Effect of the Porosity, Roughness, Wettability, and Charge of Micro-Arc Coatings on the Efficiency of Doxorubicin Delivery and Suppression of Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Experimental Methods
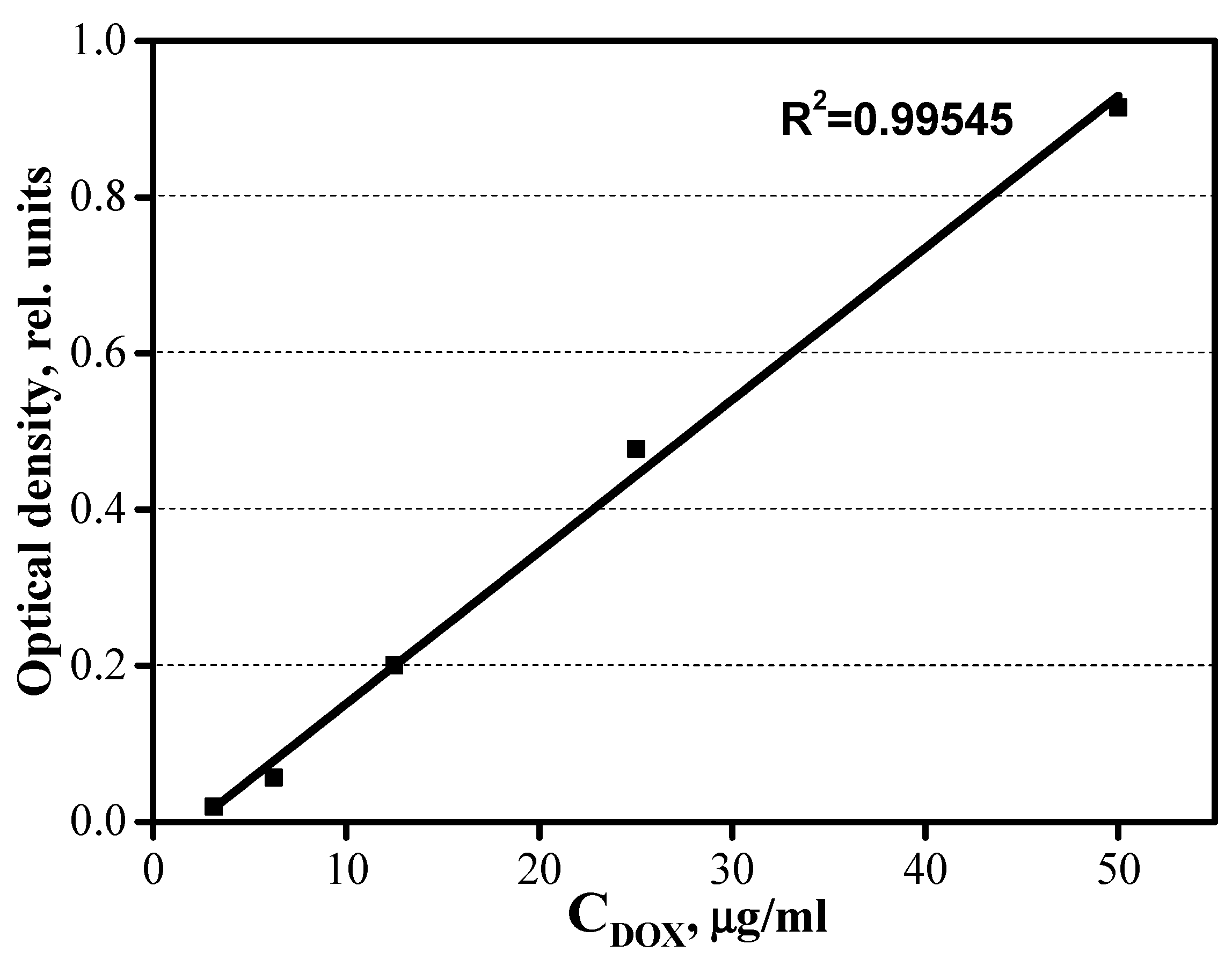
2.3. Kinetic Curves of the Sdsorption of DOX by the CaP Coating Samples
2.4. Kinetic Curves of the Desorption of DOX by the CaP Coating Samples
2.5. Study of the Effect of the Samples on Cell Line Viability
3. Results
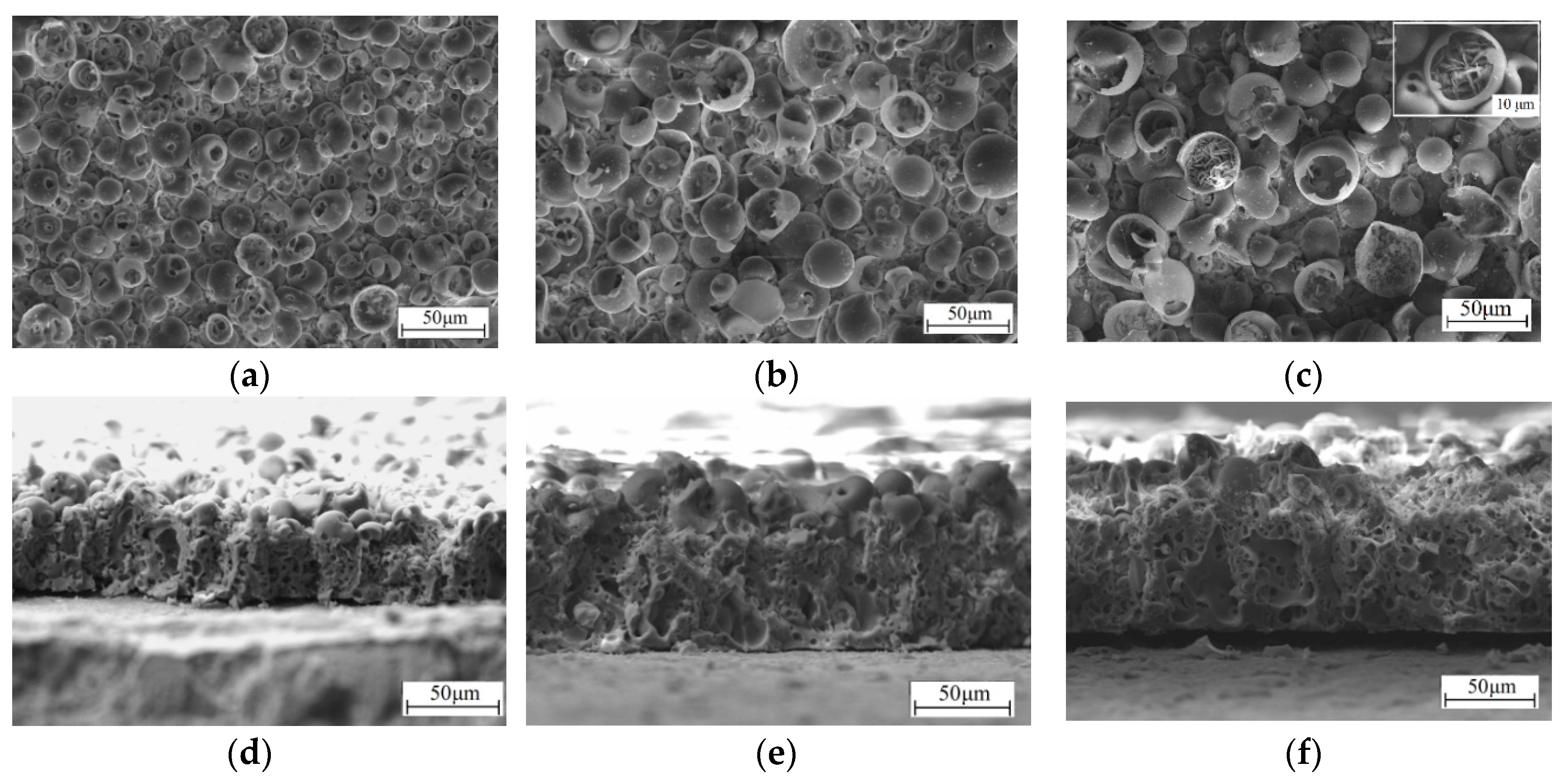
3.1. Formation of the CaP Coatings and their Structure, Morphology, and Porosity
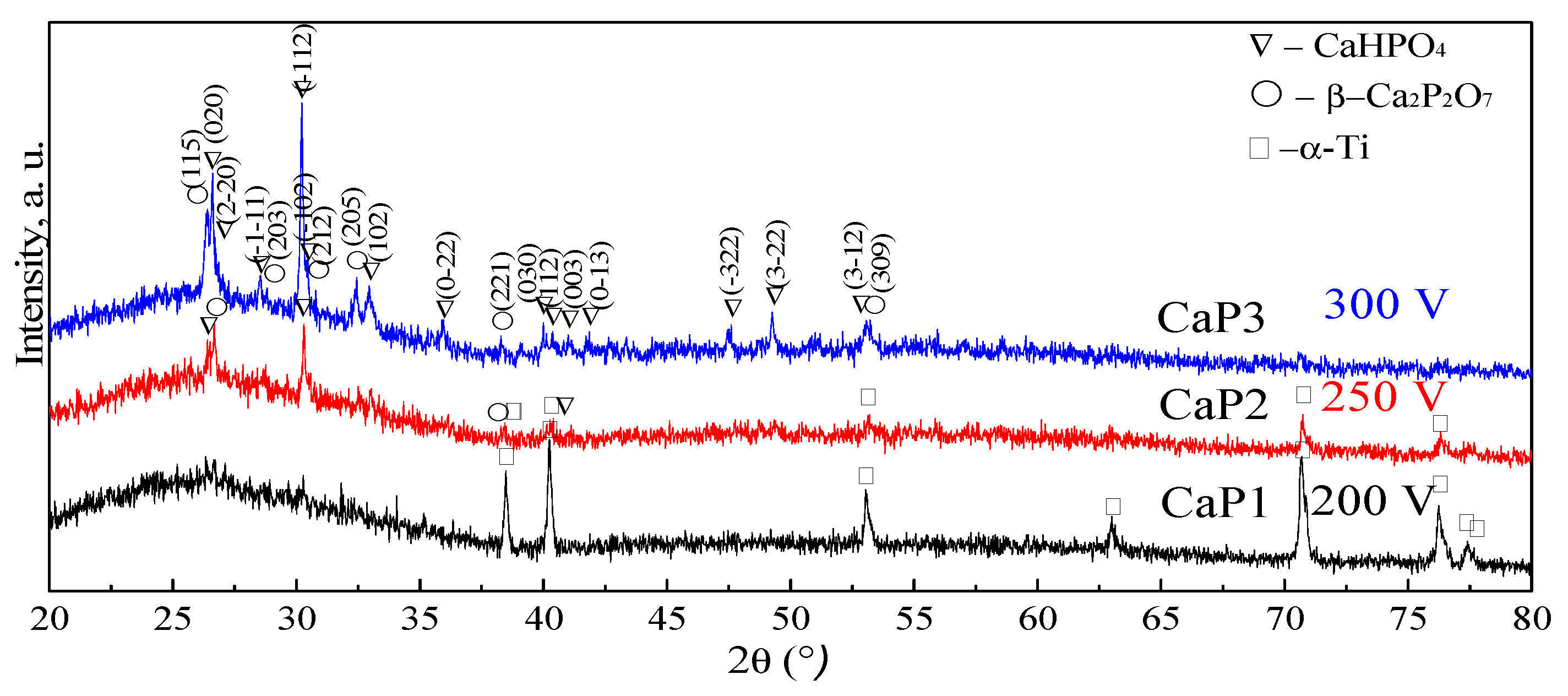
3.2. Phase Composition of CaP Coatings
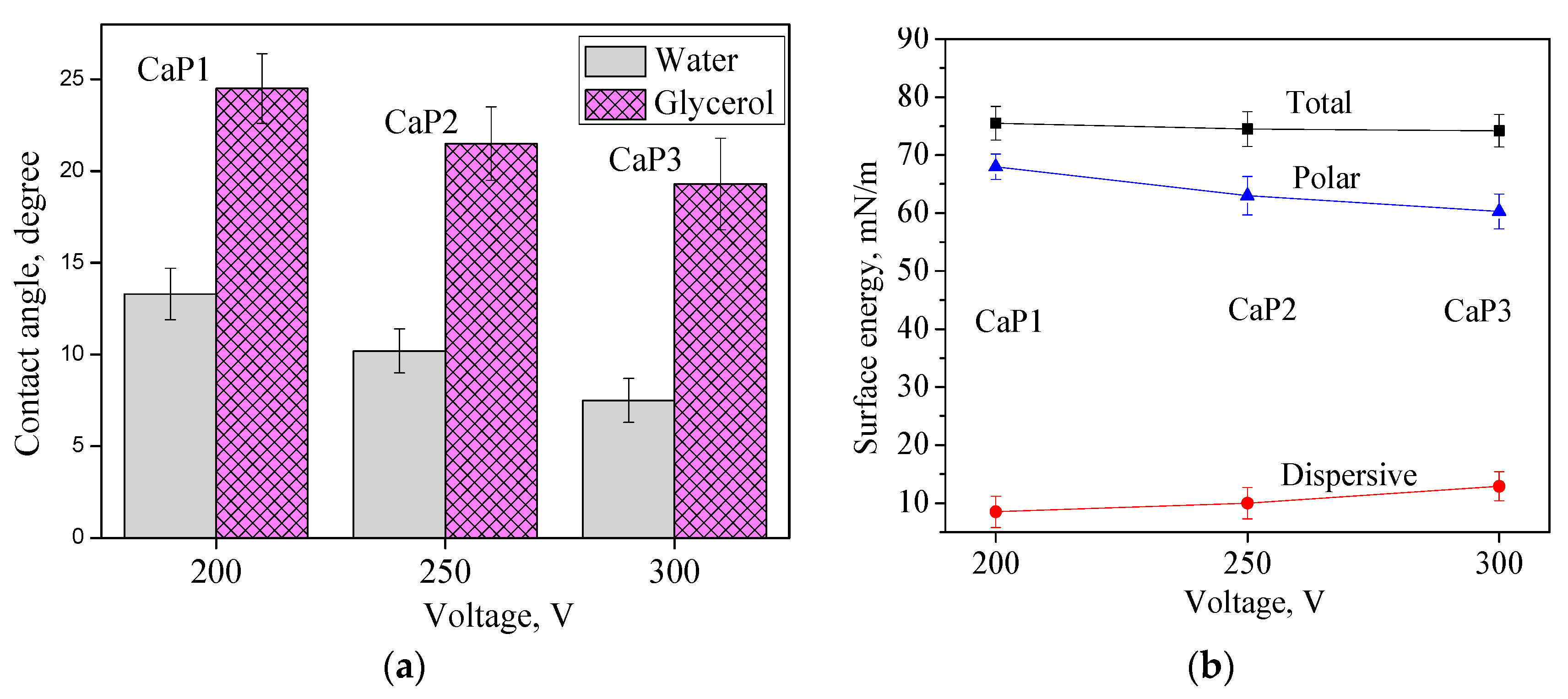
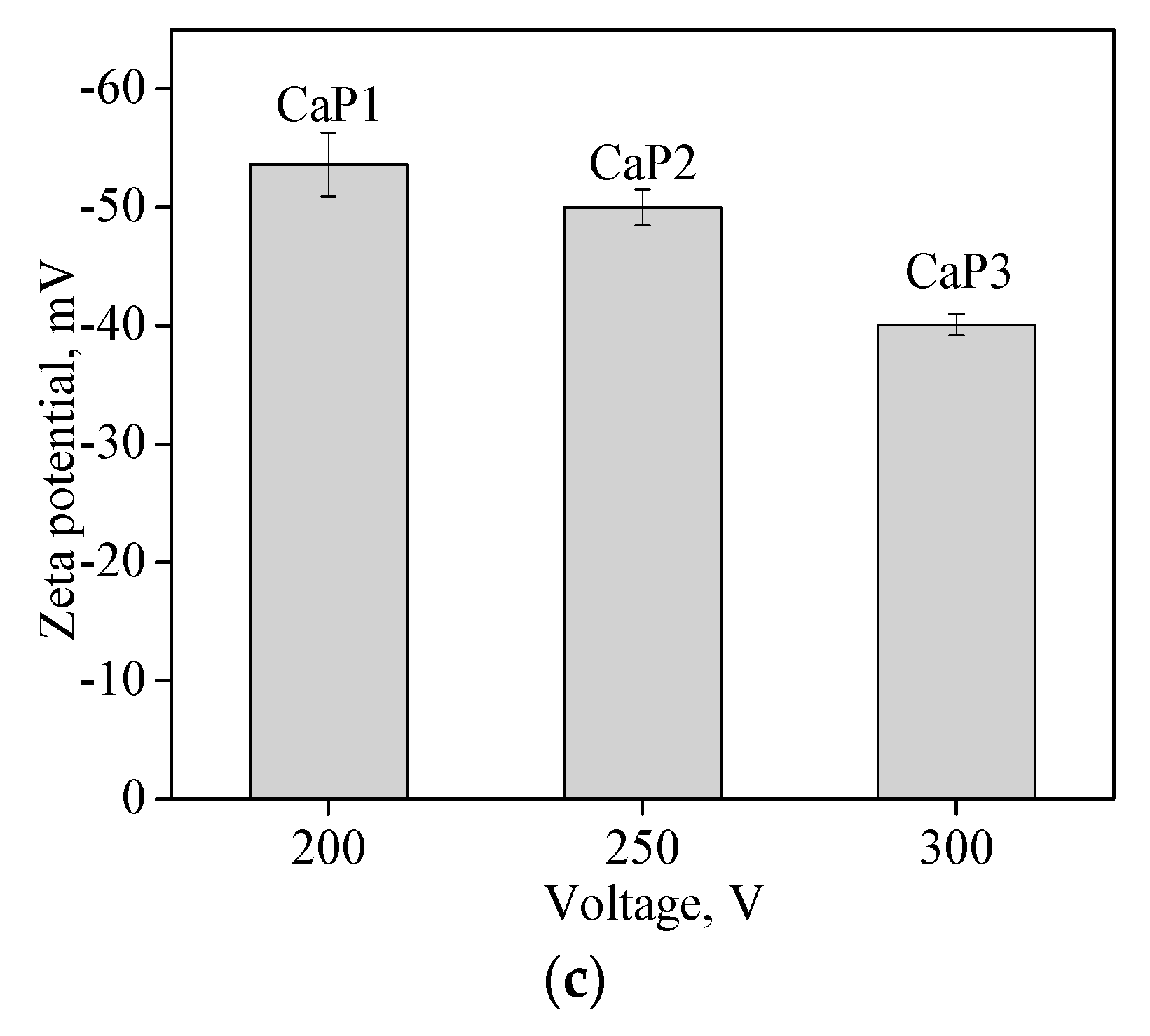
3.3. Hydrophilic Properties, Surface Energy, and Charge of CaP Coatings
3.4. Kinetics of Adsorption-Desorption
3.5. Effect of CaP Coatings Impregnated with DOX on the Viability of the Neuro-2a Cell Line
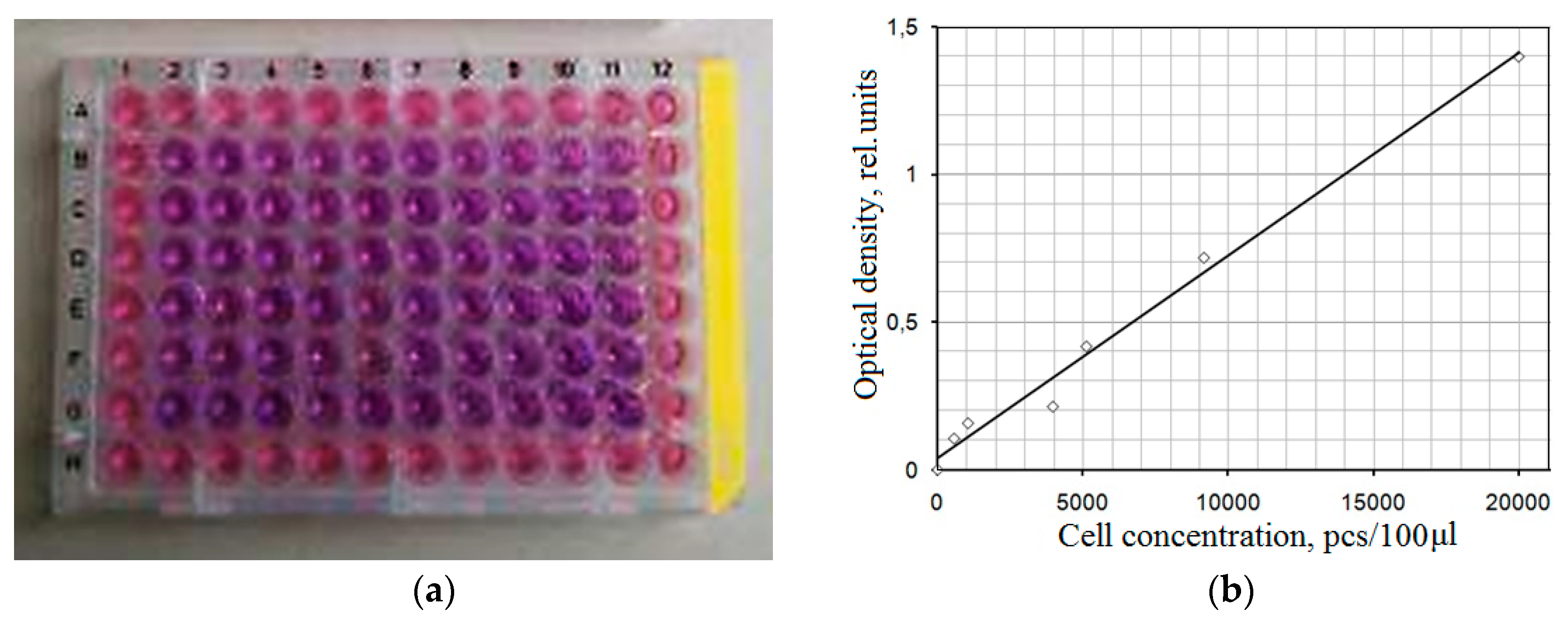
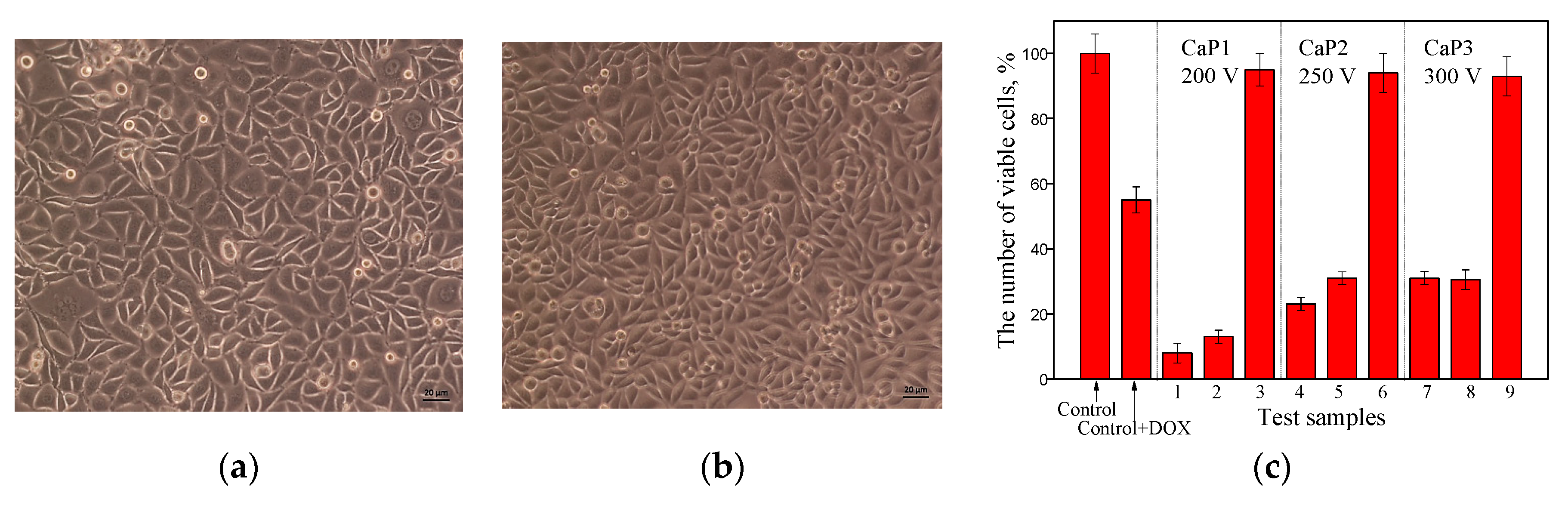
3.5.1. Study of the Effect of CaP Coatings Impregnated with DOX on the Viability of Neuro-2a Cell Line in the Goryaev Chamber
3.5.2. Study of the Effect of CaP Coatings Impregnated with DOX on the Viability of Neuro-2a Cell Line by MTT Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Campos, E.M.; Santos-Coquillat, A.; Mingo, B.; Arrabal, R.; Mohedano, M.; Pardo, A.; Ramos, V.; López Lacomba, J.L.; Matykina, E. Albumin loaded PEO coatings on Ti—Potential as drug eluting systems. Surf. Coat. Technol. 2015, 283, 44–51. [Google Scholar] [CrossRef]
- Tanzi, M.C.; Bozzini, S.; Candiani, G.; Cigada, A.; de Nardo, L.; Farè, S.; Ganazzoli, F.; Gastaldi, D.; Levi, M.; Metrangolo, P.; et al. Trends in biomedical engineering: Focus on smart bio-materials and drug delivery. J. Appl. Biomater. Biomech. 2011, 9, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; van der Mei, H.C.; Kuijer, R.; Busscher, H.J.; Rochford, E.T. Mechanism of cell integration on biomaterial implant surfaces in the presence of bacterial contamination. J. Biomed. Mater. Res. Part A 2015, 103, 3590–3598. [Google Scholar] [CrossRef] [PubMed]
- Kunrath, M.F.; Leal, B.F.; Hubler, R.; Oliveira, S.D.; Teixeira, E.R. Antibacterial potential associated with drug-delivery built TiO2 nanotubes in biomedical implants. AMB Express. 2019, 9, 51. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, J.Y.; Li, H.Q.; Zhao, A.Z.J.; Wang, Y.; Zhang, K.Q.; Sun, H.T.; Lai, Y.K. Recent advances on smart TiO2 nanotube platforms for sustainable drug delivery applications. Int. J. Nanomed. 2017, 12, 151–165. [Google Scholar] [CrossRef] [Green Version]
- Huan, L.E.; Fratila-Apachitei, I.; Apachitei, J.; Duszczyk, J. Porous TiO2 surface formed on nickel–titanium alloy by plasma electrolytic oxidation: A prospective polymer-free reservoir for drug eluting stent applications. J. Biomed. Mater. Res. 2013, 101, 700–708. [Google Scholar] [CrossRef]
- Rizwan, M.; Alias, R.; Zaidi, U.Z.; Mahmoodian, R.; Hamd, M. Surface modification of valve metals using plasma electrolytic oxidation for antibacterial applications: A review. J. Biomed. Mater. Res. Part A 2017, 106, 590–605. [Google Scholar] [CrossRef]
- Liu, S.; Li, B.; Liang, C.; Wang, H.; Qiao, Z. Formation mechanism and adhesive strength of a hydroxyapatite/TiO2 composite coating on a titanium surface prepared by micro-arc oxidation. Appl. Surf. Sci. 2016, 362, 109–114. [Google Scholar] [CrossRef]
- Sasikumar, Y.; Kumar, A.M.; Babu, R.S.; Rahman, M.M.; Samyn, L.M.; de Barros, A.L.F. Biocompatible hydrophilic brushite coatings on AZX310 and AM50 alloys for orthopedic implants. J. Mater. Sci.: Mater. Med. 2018, 29, 123. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O. Influence of process parameters on electrolytic plasma discharging behavior and aluminum oxide coating microstructure. Surf. Coat. Technol. 2010, 205, 1659–1667. [Google Scholar] [CrossRef]
- Diefenbeck, M.; Schrader, C.; Gras, F.; Mückley, T.; Schmidt, J.; Zankovych, S.; Bossert, J.; Jandt, K.D.; Völpel, A.; Sigusch, B.W.; et al. Gentamicin coating of plasma chemical oxidized titanium alloy prevents implant-related osteomyelitis in rats. Biomaterials 2016, 101, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Wang, D.; Tian, Y.; Cao, H.; Qiao, Y.; Liu, X. Sealing the pores of PEO coating with Mg-Al layered double hydroxide: Enhanced corrosion resistance, cytocompatibility and drug delivery ability. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husak, Y.; Solodovnyk, O.; Yanovska, A.; Kozik, Y.; Liubchak, I.; Ivchenko, V.; Mishchenko, O.; Zinchenko, Y.; Kuznetsov, V.; Pogorielov, M. Degradation and in vivo response of hydroxyapatite-coated Mg alloy. Coatings 2018, 8, 375. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Ye, W.; Gao, H.; Gao, L. Improving the corrosion resistance of ZEK100 magnesium alloy by combining high-pressure torsion technology with hydroxyapatite coating. Mater. Des. 2019, 181, 107933. [Google Scholar] [CrossRef]
- Asri, R.I.M.; Harun, W.S.W.; Hassan, M.A.; Ghani, S.A.C.; Buyong, Z. A Review of hydroxyapatite-based coating techniques: Sol-gel and electrochemical depositions on biocompatible metals. J. Mech. Behav. Biomed. Mater. 2016, 57, 95–108. [Google Scholar] [CrossRef] [Green Version]
- Dorozhkin, S.V. Multiphasic calcium orthophosphate (CaPO4) bioceramics and their biomedical applications. Ceram. Int. 2016, 42, 6529–6554. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. A review on the dissolution models of calcium apatites. Prog. Cryst. Growth Charact. Mater. 2002, 44, 45–61. [Google Scholar] [CrossRef]
- Son, Y.J.; Lee, I.C.; Jo, H.H.; Chung, T.J.; Oh, K.S. Setting behavior and drug release from brushite bone cement prepared with granulated hydroxyapatite and β-tricalcium phosphate. J. Korean Ceram. Soc. 2019, 56, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.J.; Cheng, Q.; Wang, J.; Zhao, Y.B.; Han, Z.Z.; Zhang, F.; Li, S.Q.; Zeng, R.C.; Wang, Z.L. Corrosion resistance and antibacterial effects of hydroxyapatite coating induced by polyacrylic acid and gentamicin sulfate on magnesium alloy. Front. Mater. Sci. 2019, 13, 87–98. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Onafuye, H.; Pieper, S.; Mulac, D.; Cinatl, J.; Wass, M.N.; Langer, K.; Michaelis, M. Doxorubicin-loaded human serum albumin nanoparticles overcome transporter-mediated drug resistance. Biorxiv 2019, 1, 655662. [Google Scholar] [CrossRef]
- Adisa, A.O.; Lawal, O.O.; Adesunkanmi, A.R.K. Evaluation of patients’ adherrence to chemotherapy for breast cancer. Afr. J. Health Sci. 2008, 15, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Puts, M.T.E.; Tu, H.A.; Tourangeau, A.; Howell, D.; Fitch, M.; Springall, E.; Alibhai, S.M.H. Factors influencing adherence to cancer treatment in older adults with cancer: A systematic review. Ann. Oncol. 2014, 25, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Kefayat, A.; Vaezifar, S. Biodegradable PLGA implants containing doxorubicin-loaded chitosan nanoparticles for treatment of breast tumor-bearing mice. Int. J. Biol. Macromol. 2019, 136, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Qiu, K.; He, C.; Feng, W.; Wang, W.; Zhou, X.; Yin, Z.; Chen, L.; Wang, H.; Mo, X. Doxorubicin-loaded electrospun poly(L-lactic acid)/mesoporous silica nanoparticles composite nanofibers for potential postsurgical cancer treatment. J. Mater. Chem. B 2013, 1, 4601–4611. [Google Scholar] [CrossRef] [PubMed]
- Bi, D.; Zhao, L.; Yu, R.; Li, H.; Guo, Y.; Wang, X.; Han, M. Surface modification of doxorubicin-loaded nanoparticles based on polydopamine with pH-sensitive property for tumor targeting therapy. Drug Deliv. 2018, 25, 564–575. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, M.; Naz, F.; Dinda, A.K.; Koul, V. In vitro and in vivo efficacy of doxorubicin loaded biodegradable semi-interpenetrating hydrogel implants of poly (acrylic acid)/gelatin for post surgical tumor treatment. Biomed. Mater. 2013, 8, 045004–045012. [Google Scholar] [CrossRef]
- Ghosh, S.; Raju, R.S.K.; Ghosh, N.; Chaudhury, K.; Ghosh, S.; Banerjee, I.; Pramanika, N. Development and physicochemical characterization of doxorubicin-encapsulated hydroxyapatite–polyvinyl alcohol nanocomposite for repair of osteosarcoma-affected bone tissues. Comptes Rendus Chim. C. R. Chim. 2019, 22, 46–57. [Google Scholar] [CrossRef]
- Sedelnikova, M.B.; Komarova, E.G.; Sharkeev, Y.P.; Ugodchikova, A.V.; Tolkacheva, T.V.; Rau, J.V.; Buyko, E.E.; Ivanov, V.V.; Sheikin, V.V. Modification of titanium surface via Ag-, Sr- and Si-containing micro-arc calcium phosphate coating. Bioact. Mater. 2019, 4, 224–235. [Google Scholar] [CrossRef]
- Sedelnikova, M.B.; Komarova, E.G.; Sharkeev, Y.P.; Tolkacheva, T.V.; Sheikin, V.V.; Egorkin, V.S.; Mashtalyar, D.V.; Kazakbaeva, A.A.; Schmidt, J. Characterization of the Micro-Arc Coatings Containing β-Tricalcium Phosphate Particles on Mg-0.8Ca Alloy. Metals 2018, 8, 238. [Google Scholar] [CrossRef] [Green Version]
- Sedelnikova, M.B.; Komarova, E.G.; Sharkeev, Y.P.; Ugodchikova, A.V.; Mushtovatova, L.S.; Karpova, M.R.; Sheikin, V.V.; Litvinova, L.S.; Khlusov, I.A. Zn-, Cu- or Ag-incorporated micro-arc coatings on titanium alloys: Properties and behavior in synthetic biological media. Surf. Coat. Technol. 2019, 369, 52–68. [Google Scholar] [CrossRef]
- ASTM E1382–97. Standard Test Methods for Determining Average Grain Size Using Semiautomatic and Automatic Image Analysis; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- BS DD ENV 1071-5 Advanced Technical Ceramics—Methods of Test for Ceramic Coatings Part 5: Determination of Porosity; British Standards Institution (BSI): London, UK, 1995.
- Sharkeev, Y.; Komarova, E.; Sedelnikova, M.; Sun, Z.; Zhu, Q.; Zhang, J.; Tolkacheva, T.; Uvarkin, P. Structure and properties of micro-arc calcium phosphate coatings on pure titanium and Ti–40Nb alloy. Trans. Nonferrous Met. Soc. China 2017, 27, 125–133. [Google Scholar] [CrossRef]
- Rudawska, A.; Jacniacka, E. Analysis for determining surface energy uncertainty by the Owens-Wendt method. Int. J. Adhes. Adhes. 2009, 29, 451–457. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell viability assays assay guidance manual. In Assay Guidance Manual; Sittampalam, G.S., Grossman, A., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004; pp. 1–23. [Google Scholar]
- Khlusov, I.A.; Khlusova, M.Y.; Zaitsev, K.V.; Kolokol’tseva, T.D.; Sharkeev, Y.P.; Pichugin, V.F.; Legostaeva, E.V.; Trofimova, I.E.; Klimov, A.S.; Zhdanova, A.I. Pilot in vitro study of the parameters of artificial niche for osteogenic differentiation of human stromal stem cell pool. Bull. Exp. Biol. Med. 2011, 150, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Komarova, E.G.; Sedelnikova, M.B.; Kondranova, A.M.; Kazantsev, S.O.; Sharkeev, Y.P. Development of calcium phosphate coatings with regulated porous structure as drug carrier systems. IOP J. Phys. Conf. Ser. 2019, 1281, 012037. [Google Scholar] [CrossRef] [Green Version]
- Harding, I.S.; Rashid, N.; Hing, K.A. Surface charge and the effect of excess calcium ions on the hydroxyapatite surface. Biomaterials 2005, 26, 6818–6826. [Google Scholar] [CrossRef]
- Desai, T.R.; Bhaduri, S.B.; Tas, A.C. A Self-setting monetite (CaHPO4) cement for skeletal repair. Adv. Biocer. Biocomp. 2007, 27, 61–69. [Google Scholar] [CrossRef]
- Park, J.W.; Jang, J.H.; Lee, C.S.; Hanawa, T. Osteoconductivity of hydrophilic microstructured titanium implants with phosphate ion chemistry. Acta Biomater. 2009, 5, 2311–2321. [Google Scholar] [CrossRef]
- Harnett, E.M.; Alderman, J.; Wood, T. The surface energy of various biomaterials coated with adhesion molecules used in cell culture. Colloids Surf. B Biointerf. 2007, 55, 90–97. [Google Scholar] [CrossRef]
- Dekhtyar, Y.; Dvornichenko, M.V.; Karlov, A.V.; Khlusov, I.A.; Polyaka, N.; Sammons, R.; Zaytsev, K.V. Electrically functionalized hydroxyapatite and calcium phosphate surfaces to enhance immobilization and proliferation of osteoblasts in vitro and modulate osteogenesis in vivo. IFMBE Proc. 2009, 25, 245–248. [Google Scholar] [CrossRef]
- Thian, E.S.; Ahmad, Z.; Huang, J.; Edirisinghe, M.J.; Jayasinghe, S.N.; Ireland, D.C.; Brooks, R.A.; Rushton, N.; Bonfield, W.; Best, S.M. The role of surface wettability and surface charge of electrosprayed nanoapatites on the behaviour of osteoblasts. Acta Biomater. 2010, 6, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.O.; Baumann, M.J.; McCabe, L.R. Electrostatic inter-actions as a predictor for osteoblast attachment to biomate-rials. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2004, 70, 436–441. [Google Scholar] [CrossRef]
- Ohgaki, M.; Kizuki, T.; Katsura, M.; Yamashita, K. Manipulation of selective cell adhesion and growth by surface charges of electrically polarized hydroxyapatite. J. Biomed. Mater. Res. 2001, 57, 366–373. [Google Scholar] [CrossRef]
- Masato Ueshima, Satoshi Tanaka, Satoshi Nakamura, Kimihiro Yamashita, Manipulation of bacterial adhesion and proliferation by surface charges of electrically polarized hydroxyapatite. J. Biomed. Mater. Res. 2002, 60, 578–584. [CrossRef]



| Properties of the Coatings | Voltage of the MAO Process | ||
|---|---|---|---|
| CaP1 −200 V | CaP2 −250 V | CaP3 −300 V | |
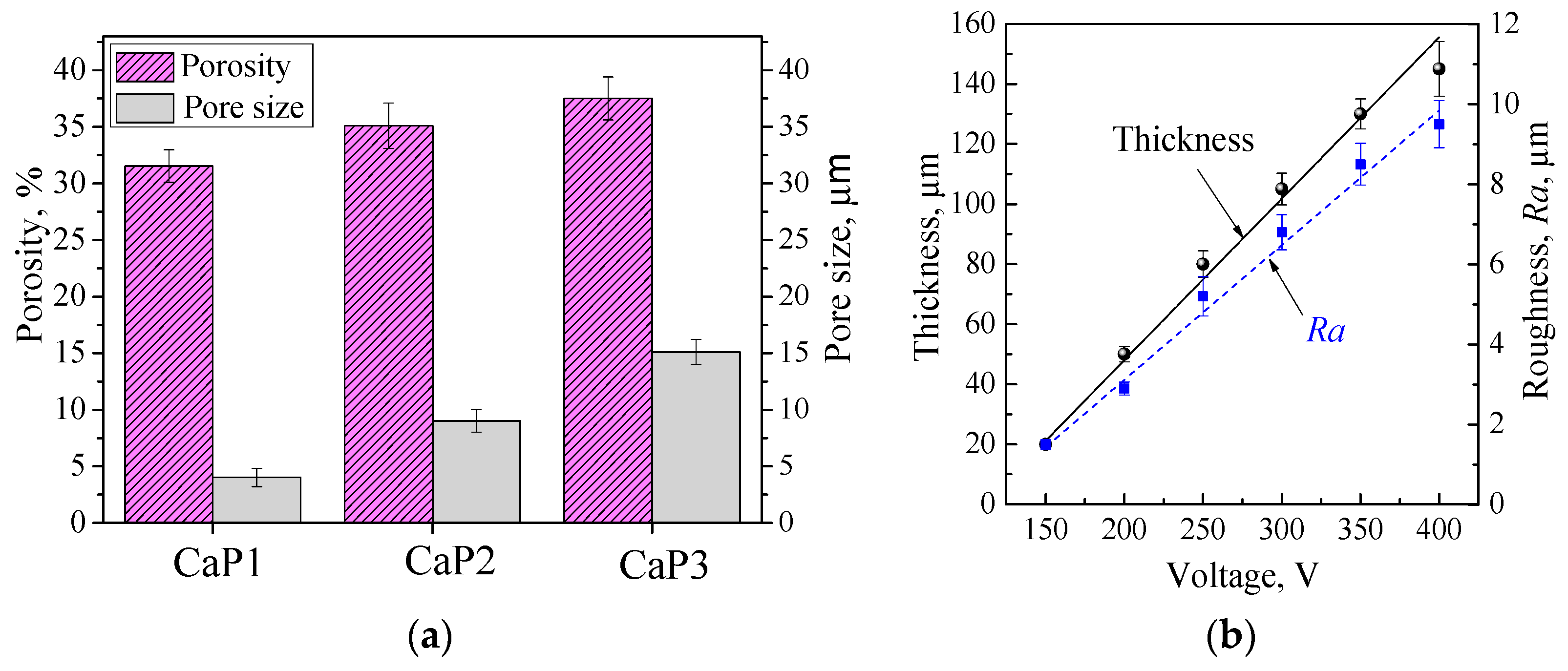
| Pore size, μm | 4.0 ± 0.8 | 9.0 ± 1.0 | 15.1 ± 1.1 |
| Porosity, % | 31.5 ± 1.5 | 35.1 ± 2.0 | 37.5 ± 1.9 |
| Thickness, μm | 50 ± 2.5 | 80 ± 4.4 | 105 ± 5.3 |
| Roughness, Ra, μm | 2.9 ± 0.16 | 5.2 ± 0.49 | 6.8 ± 0.44 |
| Structure and the presence of crystalline compounds | Amorphous | Amorphous crystalline (monetite, calcium β-pyrophosphate) | Amorphous crystalline (monetite, calcium β-pyrophosphate) |
| Contact angle, water, ° | 13.3 ± 1.4 | 10.2 ± 1.2 | 7.5 ± 1.2 |
| Contact angle, glycerol, ° | 24.5 ± 1.9 | 21.5 ± 2 | 19.3 ± 2.5 |
| Surface energy, mN/m | 75.5 ± 2.9 | 74.5 ± 3 | 74.2 ± 2.8 |
| Zeta potential, mV | −53.6 ± 2.7 | −50.0 ± 1.5 | −40.1 ± 0.9 |
| Type of the Coating | Samples with DOX | Samples without DOX |
|---|---|---|
| CaP1 200 V | 1 | - |
| 2 | - | |
| - | 3 | |
| CaP2 250 V | 4 | - |
| 5 | - | |
| - | 6 | |
| CaP3 300 V | 7 | - |
| 8 | - | |
| - | 9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedelnikova, M.B.; Komarova, E.G.; Sharkeev, Y.P.; Chebodaeva, V.V.; Tolkacheva, T.V.; Kondranova, A.M.; Zakharenko, A.M.; Bakina, O.V. Effect of the Porosity, Roughness, Wettability, and Charge of Micro-Arc Coatings on the Efficiency of Doxorubicin Delivery and Suppression of Cancer Cells. Coatings 2020, 10, 664. https://doi.org/10.3390/coatings10070664
Sedelnikova MB, Komarova EG, Sharkeev YP, Chebodaeva VV, Tolkacheva TV, Kondranova AM, Zakharenko AM, Bakina OV. Effect of the Porosity, Roughness, Wettability, and Charge of Micro-Arc Coatings on the Efficiency of Doxorubicin Delivery and Suppression of Cancer Cells. Coatings. 2020; 10(7):664. https://doi.org/10.3390/coatings10070664
Chicago/Turabian StyleSedelnikova, Mariya Borisovna, Ekaterina G. Komarova, Yurii P. Sharkeev, Valentina V. Chebodaeva, Tatiana V. Tolkacheva, Anastasia M. Kondranova, Alexander M. Zakharenko, and Olga V. Bakina. 2020. "Effect of the Porosity, Roughness, Wettability, and Charge of Micro-Arc Coatings on the Efficiency of Doxorubicin Delivery and Suppression of Cancer Cells" Coatings 10, no. 7: 664. https://doi.org/10.3390/coatings10070664
APA StyleSedelnikova, M. B., Komarova, E. G., Sharkeev, Y. P., Chebodaeva, V. V., Tolkacheva, T. V., Kondranova, A. M., Zakharenko, A. M., & Bakina, O. V. (2020). Effect of the Porosity, Roughness, Wettability, and Charge of Micro-Arc Coatings on the Efficiency of Doxorubicin Delivery and Suppression of Cancer Cells. Coatings, 10(7), 664. https://doi.org/10.3390/coatings10070664








