of Nanocrystalline-Doped Ceria Oxide Thin Films" Coatings 10, no. 5: 432. https://doi.org/10.3390/coatings10050432





