Influence of Molybdenum and Tungsten on the Formation of Zirconium Oxide Coatings on a Steel Base
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Sample Preparation
2.3. Methods
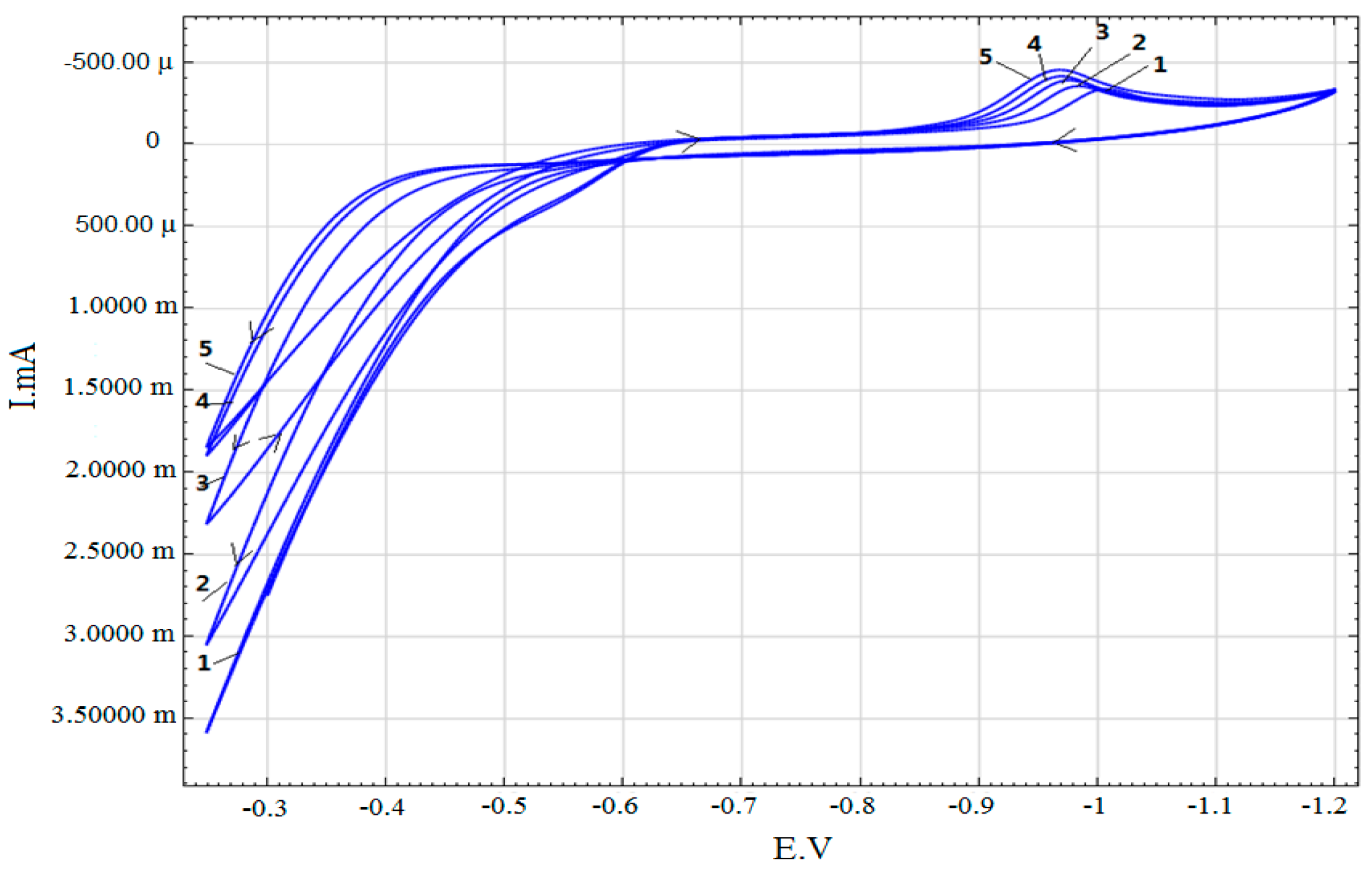
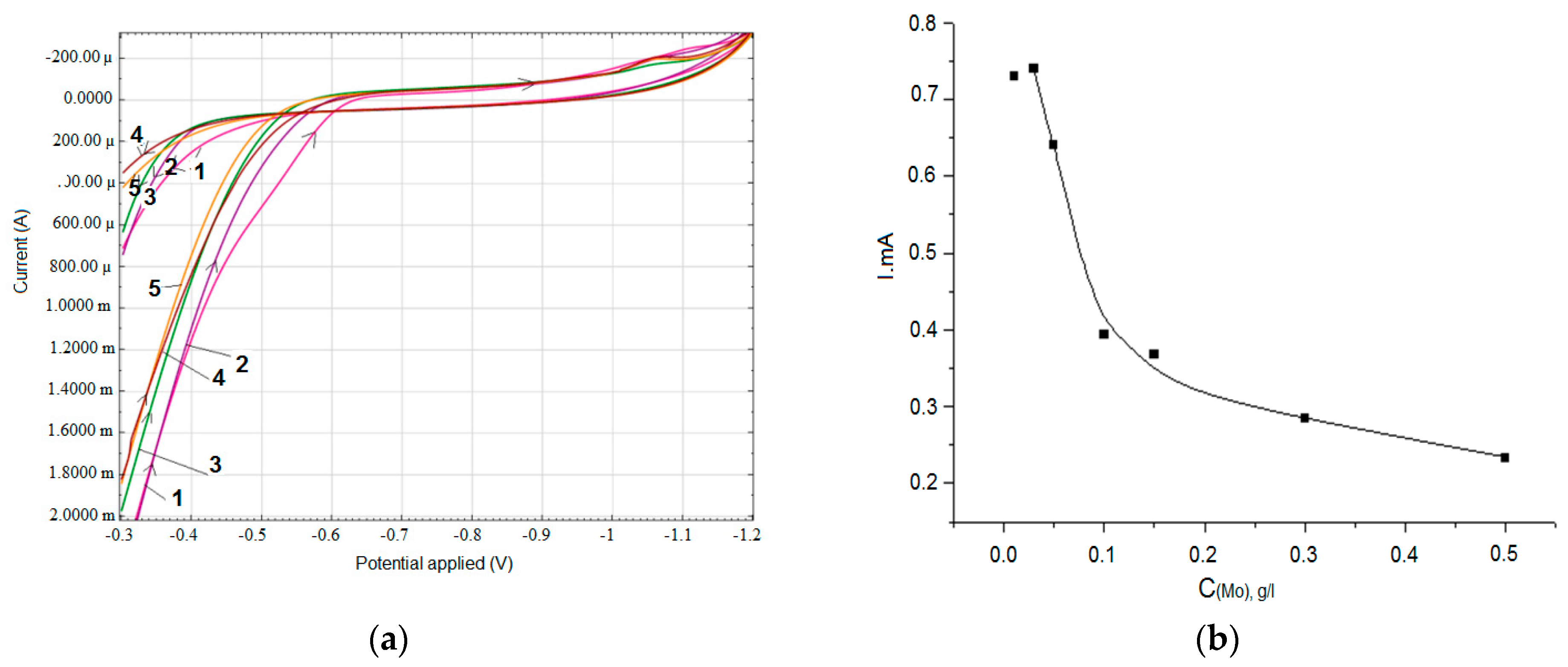
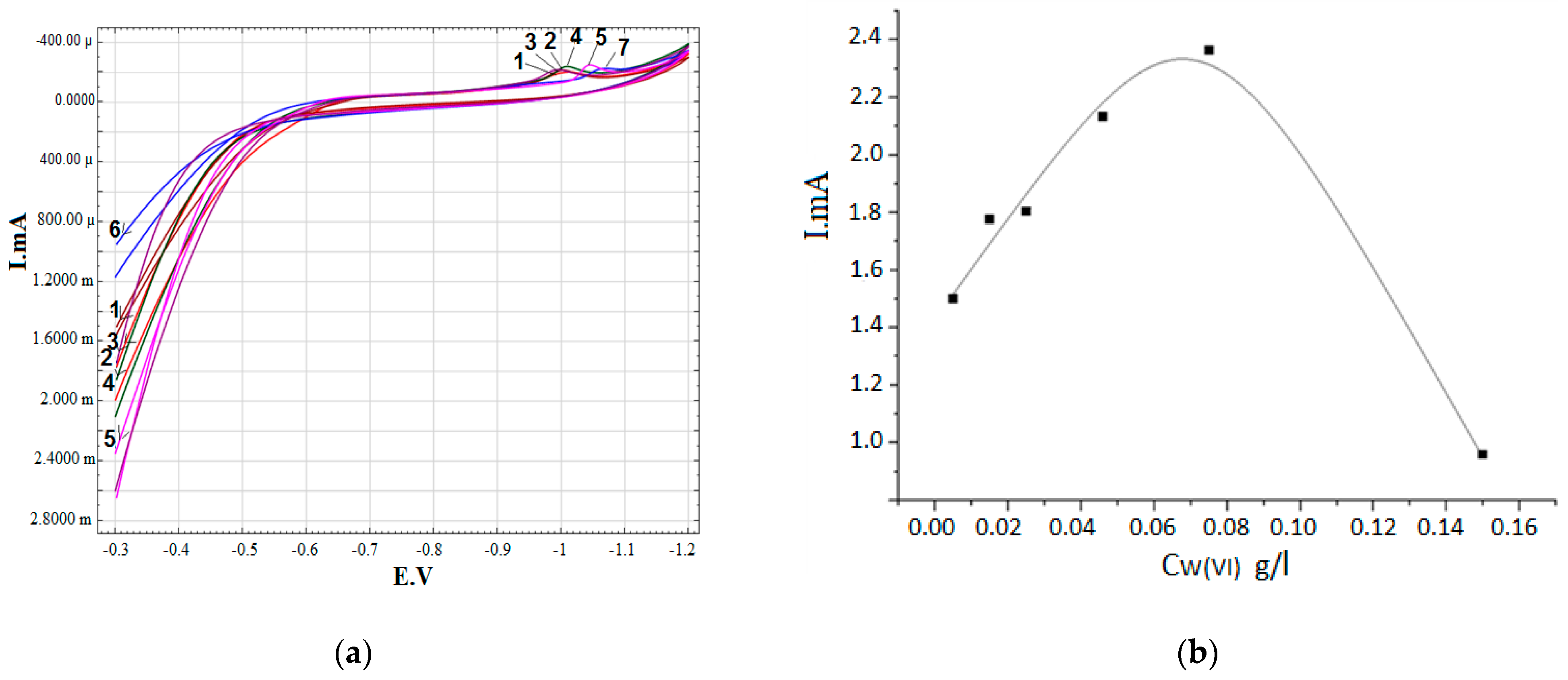
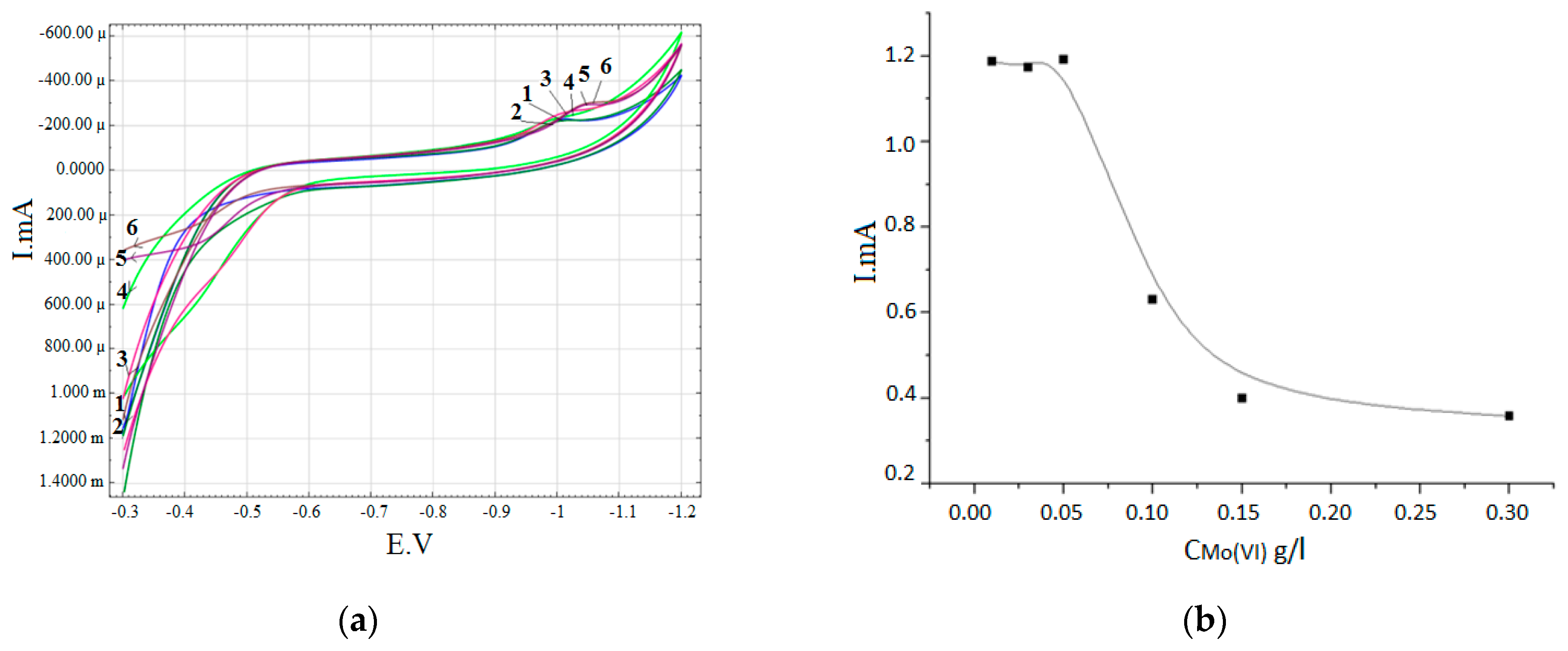
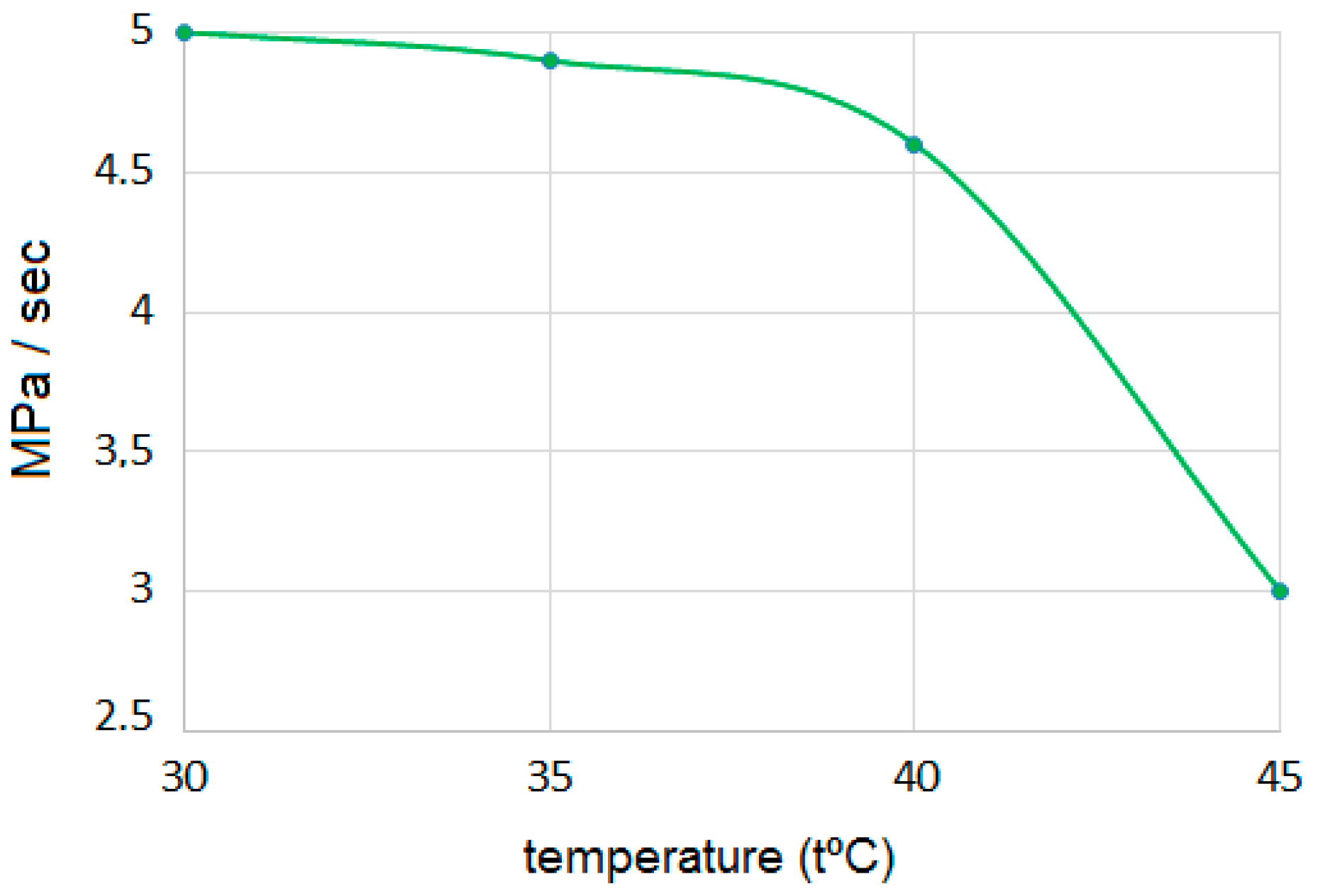
3. Results
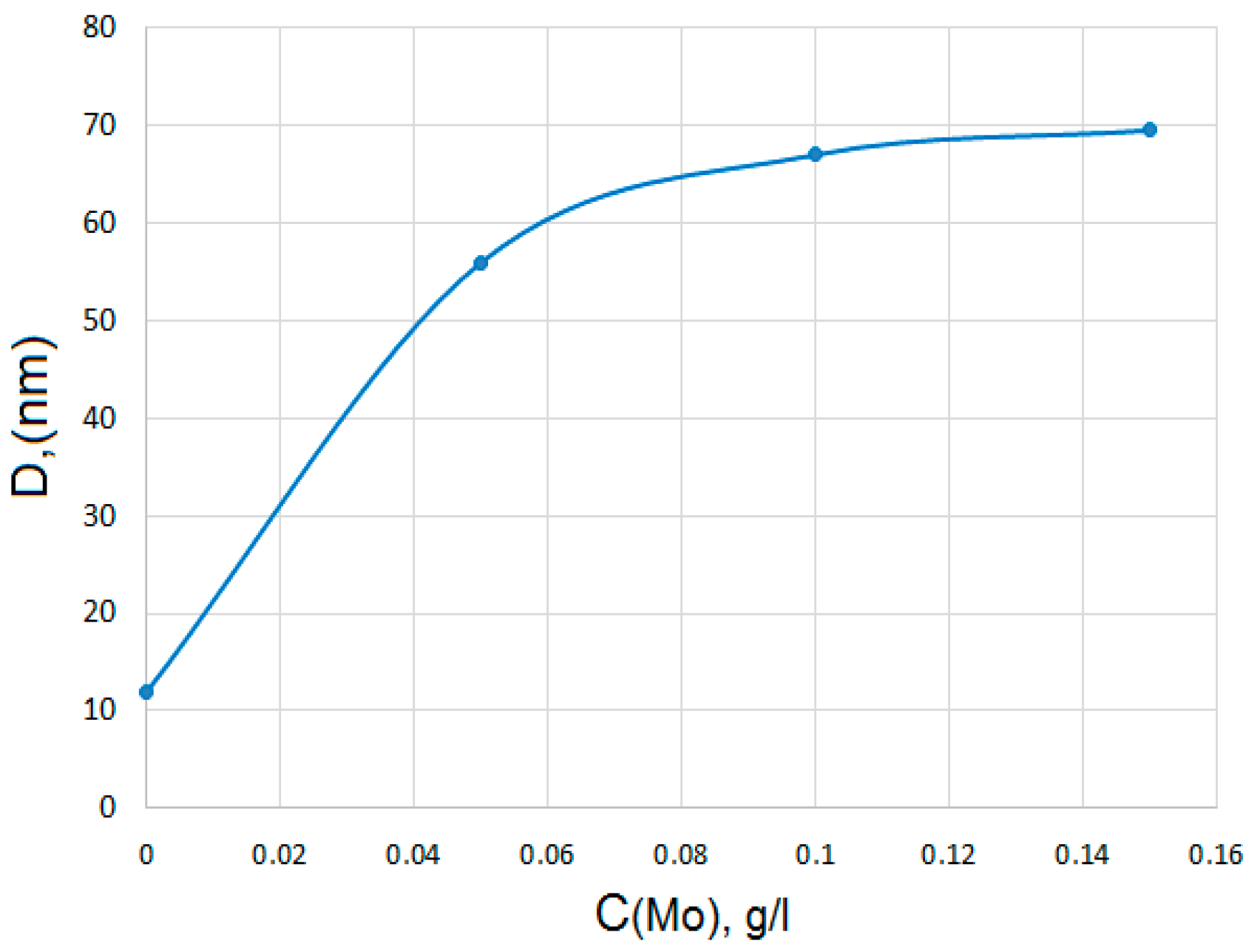
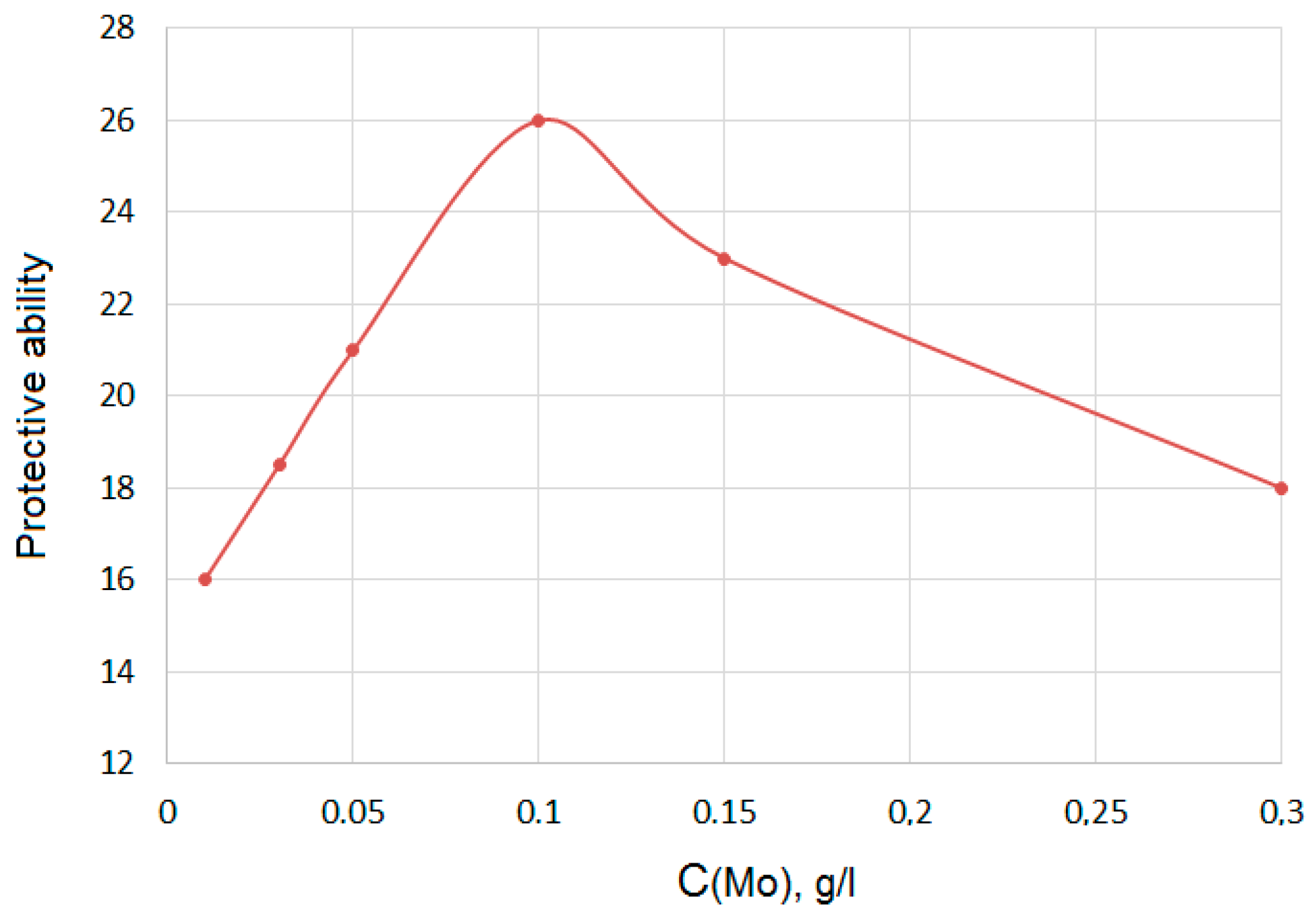
- 0.3 M Na2SO4 + 0.2 g/L Zr (IV) + (0.01–0.2 g/L) Mo (VI)
- 0.3 M Na2SO4 + 0.2 g/L Zr (IV) + 0.15 g/L W (VI)+ (0.01–0.3 g/L) Mo (VI)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kerstnera, E.K.; Kunsta, S.R.; Beltramia, L.V.R.; Vega, M.R.O. Anticorrosive performance of commercial nanoceramic coatings on AISI 1010 steel. Mater. Res. 2014, 17, 1497–1506. [Google Scholar] [CrossRef] [Green Version]
- Yi, A.; Li, W.; Du, J.; Mu, S. Preparation and properties of chrome-free colored Ti/Zr based conversion coating on aluminum alloy. Appl. Surf. Sci. 2012, 258, 5960–5964. [Google Scholar] [CrossRef]
- Cui, C.; Wang, D.; Li, X.; Qu, F.; Xu, T. A room temperature pre-treatment process: Ce3+ doted ZrO2. Nanofilm coated on the cold-rolled plate surface. Adv. Mater. Res. 2012, 347, 147–152. [Google Scholar]
- Zhai, Y.; Zhao, Z.; Frankel, G.S.; Zimmerman, J.; Bryden, T.; Fristad, W. Surface Pretreatment Based on Dilute Hexafluorozirconic Acid. In Proceedings of the 2007 Three-Service Corrosion Conference, Denver, CO, USA, 3–6 December 2007; pp. 1–16. [Google Scholar]
- Adhikaria, S.; Unocica, K.A.; Zhaia, Y.; Frankela, G.S.; Zimmermanb, J.; Fristad, W. Hexafluorozirconic acid based surface pretreatments: Characterization and performance assessment. Electrochim. Acta. 2011, 56, 1912–1924. [Google Scholar] [CrossRef]
- Nela, J.T.; Plessisa, W.D.; Nhlabathia, T.N.; Pretoriusa, C.J.; Jansena, A.A.; Crouse, P.L. Reaction kinetics of the microwave enhanced digestion of zircon with ammonium acid fluoride. J. Fluor. Chem. 2011, 132, 258–262. [Google Scholar] [CrossRef]
- Zhurinov, M.Z.; Statsyuk, V.N.; Fogel, L.A.; Bold, A.; Abrashov, A.A.; Kostiuk, A. Determination of the optimal deposition conditions oxide-zirconium coating on steel base. Rasayan J. Chem. 2019, 12, 1287–1293. [Google Scholar] [CrossRef]
- Fedrizzi, L.; Rodriguez, F.J.; Rossi, S.; Deflorian, F.; Maggio, R.D. The use of electrochemical techniques to study the corrosion behaviour of organic coatings on steel pretreated with sol-gel zirconia films. Electrochim. Acta 2001, 46, 3715–3724. [Google Scholar] [CrossRef]
- Dunham, B.; Chalk, D.D. Non-phosphate transition metal coatings. Clean. Pretreat. Surf. Prep. 2001, 1, 112–118. [Google Scholar]
- Abrashov, A.A.; Grigoryan, N.S.; Vagramyan, T.A.; Meshalkin, V.P.; Kotel’nikova, A.V.; Gribanova, A.A. Protective adhesive zirconium oxide coatings. Prot. Met. Phys. Chem. Surf. 2016, 52, 1170–1174. [Google Scholar] [CrossRef]
- Mohammadloo, H.E.; Sarabi, A.A. Titanium composite conversion coating formation on CRS in the presence of Mo and Ni ions: Electrochemical and microstructure characterizations. Appl. Surf. Sci. 2016, 387, 252–259. [Google Scholar] [CrossRef]
- Shcram, T.; Goeminne, G.; Terryn, H.; Vanhools, W. Anticorrosive coating based on zirconium. Inst. Met. Finish. 1995, 73, 91–97. [Google Scholar]
- Mohammadloo, H.E.; Sarabi, A.A.; Alvani, A.A.S.; Sameie, H.; Salimi, R. Nano-ceramic hexafluorozirconic acid based conversion thin film: Surface characterization and electrochemical study. Surf. Coat. Technol. 2012, 206, 4132–4139. [Google Scholar] [CrossRef]
- Milosev, I.; Frankel, G.S. Review—Conversion coatings based on zirconium and/or titanium. J. Electrochem. Soc. 2018, 165, 127–144. [Google Scholar] [CrossRef]
- Guan, Y.; Liu, J.; Yan, C. Novel Ti/Zr based non-chromium chemical conversion coating for the corrosion protection of electrogalvanized steel. Int. J. Electrochem. Sci. 2011, 6, 4853–4867. [Google Scholar]
- Statsyuk, V.N.; Bold, A.; Zhurinov, M.Z.; Fogel, L.A.; Sassykova, L.R.; Vagramyan, T.A.; Abrashov, A.A. Using cyclic voltammetry to determine the protective ability of phosphate coatings. Funct. Mater. 2020, 27, 605–610. [Google Scholar]
- ASTM B117-11. Standard Practice for Operating Salt Spray (Fog) Apparatus. ASTM International: West Conshohocken, PA, USA, 2011.
- Laha, P.; Schram, T.; Terry, H. Use of spectroscopic ellipsometry to study Zr/Ti film son Al. Surf. Interface Anal. 2002, 34, 677–680. [Google Scholar] [CrossRef]
- State Standard (SS) 9.302-88. ESZKS. Metallic and Nonmetallic Coatings. Methods of Accelerated Corrosion Tests.
- Abrashov, A.; Grigoryan, N.; Vagramyan, T.; Asnis, N. On the mechanism of formation of conversion titanium-containing coatings. Coatings 2020, 10, 328. [Google Scholar] [CrossRef] [Green Version]

| Chemical Element | Content, % |
|---|---|
| Iron (Fe) | 60.8 |
| Chromium (Cr) | 17–19 |
| Nickel (Ni) | 9–11 |
| Manganese (Mn) | ≤2 |
| Silicon (Si) | ≤0.8 |
| Copper (Cu) | ≤0.3 |
| Carbon (C) | ≤0.08 |
| Phosphorus (P) | ≤0.035 |
| Sulfur (S) | ≤0.02 |
| Electrolyte Composition | Ionization Current, mA |
|---|---|
| 0.3 M Na2SO4 + (0.02–1.0 g/L) Zr (IV) | 3.3–1.5 |
| 0.3 M Na2SO4 + 0.2 g/L Zr (IV) + (0.01–0.2 g/L) Mo (VI) | 0.75–0.25 |
| 0.3 M Na2SO4 + 0.2 g/L Zr (IV) + 0.15 g/L W(VI) | 0.9 |
| 0.3 M Na2SO4 + 0.2 g/L Zr (IV) + 0.075 g/L W (VI) + (0.01–0.3 g/L) Mo (VI) | 1.3–0.4 |
| 0.3 M Na2SO4 + 0.2 g/L Zr (IV) + 0.15 g/L W (VI) + (0.01–0.3 g/L) Mo (VI) | 1.2–0.35 |
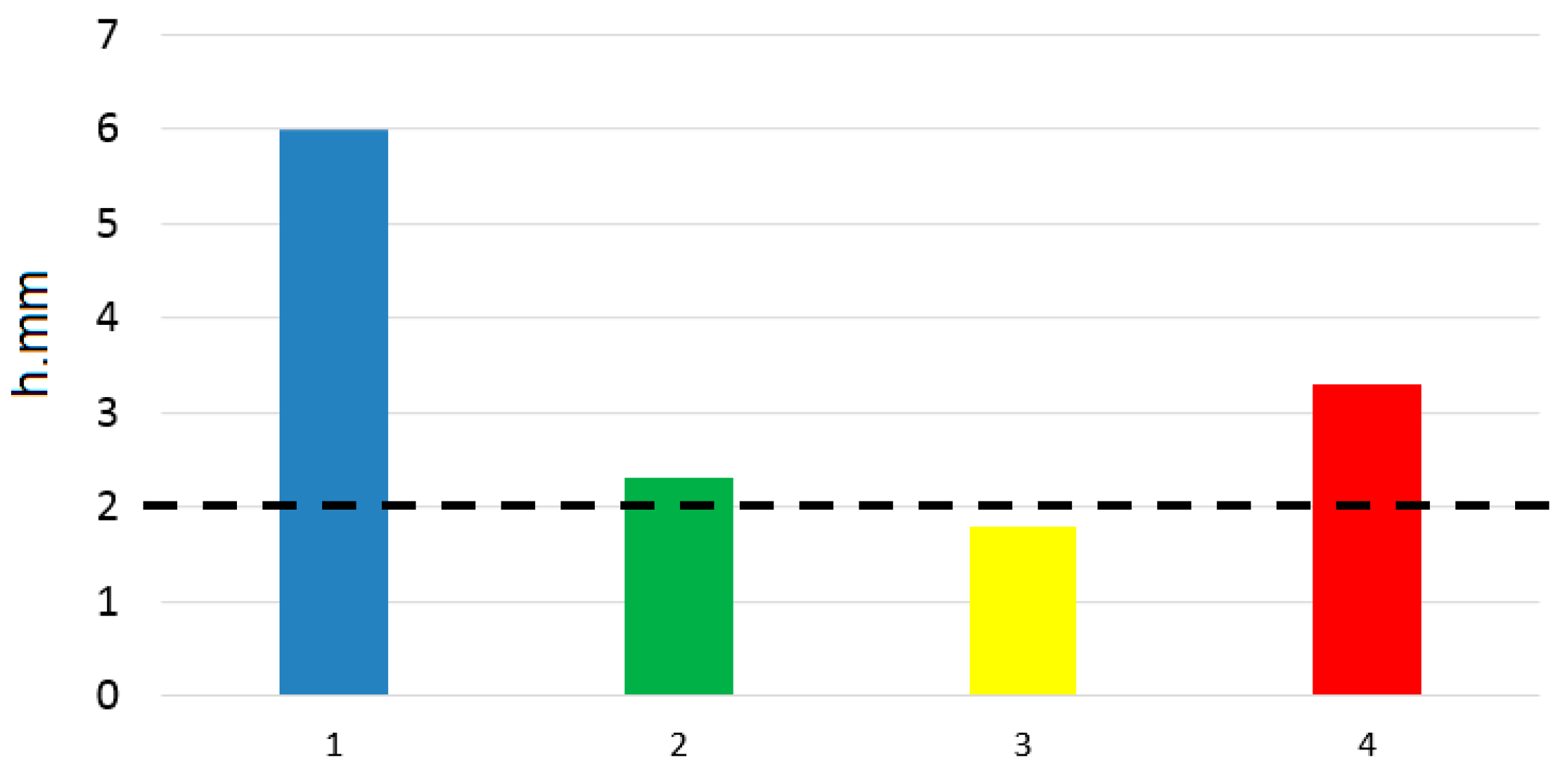
| Concentration Mo (VI), g/L | 0 | 0.05 | 0.1 | 0.15 |
| Depth of corrosive penetration, mm | 6 | 2.3 | 1.8 | 3.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bold, A.; Sassykova, L.; Fogel, L.; Vagramyan, T.; Abrashov, A. Influence of Molybdenum and Tungsten on the Formation of Zirconium Oxide Coatings on a Steel Base. Coatings 2021, 11, 42. https://doi.org/10.3390/coatings11010042
Bold A, Sassykova L, Fogel L, Vagramyan T, Abrashov A. Influence of Molybdenum and Tungsten on the Formation of Zirconium Oxide Coatings on a Steel Base. Coatings. 2021; 11(1):42. https://doi.org/10.3390/coatings11010042
Chicago/Turabian StyleBold, Amangul, Larissa Sassykova, Lidiya Fogel, Tigran Vagramyan, and Aleksey Abrashov. 2021. "Influence of Molybdenum and Tungsten on the Formation of Zirconium Oxide Coatings on a Steel Base" Coatings 11, no. 1: 42. https://doi.org/10.3390/coatings11010042
APA StyleBold, A., Sassykova, L., Fogel, L., Vagramyan, T., & Abrashov, A. (2021). Influence of Molybdenum and Tungsten on the Formation of Zirconium Oxide Coatings on a Steel Base. Coatings, 11(1), 42. https://doi.org/10.3390/coatings11010042







