Solar-Blind Photodetector Based on NaTaO3/TiO2 Composite Film with Enhanced Photoelectric Performance
Abstract
:1. Introduction
2. Experiment
3. Results and Discussion
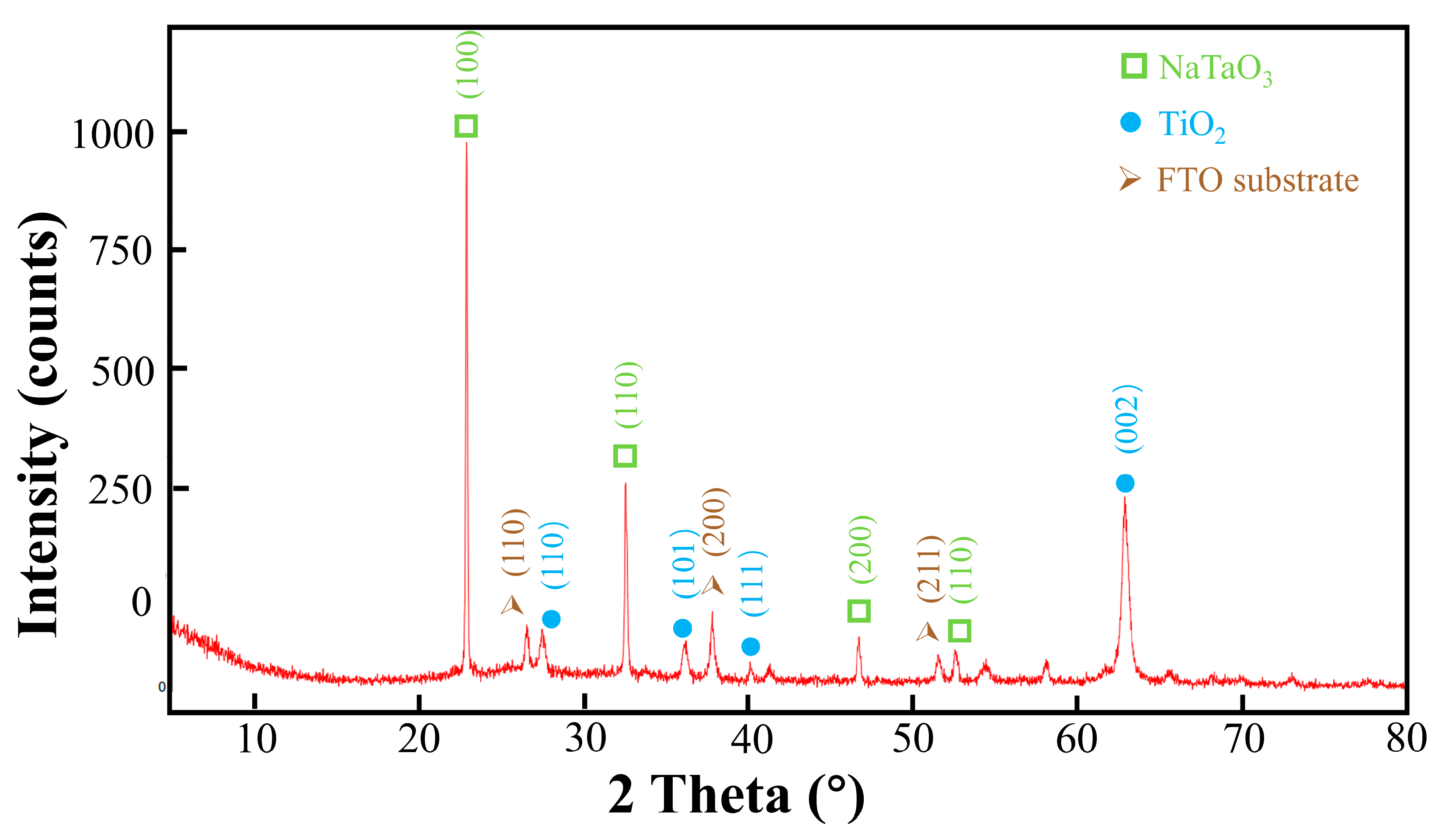
3.1. Structure Analysis
3.2. Morphology Analysis
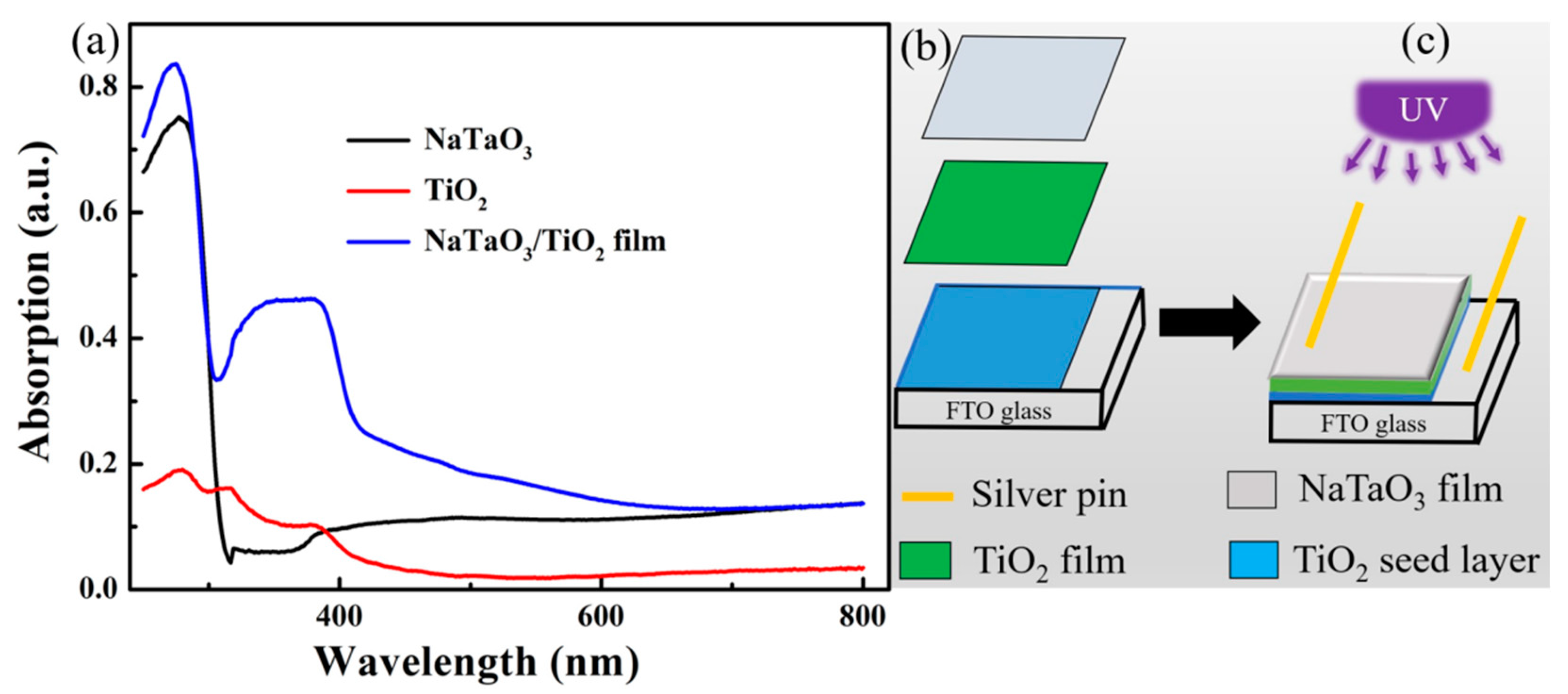
3.3. Absorption Spectrum and Device Structure
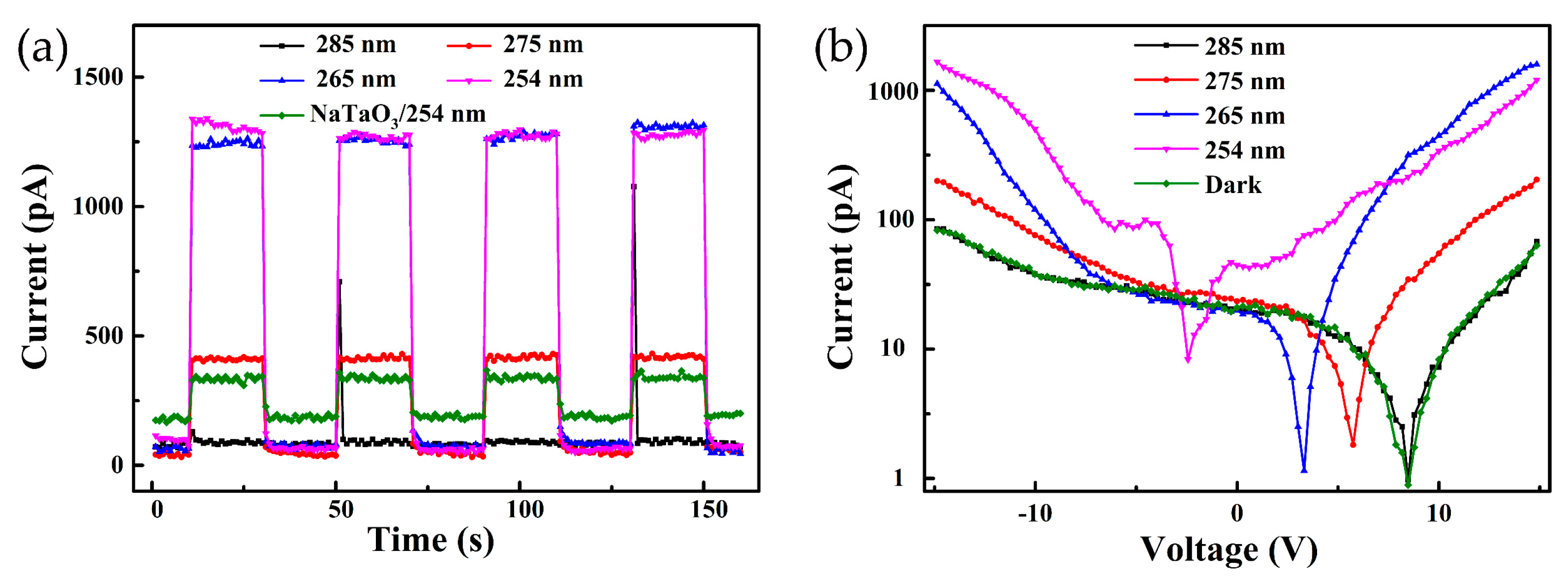
3.4. I–T and I-V Characteristics
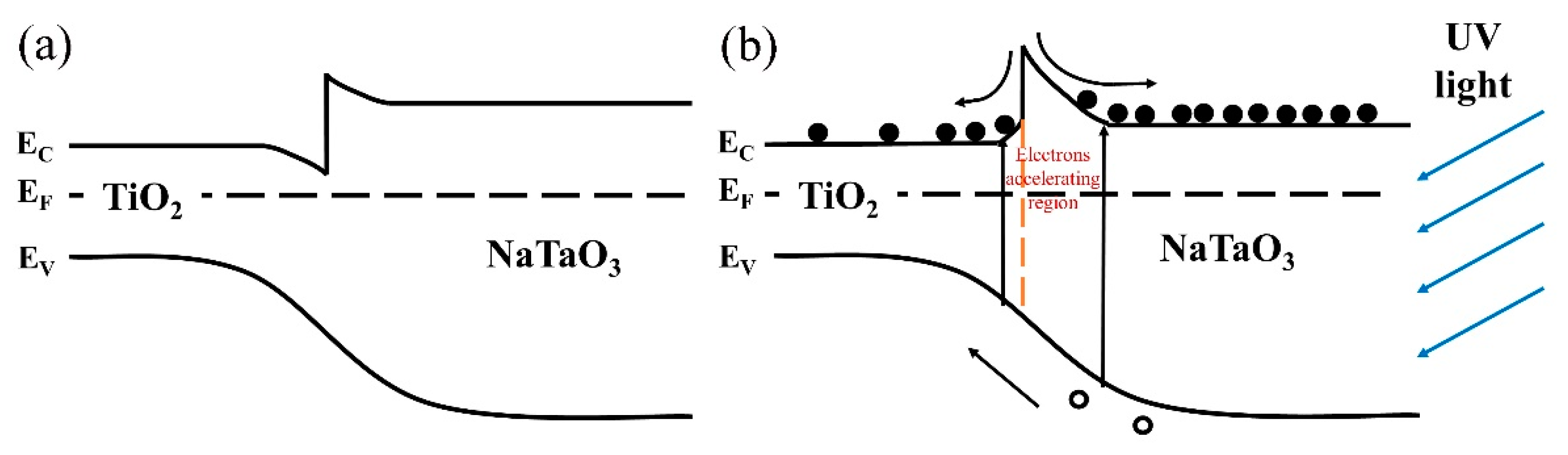
3.5. Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turner, J.; Igoe, D.; Parisi, A.V.; McGonigle, A.J.; Amar, A.; Wainwright, L. A review on the ability of smartphones to detect ultraviolet (UV) radiation and their potential to be used in UV research and for public education purposes. Sci. Total Environ. 2020, 706, 135873–135925. [Google Scholar] [CrossRef] [PubMed]
- Mares, J.W.; Boutwell, C.R.; Scheurer, A.; Falanga, M.; Schoenfeld, W.V. In Cubic ZnxMg1−xO and NixMg1−xO thin films grown by molecular beam epitaxy for deep-UV optoelectronic applications. Proc. SPIE 2010, 7603, 76031B. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, J.; Ye, W.; Li, Y.; Qi, Z.; Dai, J.; Wu, Z.; Chen, C.; Yin, J.; Li, J.; et al. High-performance AlGaN metal–semiconductor–metal solar-blind ultraviolet photodetectors by localized surface plasmon enhancement. Appl. Phys. Lett. 2015, 106, 021112–021117. [Google Scholar] [CrossRef]
- Ren, Q.; Xu, W.; Shen, Z.; You, T.; Liu, Q.; Liu, C.; Zhao, L.; Chen, L.; Yu, W. Solar-blind photodetector based on single crystal Ga2O3 film prepared by a unique ion-cutting process. ACS Appl. Electron. Mater. 2021, 3, 451–460. [Google Scholar] [CrossRef]
- Foisal, A.R.M.; Nguyen, T.; Dinh, T.; Nguyen, T.K.; Tanner, P.; Streed, E.W.; Dao, D.V. 3C-SiC/Si heterostructure: An excellent platform for position-sensitive detectors based on photovoltaic effect. ACS Appl. Mater. Interfaces 2019, 11, 40980–40987. [Google Scholar] [CrossRef]
- Wright, N.G.; Horsfall, A.B. SiC sensors: A review. J. Phys. D Appl. Phys. 2007, 40, 6345–6354. [Google Scholar] [CrossRef]
- Guo, B.; Wu, G.; Chen, H.-Z.; Wang, M. Solution-processed organic/inorganic hybrid photodetector with selective deep UV sensitivity. Org. Electron. 2016, 29, 13–21. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, G.; Zhou, J.; Ma, X. NaTaO3-based ultraviolet photodetector with capacitive efficacy. IEEE Electron Device Lett. 2013, 34, 1539–1541. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, G.; Zhang, D.; Chen, Y.; Wen, S.; Ruan, S. Facile fabrication of NaTaO3 film and its photoelectric properties. J. Alloy. Compd. 2014, 602, 322–325. [Google Scholar] [CrossRef]
- Kato, H.; Kudo, A. Highly efficient decomposition of pure water into H2 and O2 over NaTaO3 photocatalysts. Catal. Lett. 1999, 58, 153–155. [Google Scholar] [CrossRef]
- Kato, H.; Kudo, A. Water Splitting into H2 and O2 on Alkali Tantalate Photocatalysts ATaO3 (A = Li, Na, and K). J. Phys. Chem. B 2001, 105, 4285–4292. [Google Scholar] [CrossRef]
- Shi, J.; Liu, G.; Wang, N.; Li, C. Microwave-assisted hydrothermal synthesis of perovskite NaTaO3 nanocrystals and their photocatalytic properties. J. Mater. Chem. 2012, 22, 18808–18813. [Google Scholar] [CrossRef]
- Ikeda, S.; Fubuki, M.; Takahara, Y.K.; Matsumura, M. Photocatalytic activity of hydrothermally synthesized tantalate pyrochlores for overall water splitting. Appl. Catal. A 2006, 300, 186–190. [Google Scholar] [CrossRef]
- Hu, C.-C.; Teng, H. Influence of structural features on the photocatalytic activity of NaTaO3 powders from different synthesis methods. Appl. Catal. A 2007, 331, 44–50. [Google Scholar] [CrossRef]
- Husin, H.; Chen, H.-M.; Su, W.-N.; Pan, C.-J.; Chuang, W.-T.; Sheu, H.-S.; Hwang, B.-J. Green fabrication of La-doped NaTaO3 via H2O2 assisted sol–gel route for photocatalytic hydrogen production. Appl. Catal. B 2011, 102, 343–351. [Google Scholar] [CrossRef]
- Malankowska, A.; Kobylański, M.P.; Mikolajczyk, A. TiO2 and NaTaO3 Decorated by Trimetallic Au/Pd/Pt Core–Shell Nanoparticles as Efficient Photocatalysts: Experimental and Computational Studies. ACS Sustain. Chem. Eng. 2018, 6, 16665–16682. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, C.; Xu, R.; Yin, B.; Chen, Y.; Zhang, X.; Gao, F.; Ruan, S. The effect of self-depleting in UV photodetector based on simultaneously fabricated TiO2/NiO pn heterojunction and Ni/Au composite electrode. Nanotechnology 2017, 28, 365505–365517. [Google Scholar] [CrossRef]
- Darlington, N.C.; Knight, K.S. High-temperature phases of NaNbO3 and NaTaO3. Acta Crystallogr. 1999, B55, 24–30. [Google Scholar] [CrossRef]
- Kennedy, B.J.; Prodjosantoso, A.K.; Howard, C.J. Powder neutron diffraction study of the high temperature phase transitions in NaTaO3. J. Phys. Condens. Matter 1999, 11, 6319–6327. [Google Scholar] [CrossRef]
- Chen, W.; Kuang, Q.; Xie, Z. Morphology evolution of NaTaO3 submicrometer single-crystals: From cubes to quasi-spheres. Sci. China-Mater. 2015, 58, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Liu, C.; Yin, B.; Xu, R.; Zhou, J.; Zhang, X.; Ruan, S. Organics filled one-dimensional TiO2 nanowires array ultraviolet detector with enhanced photo-conductivity and dark-resistivity. Nanoscale 2017, 9, 9095–9103. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wang, F.; Chen, H.; Wang, Y.; Jiang, M.; Fang, X.; Zhao, D. Solar-Blind avalanche photodetector based on single ZnO–Ga2O3 core–shell microwire. Nano Lett. 2015, 15, 3988–3993. [Google Scholar] [CrossRef]
- Zhang, J.; Jiao, S.; Wang, D.; Ni, S.; Gao, S.; Wang, J. Solar-blind ultraviolet photodetection of an α-Ga2O3 nanorod array based on photoelectrochemical self-powered detectors with a simple, newly-designed structure. J. Mater. Chem. C 2019, 7, 6867–6871. [Google Scholar] [CrossRef]
- Li, K.; Zang, J.; Yang, X.; Tian, Y.; Lin, C.; Sun, P.; Zhang, Z.; Chen, Y.; Shan, C. Solar-Blind position-sensitive detectors fabricated from β-Ga2O3/Polycrystalline diamond heterojunctions. Phys. Status Solidi RRL 2021, 2100347–2100361. [Google Scholar] [CrossRef]
- Kumar, R.C. Current transport in isotype heterojunctions. Int. J. Electron. 1968, 25, 239–247. [Google Scholar] [CrossRef]
- Grant, F.A. Properties of Rutile (Titanium Dioxide). Rev. Mod. Phys. 1959, 31, 646–674. [Google Scholar] [CrossRef]
- Frederikse, H. Recent studies on rutile (TiO2). J. Appl. Phys. 1961, 32, 2211–2215. [Google Scholar] [CrossRef]
- Hu, C.-C.; Teng, H. Structural features of p-type semiconducting NiO as a co-catalyst for photocatalytic water splitting. J. Catal. 2010, 272, 1–8. [Google Scholar] [CrossRef]
- Choi, M.; Oba, F.; Tanaka, I. First-principles study of native defects and lanthanum impurities in NaTaO3. Phys. Rev. B 2008, 78, 014115–014122. [Google Scholar] [CrossRef] [Green Version]
- Asahi, R.; Taga, Y.; Mannstadt, W.; Freeman, A.J. Electronic and optical properties of anatase TiO2. Phys. Rev. B 2000, 61, 7459–7465. [Google Scholar] [CrossRef]
- Li, Z.H.; Chen, G.; Liu, J.W. Electron structure and optical absorption properties of cubic and orthorhombic NaTaO3 by density functional theory. Solid State Commun. 2007, 143, 295–299. [Google Scholar] [CrossRef]
- Kumar, R.C. On the solution of poisson equation for an isotype heterojunction under zero-current condition. Solid-State Electron. 1968, 11, 543–551. [Google Scholar] [CrossRef]
- Cserveny, S.I. Potential distribution and capacitance of abrupt heterojunctions. Int. J. Electron. 1968, 25, 65–80. [Google Scholar] [CrossRef]
- Li-Sheng, Y.; Cun-Da, W. Properties of p-N and n-N heterointerfaces in GaAs-AlxGa1−xAs heterostructure LED’s. IEEE Trans. Electron Devices 1983, 30, 326–329. [Google Scholar] [CrossRef]
- Oldham, W.G.; Milnes, A.G. n-n Semiconductor heterojunctions. Solid-State Electron. 1963, 6, 121–132. [Google Scholar] [CrossRef]
- Sze, S.M.; Ng, K.K. (Eds.) Physics of Semiconductor Devices, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Wu, X.; Yin, S.; Dong, Q.; Sato, T. Preparation and visible light induced photocatalytic activity of C-NaTaO3 and C-NaTaO3-Cl-TiO2 composite. Phys. Chem. Chem. Phys. 2013, 15, 20633–20640. [Google Scholar] [CrossRef]
- Kong, X.; Liu, C.; Dong, W.; Zhang, X.; Tao, C.; Shen, L.; Zhou, J.; Fei, Y.; Ruan, S. Metal-semiconductor-metal TiO2 ultraviolet detectors with Ni electrodes. Appl. Phys. Lett. 2009, 94, 123502. [Google Scholar] [CrossRef] [Green Version]


| Wavelength (nm) | Light Intensity (mW/cm2) | Responsivity (mA/W) |
|---|---|---|
| 254 | 1.02 | 1.29 |
| 265 | 0.52 | 1.65 |
| 275 | 0.43 | 0.22 |
| 285 | 0.01 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Li, Z.; Zhao, Y.; Wu, Z.; Zhang, J.; Yang, L.; Wang, S.; Li, S. Solar-Blind Photodetector Based on NaTaO3/TiO2 Composite Film with Enhanced Photoelectric Performance. Coatings 2021, 11, 1178. https://doi.org/10.3390/coatings11101178
Zhang M, Li Z, Zhao Y, Wu Z, Zhang J, Yang L, Wang S, Li S. Solar-Blind Photodetector Based on NaTaO3/TiO2 Composite Film with Enhanced Photoelectric Performance. Coatings. 2021; 11(10):1178. https://doi.org/10.3390/coatings11101178
Chicago/Turabian StyleZhang, Min, Zhenjiang Li, Yunfei Zhao, Zhaofeng Wu, Jun Zhang, Linyu Yang, Shuying Wang, and Shiqing Li. 2021. "Solar-Blind Photodetector Based on NaTaO3/TiO2 Composite Film with Enhanced Photoelectric Performance" Coatings 11, no. 10: 1178. https://doi.org/10.3390/coatings11101178






