Synthesis and Characterization of Ce3+-Doped Ni0.5Cd0.5Fe2O4 Nanoparticles by Sol–Gel Auto-Combustion Method
Abstract
:1. Introduction
2. Material Synthesis and Characterizations
3. Results and Discussion
3.1. Structural Analysis
3.2. Morphological Studies and Elemental Composition
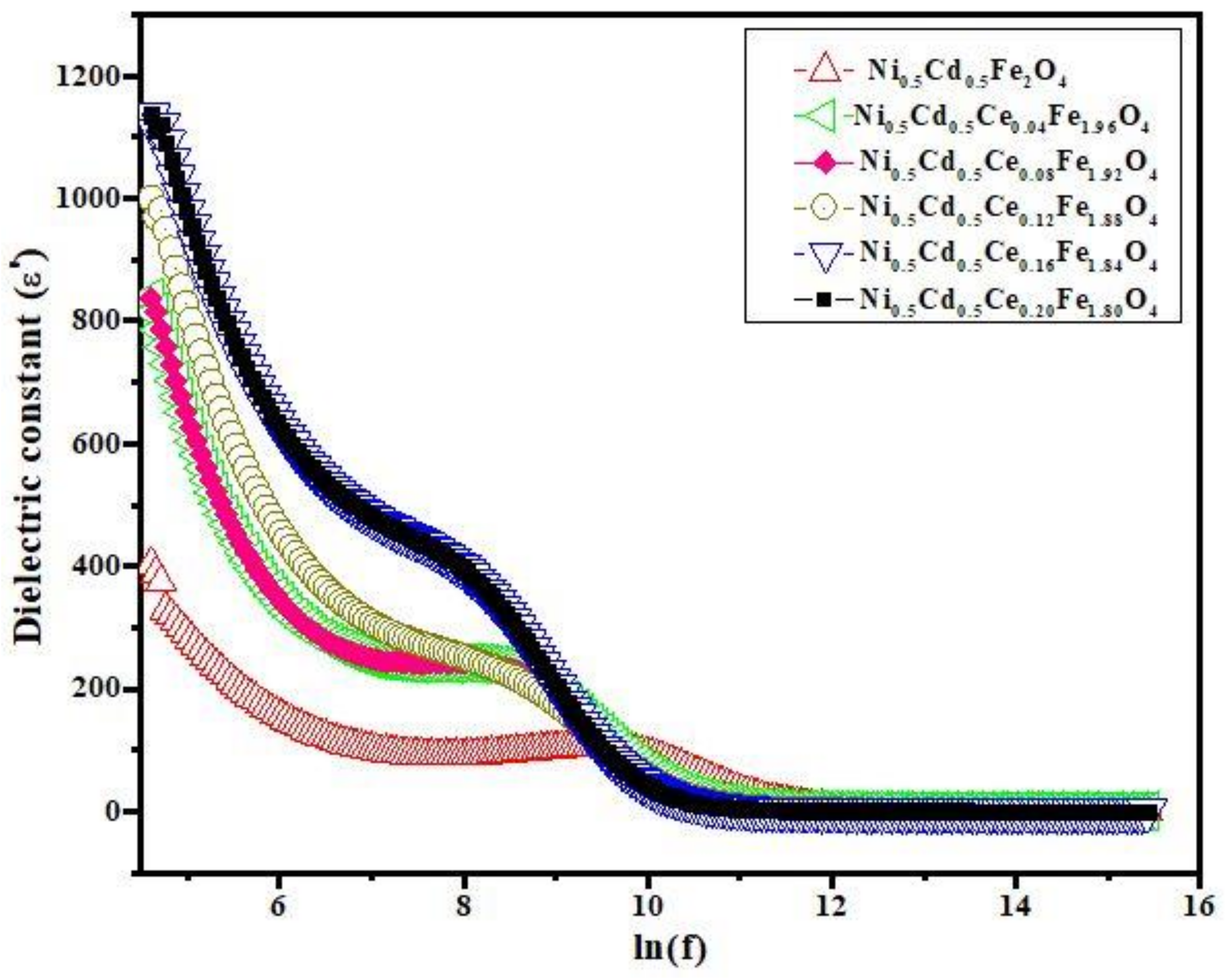
3.3. Dielectric Properties
3.4. Magnetic Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabri, N.G. The outermembrane proteins profile of Salmonella enterica serotypes Enteritidis, Muenster, Florian, Omuna and Noya and their dendrogram analysis. Int. J. Adv. Res. 2013, 2, 182–187. [Google Scholar]
- Atiq, S.; Majeed, M.; Ahmad, A.; Abbaas, S.K.; Saleem, M.; Riaz, S.; Naseem, S. Synthesis and investigation of structural, morphological, magnetic, dielectric and impedance spectroscopic characteristics of Ni-Zn ferrite nanoparticles. Ceram. Int. 2017, 43, 2486–2494. [Google Scholar] [CrossRef]
- Ditta, A.; Khan, M.A.; Junaid, M.; Khalil, R.M.A.; Warsi, M.F. Structural, magnetic and spectral properties of Gd and Dy co-doped dielectrically modified Co-Ni (Ni0.4Co0.6Fe2O4) ferrites. Physica B 2017, 507, 27–34. [Google Scholar] [CrossRef]
- Jalaiah, K.; Babu, K.V. Structural, magnetic and electrical properties of nickel doped Mn-Zn spinel ferrite synthesized by sol-gel method. J. Magn. Magn. Mater. 2017, 423, 275–280. [Google Scholar] [CrossRef]
- Heiba, Z.K.; Mohamed, M.B.; Hamdeh, H.H.; Ahmed, M.A. Structural analysis and cations distribution of nanocrystalline Ni1−xZnxFe1.7Ga0.3O4. J. Alloys Compd. 2015, 618, 755–760. [Google Scholar] [CrossRef]
- Liu, Q.; Lv, L.; Zhou, J.P.; Chen, X.M.; Bian, X.B.; Lu, P.J. Influence of nickel-zinc ratio on microstructure, magnetic and dielectric properties of Ni1−xZnxFe2O4 ferrites. Ceram. Proc. Res. 2012, 13, 110–116. [Google Scholar]
- Mukharjee, S.; Pradip, S.; Mishra, A.K.; Das, D. Zn substituted NiFe2O4 with very high saturation magnetization and negligible dielectric loss synthesized via a soft chemical route. Appl. Phys. A 2014, 114, 389–393. [Google Scholar] [CrossRef]
- Ghasemi, A.; Mousavinia, M. Structural and magnetic evaluation of substituted NiZnFe2O4 particles synthesized by conventional sol–gel method. Ceram. Int. 2014, 40, 2825–2834. [Google Scholar] [CrossRef]
- Wu, X.; Chen, W.; Wu, W.; Li, H.; Lin, C. Structural and Magnetic Properties Evolution of Li-Substituted Co0.5Ni0.5Fe2O4 Ferrite. J. Electron. Mater. 2017, 46, 199–207. [Google Scholar] [CrossRef]
- Xie, J.L.; Han, M.; Chen, L.; Kuang, R.; Deng, L.J. Microwave-absorbing properties of NiCoZn spinel ferrites. Magn. Magn. Mater. 2007, 314, 37–42. [Google Scholar] [CrossRef]
- Olsen, E.; Thonstad, J. Nickel ferrite as inert anodes in aluminium electrolysis: Part I Material fabrication and preliminary testing. J. Appl. Electrochem. 1999, 29, 293–299. [Google Scholar] [CrossRef]
- Berchmans, L.J.; Selvan, R.K.; Augustin, C.O. Evaluation of Mg2+-substituted NiFe2O4 as a green anode material. Mater. Lett. 2004, 58, 1928–1933. [Google Scholar] [CrossRef]
- Sugimoto, M. The past, present, and future of ferrites. J. Am. Ceram. Soc. 1999, 82, 269–279. [Google Scholar] [CrossRef]
- Verwey, J.W.; Heilmann, E.L. Physical properties and cation arrangement of oxides with spinel structures II. Electronic conductivity. J. Chem. Phys. 1947, 15, 181–187. [Google Scholar] [CrossRef]
- Valenzuela, R. Magnetic Ceramics; Cambridge University Press: Cambridge, UK, 2005; Volume 4. [Google Scholar]
- Smit, J.; Wijn, H.P.J. Ferrites, Philips Technical Library; Springer: Eindhoven, The Netherlands, 1959. [Google Scholar]
- Ishaque, M.; Islam, M.U.; Khan, M.A.; Rahman, I.Z.; Genson, A.; Hampshire, S. Structural, electrical and dielectric properties of yttrium substituted nickel ferrites. Phys. B Phys. Condens. Matter 2010, 405, 1532–1540. [Google Scholar] [CrossRef]
- Grigorova, M.; Blythe, H.J.; Blaskov, V.; Rusaniv, V.; Petkov, V.; Masheva, V.; Nitianova, D.; Martinez, L.M.; Mŭnoz, J.S.; Mikhov, M. Magnetic properties and Mössbauer spectra of nanosized CoFe2O4 powders. J. Magn. Magn. Mater. 1998, 183, 163–172. [Google Scholar] [CrossRef]
- Ishino, K.; Narumiya, Y. Development of magnetic ferrites: Control and application of losses. Ceram. Soc. Bull. 1987, 66, 1469–1474. [Google Scholar]
- Maaz, K.; Khalid, W.; Mumtaz, A.; Hasanain, S.K.; Liu, J.; Duan, J.L. Magnetic characterization of Co1−xNixFe2O4 (0 ≤ x ≤ 1) nanoparticles prepared by co-precipitation route. Phys. E Low-Dimens. Syst. Nanostruct. 2009, 41, 593–599. [Google Scholar] [CrossRef]
- Maensiri, S.; Masingboon, C.; Boonchomb, B.; Seraphin, S. A simple route to synthesize nickel ferrite (NiFe2O4) nanoparticles using egg white. Scr. Mater. 2007, 56, 797–800. [Google Scholar] [CrossRef]
- Zhang, D.; Tong, Z.; Xu, G.; Li, S.; Ma, J. Templated fabrication of NiFe2O4 nanorods: Characterization, magnetic and electrochemical properties. Solid State Sci. 2009, 11, 113–117. [Google Scholar] [CrossRef]
- Batoo, K.M.; Ansari, M.S. Low temperature-fired Ni-Cu-Zn ferrite nanoparticles through auto-combustion method for multilayer chip inductor applications. Nanoscale Res. Lett. 2012, 7, 112. [Google Scholar] [CrossRef] [Green Version]
- Nikumbh, A.K.; Pawar, R.A.; Nighot, D.V.; Gugale, G.S.; Sangale, M.D.; Khanvilkar, M.B.; Nagawade, A.V. Structural, electrical, magnetic and dielectric properties of rare-earth substituted cobalt ferrites nanoparticles synthesized by the co-precipitation method. J. Magn. Magn. Mater. 2014, 355, 201–209. [Google Scholar] [CrossRef]
- Sun, G.L.; Li, J.B.; Sun, J.J.; Yang, X.Z. The influences of Zn2+ and some rare-earth ions on the magnetic properties of nickel-zinc ferrites. J. Magn. Magn. Mater. 2004, 281, 173–177. [Google Scholar] [CrossRef]
- Rezlescu, N.; Rezlescu, E.; Pasnicu, C.; Craus, M.L. Effects of the rare-earth ions on some properties of a nickel-zinc ferrite. J. Phys. Condens. Matter. 1994, 6, 5707–5716. [Google Scholar] [CrossRef]
- Hochschild, R.; Fuess, H. Rare-earth doping of nickel zinc ferrites. J. Mater. Chem. 2000, 10, 539–542. [Google Scholar] [CrossRef]
- Kahn, M.L.; Zhang, Z. Synthesis and magnetic properties of CoFe2O4 spinel ferrite nanoparticles doped with lanthanide ions. J. Appl. Phys. Lett. 2001, 78, 3651–3653. [Google Scholar] [CrossRef]
- Jiang, J.; Li, L.C.; Xu, F.; Xie, Y.L. Preparation and magnetic properties of Zn-Cu-Cr- Sm ferrite via a rheological phase reaction method. Mater. Sci. Eng. B 2007, 137, 166–169. [Google Scholar] [CrossRef]
- Jiang, J.; Li, L.C.; Xu, F. Structural analysis and magnetic properties of Gd-doped Li-Ni ferrites prepared by the rheological phase method. J. Rare Earths 2007, 25, 79–83. [Google Scholar] [CrossRef]
- Jiang, J.; Li, L.C.; Xu, F.; Li, Z.T. Structural and magnetic properties of La-doped Li- Ni ferrite. J. Rare Earths 2005, 23, 259–262. [Google Scholar]
- Chand, J.; Singh, M. Electric and dielectric properties of MgGd0.1Fe1.9O4 ferrite. J. Alloys Compd. 2009, 486, 376–379. [Google Scholar] [CrossRef]
- Sattar, A.A.; El-Shokrofy, K.M. Rare earth doping effect on the electrical properties of Cu-Zn ferrites. Le J. De Phys. IV 1997, 7, C1-245–C1-246. [Google Scholar]
- Verma, V.; Kotnala, R.K.; Pandey, V.; Kothari, P.C.; Radhapiyari, L.; Matheru, B.S. The effect on dielectric losses in lithium ferrite by cerium substitution. J. Alloys Compd. 2008, 466, 404–407. [Google Scholar] [CrossRef]
- Xiang, J.; Shen, X.; Zhu, Y. Effects of Ce3+ doping on the structure and magnetic properties of Mn-Zn ferrite fibers. Rare Met. 2009, 28, 151–155. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Zarrintaj, P.; Ganjali, M.R.; Ali, J.A.; Karimzadeh, I.; Aghazadeh, M.; Ghaffari, M.; Saeb, M.R. Curing epoxy with electrochemically synthesized GdxFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105245. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ali, J.A.; Aghazadeh, M.; Formela, K.; Saeb, M.R.; Ranjbar, Z.; Ganjali, M.R. Curing epoxy with electrochemically synthesized ZnxFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105246. [Google Scholar] [CrossRef]
- Sharifi, S.; Yazdani, A.; Rahimi, K. Incremental substitution of Ni with Mn in NiFe2O4 to largely enhance its supercapacitance properties. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Zaman, A.; Uddin, S.; Mehboob, N.; Ali, A.; Ahmad, A.; Bashir, K. Effect of Zr4+ on the structural and microwave dielectric properties of CaTiO3 ceramics. Ferroelectrics 2021, 577, 143–152. [Google Scholar] [CrossRef]
- Zaman, A.; Uddin, S.; Mehboob, N.; Ali, A. Structural investigation and improvement of microwave dielectric properties in Ca (HfxTi1-x)O3 ceramics. Phys. Scr. 2020, 96, 025701. [Google Scholar] [CrossRef]
- Ansari, M.M.N.; Khan, S.; Ahmad, N. Effect of R3+ (R = Pr, Nd, Eu and Gd) substitution on the structural, electrical, magnetic, and optical properties of Mn-ferrite nanoparticles. J. Magn. Magn. Mater. 2018, 465, 81–87. [Google Scholar] [CrossRef]
- Abbas, M.; Ullah, R.; Ullah, K.; Sultana, F.; Mahmood, A.; Mateen, A.; Zhang, Y.; Ali, A.; Althubeiti, K.; Mushtaq, M.; et al. Structural, optical, electrical and dielectric properties of (Sr1-xMgx)(Sn0.5Ti0.5)O3 (X = 0.00, 0.25, 0.50, 0.75) ceramics via solid state route. Ceram. Int. 2021, 47, 30129–30136. [Google Scholar] [CrossRef]
- Koops, C.G. On the dispersion of resistivity and dielectric constant of somesemiconductors at audio frequencies. Phys. Rev. 1951, 83, 121–124. [Google Scholar] [CrossRef]
- Iwamoto, M. Maxwell–Wagner Effect. In Encyclopedia of Nanotechnology; Springer: Dordrecht, The Netherlands, 2015. [Google Scholar]
- Dixit, G.; Singh, J.P.; Chen, C.L.; Dong, C.L.; Srivastava, R.C.; Agrawal, H.M.; Pong, W.F.; Asokan, K. Study of structural, morphological and electrical properties of Ce doped NiFe2O4 nanoparticles and their electronic structure investigation. J. Alloys Compd. 2013, 581, 178–185. [Google Scholar] [CrossRef]
- Reetu; Agarwal, A.; Sanghi, S.; Ashima; Ahlawat, N.; Monica. Phase transformation, dielectric and magnetic properties of Nb doped Bi0.8Sr0.2FeO3 multiferroics. J. Appl. Phys. 2012, 111, 113917. [Google Scholar] [CrossRef]
- George, L.; Viji, C.; Mathew, H.; Mohammed, E.M. Structural, Dielectric, Magnetic and Optical Properties of Cerium Substituted Ni-Zn Mixed Ferrite. Mater. Sci. Res. India 2017, 14, 133–139. [Google Scholar] [CrossRef]
- Hashim, M.; Raghasudha, M.; Meena, S.S.; Shah, J.; Shirsath, S.E.; Kumar, S.; Ravinder, D.; Bhatt, P.; Kumar, R.; Kotnala, R.K. Influence of rare earth ion doping (Ce and Dy) on electrical and magnetic properties of cobalt ferrites. J. Magn. Magn. Mater. 2018, 449, 319–327. [Google Scholar] [CrossRef]





| Samples | Lattice Parameter(a) (Å)3 | Crystallite Size(D) (nm) | Volume (Å)3 | X-ray Density (g/cm3) | Mass Density (g/cm3) | Porosity (P) (%) |
|---|---|---|---|---|---|---|
| Ni0.5Cd0.5Fe2O4 | 8.372 | 21.76 | 586.86 | 5.916 | 3.304 | 31 |
| Ni0.5Cd0.5Ce0.04Fe1.96O4 | 8.385 | 23.99 | 589.74 | 5.962 | 3.381 | 43 |
| Ni0.5Cd0.5Ce0.08Fe1.92O4 | 8.385 | 21.76 | 589.74 | 6.038 | 3.134 | 47 |
| Ni0.5Cd0.5Ce0.12Fe1.88O4 | 8.372 | 20.33 | 586.85 | 6.144 | 3.647 | 41 |
| Ni0.5Cd0.5Ce0.16Fe1.84O4 | 8.381 | 22.92 | 588.77 | 6.201 | 3.051 | 49 |
| Ni0.5Cd0.5Ce0.20Fe1.80O4 | 8.385 | 21.84 | 589.74 | 6.266 | 3.279 | 47 |
| Sample Name | Particles Size (µm) | Deviation in Particle Size (nm) |
|---|---|---|
| Ni0.5Cd0.5Fe2O4 | 0.24 | 1.489 |
| Ni0.5Cd0.5Ce0.04Fe1.96O4 | 0.25 | 5.962 |
| Ni0.5Cd0.5Ce0.08Fe1.92O4 | 0.23 | 2.983 |
| Ni0.5Cd0.5Ce0.12Fe1.88O4 | 0.23 | 2.983 |
| Ni0.5Cd0.5Ce0.16Fe1.84O4 | 0.23 | 2.983 |
| Ni0.5Cd0.5Ce0.20Fe1.80O4 | 0.24 | 1.489 |
| Elements (At %) | Ni0.5Cd0.5CexFe2−xO4 | |||||
|---|---|---|---|---|---|---|
| X = 0 | X = 0.04 | X = 0.08 | X = 0.12 | X = 0.16 | X = 0.20 | |
| O | 24.89 | 23.69 | 27.12 | 21.71 | 28.52 | 28.42 |
| Fe | 60.84 | 57.66 | 53.59 | 52.71 | 45.13 | 44.01 |
| Ni | 12.34 | 12.61 | 12.71 | 12.77 | 12.61 | 11.47 |
| Cd | 1.93 | 2.30 | 1.48 | 4.69 | 2.67 | 1.95 |
| Ce | - | 3.74 | 5.10 | 8.13 | 11.08 | 14.14 |
| Composition | Saturation Magnetization Ms (emu/g) | Remanent Magnetization Mr (emu/g) | Coercivity Hc (Oe) | Remanence Ratio Mr/Ms |
|---|---|---|---|---|
| Ni0.5Cd0.5Fe2O4 | 40.7 | 11.8 | 227 | 0.29 |
| Ni0.5Cd0.5Ce0.04Fe1.96O4 | 41.3 | 9.3 | 174 | 0.23 |
| Ni0.5Cd0.5Ce0.08Fe1.92O4 | 30.5 | 7.3 | 185 | 0.24 |
| Ni0.5Cd0.5Ce0.12Fe1.88O4 | 33.7 | 7.6 | 178 | 0.23 |
| Ni0.5Cd0.5Ce0.16Fe1.84O4 | 26.5 | 5.8 | 170 | 0.22 |
| Ni0.5Cd0.5Ce0.20Fe1.80O4 | 32.2 | 6.8 | 164 | 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, D.; Mehboob, N.; Zaman, A.; Ahmed, N.; Ahmed, K.; Mushtaq, M.; Althubeiti, K.; Ali, A.; Sultana, F.; Bashir, K. Synthesis and Characterization of Ce3+-Doped Ni0.5Cd0.5Fe2O4 Nanoparticles by Sol–Gel Auto-Combustion Method. Coatings 2021, 11, 1156. https://doi.org/10.3390/coatings11101156
Ahmad D, Mehboob N, Zaman A, Ahmed N, Ahmed K, Mushtaq M, Althubeiti K, Ali A, Sultana F, Bashir K. Synthesis and Characterization of Ce3+-Doped Ni0.5Cd0.5Fe2O4 Nanoparticles by Sol–Gel Auto-Combustion Method. Coatings. 2021; 11(10):1156. https://doi.org/10.3390/coatings11101156
Chicago/Turabian StyleAhmad, Danyal, Nasir Mehboob, Abid Zaman, Nabeel Ahmed, Kashif Ahmed, Muhammad Mushtaq, Khaled Althubeiti, Asad Ali, Fozia Sultana, and Khalid Bashir. 2021. "Synthesis and Characterization of Ce3+-Doped Ni0.5Cd0.5Fe2O4 Nanoparticles by Sol–Gel Auto-Combustion Method" Coatings 11, no. 10: 1156. https://doi.org/10.3390/coatings11101156






