A Review on Graphene Based Materials and Their Antimicrobial Properties
Abstract
1. Introduction
2. Preparation Methods of Graphene from Waste and Bioprecursors
2.1. Significance of Preparation of Graphene from Bioresources
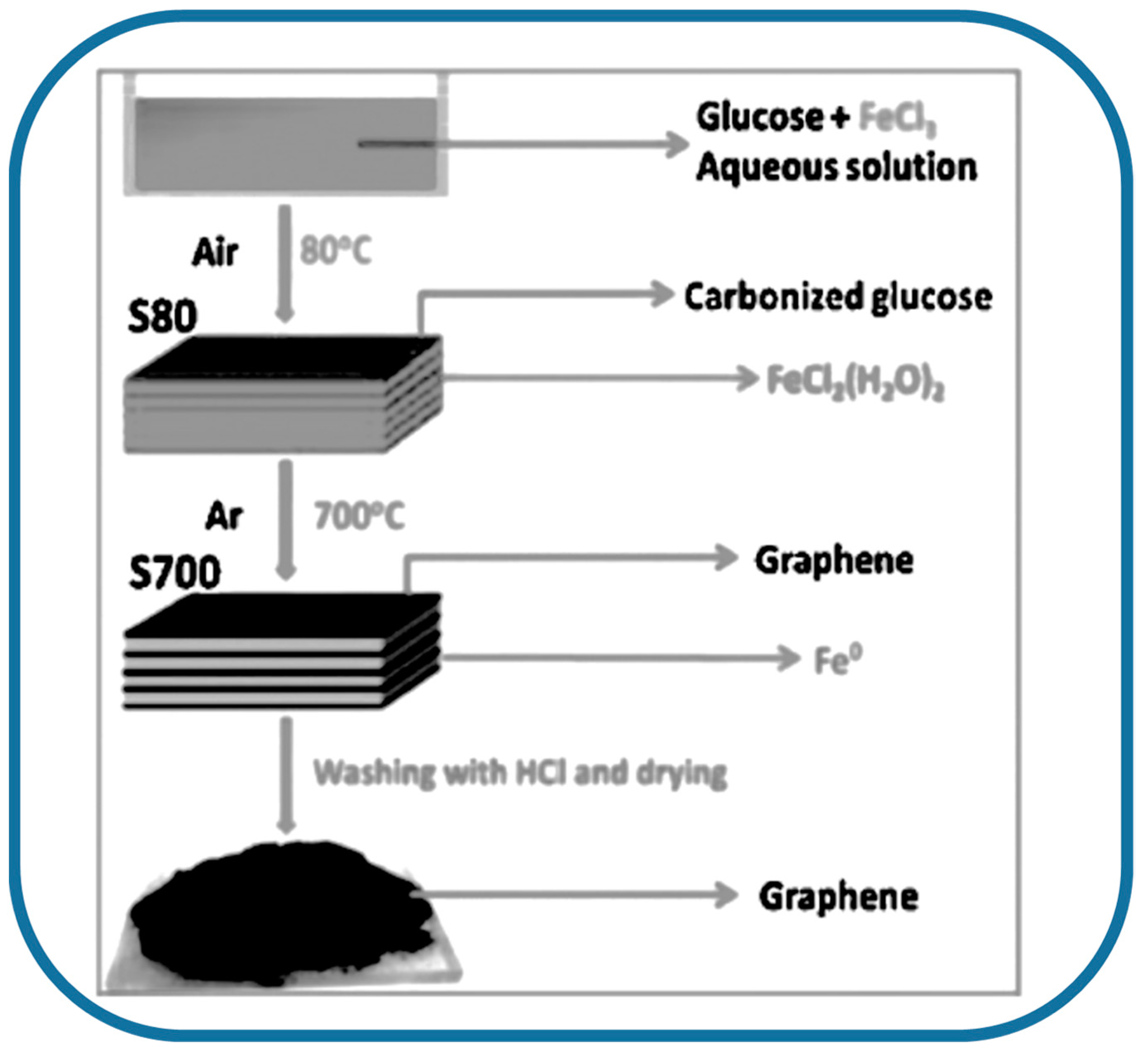
2.2. Different Bioprecursors
3. Antimicrobial Mechanism of Graphene Materials
Antibacterial Activity Mechanism of Graphene-Based Materials (GBMs)

4. Factors Affecting the Antibacterial Activity of Graphene and Its Derivatives
4.1. Bacteria Shape and Type
4.2. Number of Layers (Graphene)
4.3. Graphene Sheet Size
4.4. Concentration of Graphene-Based Materials
5. Antimicrobial Applications of Graphene and Its Composites
5.1. Graphene-Based Antimicrobial Hydrogels
5.2. Packaging with Antimicrobial Ability
5.3. Wound Dressing and Bandages
5.4. Antimicrobial Films and Coatings
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Seiji, H. Adverse effects of antimicrobial agents: The mechanisms of their concentration-dependent effects. Jpn. J. Chemother 2004, 52, 293–303. [Google Scholar]
- Anand, A.; Unnikrishnan, B.; Wei, S.-C.; Chou, C.P.; Zhang, L.-Z.; Huang, C.-C. Graphene oxide and carbon dots as broad-spectrum antimicrobial agents—A minireview. Nanoscale Horiz. 2019, 4, 117–137. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. HHS public access mechanisms of antibiotic resistance. Microbiol. Spectr. 2016, 4, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-Z.; Mu, C.; Zhu, W.; Yan, X.; Hu, X.; Yang, J. Flexible polyurethane composites prepared by incorporation of polyethylenimine-modified slightly reduced graphene oxide. Carbon 2016, 98, 432–440. [Google Scholar] [CrossRef]
- Yaragalla, S.; Rajendran, R.; AlMaadeed, M.A.; Kalarikkal, N.; Thomas, S. Chemical modification of graphene with grape seed extract: Its structural, optical and antimicrobial properties. Mater. Sci. Eng. C 2019, 102, 305–314. [Google Scholar] [CrossRef]
- Yaragalla, S.; Rajendran, R.; Jose, J.; AlMaadeed, M.A.; Kalarikkal, N.; Thomas, S. Preparation and characterization of green graphene using grape seed extract for bioapplications. Mater. Sci. Eng. C 2016, 65, 345–353. [Google Scholar] [CrossRef]
- Yaragalla, S. Preparation of epoxy graphene and its structural and optical properties. Adv. Mater. Lett. 2015, 6, 848–852. [Google Scholar] [CrossRef]
- Yazyev, O.V.; Louie, S.G. Electronic transport in polycrystalline graphene. Nat. Mater. 2010, 9, 806–809. [Google Scholar] [CrossRef] [PubMed]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Yaragalla, S.; Dussoni, S.; Zahid, M.; Maggiali, M.; Metta, G.; Athanasiou, A.; Bayer, I.S. Stretchable graphene and carbon nanofiber capacitive touch sensors for robotic skin applications. J. Ind. Eng. Chem. 2021, 101, 348–358. [Google Scholar] [CrossRef]
- Pop, E.; Varshney, V.; Roy, A.K. Thermal properties of graphene: Fundamentals and applications. MRS Bull. 2012, 37, 1273–1281. [Google Scholar] [CrossRef]
- Zarafu, I.; Turcu, I.; Culiță, D.; Petrescu, S.; Popa, M.; Chifiriuc, M.C.; Limban, C.; Telehoiu, A.; Ioniță, P. Antimicrobial features of organic functionalized graphene-oxide with selected amines. Materials 2018, 11, 1704. [Google Scholar] [CrossRef]
- Pulingam, T.; Thong, K.L.; Ali, M.E.; Appaturi, J.N.; Dinshaw, I.J.; Ong, Z.Y.; Leo, B.F. Graphene oxide exhibits differential mechanistic action towards Gram-positive and Gram-negative bacteria. Colloids Surf. B Biointerfaces 2019, 181, 6–15. [Google Scholar] [CrossRef]
- Jia, X.; Ahmad, I.; Yang, R.; Wang, C. Versatile graphene-based photothermal nanocomposites for effectively capturing and killing bacteria, and for destroying bacterial biofilms. J. Mater. Chem. B 2017, 5, 2459–2467. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, K.; Veerapandian, M.; Zhang, L.-H.; Yun, K.; Kim, S.J. Antibacterial efficiency of graphene nanosheets against pathogenic bacteria via lipid peroxidation. J. Phys. Chem. C 2012, 116, 17280–17287. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Ye, X.; Hu, K.; Zhong, H.; Yuan, X.; Xiong, H.; Guo, Z. A facile one-pot method to two kinds of graphene oxide-based hydrogels with broad-spectrum antimicrobial properties. Chem. Eng. J. 2015, 260, 331–337. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.B.; Evmenenko, G.; Nguyen, S.T.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Wen, S.; Chen, Y.; Zhang, F.; Panine, P.; Chan, T.W.; Zhang, L.; Liang, Y.; Liu, L. High performance graphene oxide based rubber composites. Sci. Rep. 2013, 3, 2508. [Google Scholar] [CrossRef]
- Yaragalla, S.; Mishra, R.K.; Thomas, S.; Kalarikkal, N.; Maria, H.J. Carbon-Based Nanofillers and Their Rubber Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Ponnamma, D.; Jose Chirayil, C.; Sadasivuni, K.K.; Somasekharan, L.; Yaragalla, S.; Abraham, J.; Thomas, S. Special Purpose Elastomers: Synthesis, Structure-Property Relationship, Compounding, Processing and Applications; Springer: Berlin/Heidelberg, Germany, 2013; pp. 47–82. [Google Scholar]
- Yaragalla, S.; Zahid, M.; Panda, J.K.; Tsagarakis, N.; Cingolani, R.; Athanassiou, A. Comprehensive enhancement in thermomechanical performance of melt-extruded peek filaments by graphene incorporation. Polymers 2021, 13, 1425. [Google Scholar] [CrossRef]
- Kommu, A.; Singh, J.K. A review on graphene-based materials for removal of toxic pollutants from wastewater. Soft Mater. 2020, 18, 297–322. [Google Scholar] [CrossRef]
- Suvarnaphaet, P.; Pechprasarn, S. Graphene-based materials for biosensors: A review. Sensors 2017, 17, 2161. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhao, T.S. Graphene sheets fabricated from disposable paper cups as a catalyst support material for fuel cells. J. Mater. Chem. A 2013, 1, 183–187. [Google Scholar] [CrossRef]
- Le Van, K.; Luong, T.T.T. Activated carbon derived from rice husk by NaOH activation and its application in supercapacitor. Prog. Nat. Sci. Mater. Int. 2014, 24, 191–198. [Google Scholar] [CrossRef]
- Seitzhanova, M.A.; Mansurov, Z.A.; Yeleuov, M.; Roviello, V.; Di Capua, R. The characteristics of graphene obtained from rice husk and graphite. Eurasian Chem. J. 2019, 21, 149. [Google Scholar] [CrossRef]
- Kumar, M.; Sachdeva, A.; Garg, R.K.; Singh, S. Synthesis and characterization of graphene prepared from rice husk by a simple microwave process. Nano Hybrids Compos. 2020, 29, 74–83. [Google Scholar] [CrossRef]
- Seitzhanova, M.; Chenchik, D.; Yeleuov, M.; Mansurov, Z.A.; Di Capua, R.; Elibaeva, N.S. Synthesis and characterization of graphene layers from rice husks. Chem. Bull. Kazakh Natl. Univ. 2018, 89, 12–18. [Google Scholar] [CrossRef][Green Version]
- Muramatsu, H.; Kim, Y.A.; Hayashi, T. Synthesis and characterization of graphene from rice husks. Carbon 2017, 114, 750. [Google Scholar] [CrossRef]
- Bakar, R.A.; Yahya, R.; Gan, S.N. Production of high purity amorphous silica from rice husk. Procedia Chem. 2016, 19, 189–195. [Google Scholar] [CrossRef]
- Liu, N.; Huo, K.; McDowell, M.T.; Zhao, J.; Cui, Y. Rice husks as a sustainable source of nanostructured silicon for high performance Li-ion battery anodes. Sci. Rep. 2013, 3, 1919. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, J.; Zhang, X.; Li, N.; Liu, B.; Li, Y.; Wang, Y.; Wang, W.; Li, Y.; Zhang, L.; et al. Large-scale and controllable synthesis of graphene quantum dots from rice husk biomass: A comprehensive utilization strategy. ACS Appl. Mater. Interfaces 2016, 8, 1434–1439. [Google Scholar] [CrossRef]
- Muramatsu, H.; Kim, Y.A.; Yang, K.-S.; Cruz-Silva, R.; Toda, I.; Yamada, T.; Terrones, M.; Endo, M.; Hayashi, T.; Saitoh, H. Rice husk-derived graphene with nano-sized domains and clean edges. Small 2014, 10, 2766–2770. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ogata, H.; Morimoto, S.; Ortiz-Medina, J.; Fujishige, M.; Takeuchi, K.; Muramatsu, H.; Hayashi, T.; Terrones, M.; Hashimoto, Y.; et al. Nanocarbons from rice husk by microwave plasma irradiation: From graphene and carbon nanotubes to graphenated carbon nanotube hybrids. Carbon 2015, 94, 479–484. [Google Scholar] [CrossRef]
- Wang, F.; Liu, L.; Li, W.J. Graphene-based glucose sensors: A brief review. IEEE Trans. Nanobioscience 2015, 14, 818–834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Song, J.; Yang, G.; Han, B. Large-scale production of high-quality graphene using glucose and ferric chloride. Chem. Sci. 2014, 5, 4656–4660. [Google Scholar] [CrossRef]
- Li, X.-H.; Kurasch, S.; Kaiser, U.; Antonietti, M. Synthesis of monolayer-patched graphene from glucose. Angew. Chemie Int. Ed. 2012, 51, 9689–9692. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, Z.; Kohandehghan, A.; Li, Z.; Cui, K.; Tan, X.; Stephenson, T.J.; King’ondu, C.K.; Holt, C.M.B.; Olsen, B.C.; et al. Interconnected carbon nanosheets derived from hemp for ultrafast supercapacitors with high energy. ACS Nano 2013, 7, 5131–5141. [Google Scholar] [CrossRef]
- Hegab, H.M.; ElMekawy, A.; Zou, L.; Mulcahy, D.; Saint, C.P.; Ginic-Markovic, M. The controversial antibacterial activity of graphene-based materials. Carbon 2016, 105, 362–376. [Google Scholar] [CrossRef]
- Szunerits, S.; Boukherroub, R. Antibacterial activity of graphene-based materials. J. Mater. Chem. B 2016, 4, 6892–6912. [Google Scholar] [CrossRef]
- Djurabekova, F.; Kotomin, E.; Ridgway, M.C.; Sobolev, N.A. Defect-induced effects in nanomaterials. Phys. Status. Solidi. 2015, 12, 9. [Google Scholar] [CrossRef]
- de Heer, W.A. The invention of graphene electronics and the physics of epitaxial graphene on silicon carbide. Phys. Scr. 2012, T146, 014004. [Google Scholar] [CrossRef]
- Tu, Y.; Lv, M.; Xiu, P.; Huynh, T.; Zhang, M.; Castelli, M.; Liu, Z.; Huang, Q.; Fan, C.; Fang, H.; et al. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat. Nanotechnol. 2013, 8, 594–601. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, L.; Li, M.; Zhao, R.; Yang, X.; Ji, T.; Gu, Z.; Yin, J.-J.; Gao, X.; Nie, G. Deciphering the underlying mechanisms of oxidation-state dependent cytotoxicity of graphene oxide on mammalian cells. Toxicol. Lett. 2015, 237, 61–71. [Google Scholar] [CrossRef]
- Hernandez, V.; Crépin, T.; Palencia, A.; Cusack, S.; Akama, T.; Baker, S.J.; Wei, B.; Feng, L.; Freund, Y.R.; Liu, L.; et al. Discovery of a novel class of boron-based antibacterials with activity against gram-negative bacteria. Antimicrob. Agents Chemother. 2013, 57, 1394–1403. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against Bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.Y.; Laurent, S.; Chen, W.; Akhavan, O.; Imani, M.; Ashkarran, A.A.; Mahmoudi, M. Graphene: Promises, facts, opportunities, and challenges in nanomedicine. Chem. Rev. 2013, 113, 3407–3424. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Gao, H. Cell interaction with graphene microsheets: Near-orthogonal cutting versus parallel attachment. Nanoscale 2015, 7, 5457–5467. [Google Scholar] [CrossRef] [PubMed]
- Dallavalle, M.; Calvaresi, M.; Bottoni, A.; Melle-Franco, M.; Zerbetto, F. Graphene can wreak havoc with cell membranes. ACS Appl. Mater. Interfaces 2015, 7, 4406–4414. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.H.; Truong, V.K.; Quinn, M.D.J.; Notley, S.M.; Guo, Y.; Baulin, V.A.; al Kobaisi, M.; Crawford, R.J.; Ivanova, E.P. Graphene induces formation of pores that kill spherical and rod-shaped bacteria. ACS Nano 2015, 9, 8458–8467. [Google Scholar] [CrossRef] [PubMed]
- Luan, B.; Huynh, T.; Zhao, L.; Zhou, R. Potential toxicity of graphene to cell functions via disrupting protein–protein interactions. ACS Nano 2015, 9, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Mangadlao, J.D.; Santos, C.M.; Felipe, M.J.L.; de Leon, A.C.C.; Rodrigues, D.F.; Advincula, R.C. On the antibacterial mechanism of graphene oxide (GO) Langmuir–Blodgett films. Chem. Commun. 2015, 51, 2886–2889. [Google Scholar] [CrossRef]
- Shi, L.F.; Liu, J.Z.; Yang, J.H.; Cai, L.F.; Shi, L.Y.; Qiu, H.X. Langmuir-Blodgett assembly of transparent graphene oxide-silver microwire hybrid films with an antibacterial property. New Carbon Mater. 2017, 32, 344–351. [Google Scholar] [CrossRef]
- Hui, L.; Piao, J.-G.; Auletta, J.; Hu, K.; Zhu, Y.; Meyer, T.; Liu, H.; Yang, L. Availability of the basal planes of graphene oxide determines whether it is antibacterial. ACS Appl. Mater. Interfaces 2014, 6, 13183–13190. [Google Scholar] [CrossRef]
- Xia, M.-Y.; Xie, Y.; Yu, C.-H.; Chen, G.-Y.; Li, Y.-H.; Zhang, T.; Peng, Q. Graphene-based nanomaterials: The promising active agents for antibiotics-independent antibacterial applications. J. Control. Release 2019, 307, 16–31. [Google Scholar] [CrossRef]
- Liu, S.; Hu, M.; Zeng, T.H.; Wu, R.; Jiang, R.; Wei, J.; Wang, L.; Kong, J.; Chen, Y. Lateral dimension-dependent antibacterial activity of graphene oxide sheets. Langmuir 2012, 28, 12364–12372. [Google Scholar] [CrossRef]
- Bondarenko, O.; Ivask, A.; Käkinen, A.; Kurvet, I.; Kahru, A. Particle-cell contact enhances antibacterial activity of silver nanoparticles. PLoS ONE 2013, 8, e64060. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Ahmad, N.S.; Abdullah, N.; Yasin, F.M. Toxicity assessment of reduced graphene oxide and titanium dioxide nanomaterials on gram-positive and gram-negative bacteria under normal laboratory lighting condition. Toxicol Rep. 2020, 7, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Lekshmi, G.S.; Ostrikov, K.; Lussini, V.; Blinco, J.; Mohandas, M.; Vasilev, K.; Bottle, S.; Bazaka, K.; Ostrikov, K. Synergic bactericidal effects of reduced graphene oxide and silver nanoparticles against Gram-positive and Gram-negative bacteria. Sci. Rep. 2017, 7, 1591. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Tan, S.; Lin, M.; Xie, A.; Mai, W.; Zhang, X.; Lin, Z.; Wu, T.; Liu, Y. Synergistic antibacterial brilliant blue/reduced graphene oxide/quaternary phosphonium salt composite with excellent water solubility and specific targeting capability. Langmuir 2011, 27, 7828–7835. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, I.; Bhattacharya, P.; Talukdar, M.; Neogi, S.; Pal, S.K.; Chakraborty, S. Bactericidal effect of graphene oxide and reduced graphene oxide: Influence of shape of bacteria. Colloid Interface Sci. Commun. 2019, 28, 60–68. [Google Scholar] [CrossRef]
- Makhetha, T.A.; Ray, S.C.; Moutloali, R.M. Zeolitic imidazolate framework-8-encapsulated nanoparticle of ag/cu composites supported on graphene oxide: Synthesis and antibacterial activity. ACS Omega 2020, 5, 9626–9640. [Google Scholar] [CrossRef] [PubMed]
- Cobos, M.; De-La-Pinta, I.; Quindós, G.; Fernández, M.J.; Fernández, M.D. Graphene oxide–silver nanoparticle nanohybrids: Synthesis, characterization, and antimicrobial properties. Nanomaterials 2020, 10, 376. [Google Scholar] [CrossRef] [PubMed]
- Young, K.D. The selective value of bacterial shape. Microbiol. Mol. Biol. Rev. 2006, 70, 660–703. [Google Scholar] [CrossRef] [PubMed]
- Marković, Z.M.; Jovanović, S.P.; Mašković, P.Z.; Danko, M.; Mičušík, M.; Pavlović, V.B.; Milivojević, D.D.; Kleinová, A.; Špitalský, Z.; Marković, B.M.T. Photo-induced antibacterial activity of four graphene based nanomaterials on a wide range of bacteria. RSC Adv. 2018, 8, 31337–31347. [Google Scholar] [CrossRef]
- Wang, J.; Wei, Y.; Shi, X.; Gao, H. Cellular entry of graphene nanosheets: The role of thickness, oxidation and surface adsorption. RSC Adv. 2013, 3, 15776. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.-H.; Jeyaraj, M.; Kim, J.-H. Differential cytotoxicity of different sizes of graphene oxide nanoparticles in leydig (TM3) and sertoli (TM4) cells. Nanomaterials 2019, 9, 139. [Google Scholar] [CrossRef]
- Shi, X.; Chang, H.; Chen, S.; Lai, C.; Khademhosseini, A.; Wu, H. Regulating cellular behavior on few-layer reduced graphene oxide films with well-controlled reduction states. Adv. Funct. Mater. 2012, 22, 751–759. [Google Scholar] [CrossRef]
- Yang, K.; Li, Y.; Tan, X.; Peng, R.; Liu, Z. Behavior and toxicity of graphene and its functionalized derivatives in biological systems. Small 2013, 9, 1492–1503. [Google Scholar] [CrossRef]
- Hu, W.; Peng, C.; Lv, M.; Li, X.; Zhang, Y.; Chen, N.; Fan, C.; Huang, Q. Protein corona-mediated mitigation of cytotoxicity of graphene oxide. ACS Nano 2011, 5, 3693–3700. [Google Scholar] [CrossRef] [PubMed]
- Tabish, T.A. Graphene-based materials: The missing piece in nanomedicine? Biochem. Biophys. Res. Commun. 2018, 504, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Liu, H.; Fan, Y. Graphene-based materials in regenerative medicine. Adv. Healthc. Mater. 2015, 4, 1451–1468. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.R.; Li, Y.-C.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Zhang, Y.S.; Tamayol, A.; Khademhosseini, A. Graphene-based materials for tissue engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274. [Google Scholar] [CrossRef]
- Han, S.; Sun, J.; He, S.; Tang, M.; Chai, R. The application of graphene-based biomaterials in biomedicine. Am. J. Transl. Res. 2019, 11, 3246. [Google Scholar]
- Li, K.; Li, P.; Fan, Y. The assembly of silk fibroin and graphene-based nanomaterials with enhanced mechanical/conductive properties and their biomedical applications. J. Mater. Chem. B 2019, 7, 6890–6913. [Google Scholar] [CrossRef]
- de Chuffa, L.G.A.; Seiva, F.R.F.; Novais, A.A.; Simão, V.A.; Giménez, V.M.M.; Manucha, W.; Zuccari, D.A.P.d.; Reiter, R.J. Melatonin-loaded nanocarriers: New horizons for therapeutic applications. Molecules 2021, 26, 3562. [Google Scholar] [CrossRef]
- Zhuang, Y.; Liu, Q.; Kong, Y.; Shen, C.; Hao, H.; Dionysiou, D.D.; Shi, B. Enhanced antibiotic removal through a dual-reaction-center Fenton-like process in 3D graphene based hydrogels. Environ. Sci. Nano 2019, 6, 388–398. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Liu, C.F.; Huang, C.Z. A facile and green method to fabricate graphene-based multifunctional hydrogels for miniature-scale water purification. RSC Adv. 2013, 3, 9240. [Google Scholar] [CrossRef]
- Abudabbus, M.M.; Jevremović, I.; Janković, A.; Perić-Grujić, A.; Matić, I.; Vukašinović-Sekulić, M.; Hui, D.; Rhee, K.Y.; Mišković-Stankovićad, V. Biological activity of electrochemically synthesized silver doped polyvinyl alcohol/graphene composite hydrogel discs for biomedical applications. Compos. Part B Eng. 2016, 104, 26–34. [Google Scholar] [CrossRef]
- Fan, Z.; Liu, B.; Wang, J.; Zhang, S.; Lin, Q.; Gong, P.; Ma, L.; Yang, S. A novel wound dressing based on ag/graphene polymer hydrogel: Effectively kill bacteria and accelerate wound healing. Adv. Funct. Mater. 2014, 24, 3933–3943. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Yang, Y.; Shi, J.; Zhang, H.; Yao, X.; Chen, W.; Zhang, X. A rose bengal/graphene oxide/PVA hybrid hydrogel with enhanced mechanical properties and light-triggered antibacterial activity for wound treatment. Mater. Sci. Eng. C 2021, 118, 111447. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Lai, J.; Zhang, N.; Liu, Y.; Liu, R.; Liu, X. Tannic acid induced self-assembly of three-dimensional graphene with good adsorption and antibacterial properties. ACS Sustain. Chem. Eng. 2016, 4, 1404–1413. [Google Scholar] [CrossRef]
- Zeng, X.; McCarthy, D.T.; Deletic, A.; Zhang, X. Silver/reduced graphene oxide hydrogel as novel bactericidal filter for point-of-use water disinfection. Adv. Funct. Mater. 2015, 25, 4344–4351. [Google Scholar] [CrossRef]
- Xue, B.; Qin, M.; Wu, J.; Luo, D.; Jiang, Q.; Li, Y.; Cao, Y.; Wang, W. Electroresponsive supramolecular graphene oxide hydrogels for active bacteria adsorption and removal. ACS Appl. Mater. Interfaces 2016, 8, 15120–15127. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, T.; Dai, B.; Zhang, H.; Chen, X.; Yang, J.; Liu, J.; Sun, D. Rapid fabrication of composite hydrogel microfibers for weavable and sustainable antibacterial applications. ACS Sustain. Chem. Eng. 2016, 4, 6534–6542. [Google Scholar] [CrossRef]
- Maji, P.K. Graphene-based polymer nanocomposites: Materials for future revolution. MOJ Polym. Sci. 2017, 1, 94–97. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Ahmed, J.; Ejaz, M.; Mullah, M. Polylactide/graphene oxide nanosheets/clove essential oil composite films for potential food packaging applications. Int. J. Biol. Macromol. 2018, 107, 194–203. [Google Scholar] [CrossRef]
- Wang, H.; Chen, M.; Jin, C.; Niu, B.; Jiang, S.; Li, X.; Jiang, S. Antibacterial [2-(methacryloyloxy) ethyl] trimethylammonium chloride functionalized reduced graphene oxide/poly(ethylene-co-vinyl alcohol) multilayer barrier film for food packaging. J. Agric. Food Chem. 2018, 66, 732–739. [Google Scholar] [CrossRef]
- Gouvêa, R.F.; Del Aguila, E.M.; Paschoalin, V.M.F.; Andrade, C.T. Extruded hybrids based on poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and reduced graphene oxide composite for active food packaging. Food Packag. Shelf Life 2018, 16, 77–85. [Google Scholar] [CrossRef]
- Barra, A.; Ferreira, N.M.; Martins, M.A.; Lazar, O.; Pantazi, A.; Jderu, A.A.; Neumayer, S.M.; Rodriguez, B.J.; Enăchescu, M.; Ferreira, P. Eco-friendly preparation of electrically conductive chitosan—Reduced graphene oxide flexible bionanocomposites for food packaging and biological applications. Compos. Sci. Technol. 2019, 173, 53–60. [Google Scholar] [CrossRef]
- Grande, C.D.; Mangadlao, J.; Fan, J.; De Leon, A.; Delgado-Ospina, J.; Rojas, J.G.; Rodrigues, D.F.; Advincula, R. Chitosan cross-linked graphene oxide nanocomposite films with antimicrobial activity for application in food industry. Macromol. Symp. 2017, 374, 1600114. [Google Scholar] [CrossRef]
- Ghanem, A.F.; Youssef, A.M.; Abdel Rehim, M.H. Hydrophobically modified graphene oxide as a barrier and antibacterial agent for polystyrene packaging. J. Mater. Sci. 2020, 55, 4685–4700. [Google Scholar] [CrossRef]
- Shams, E.; Yeganeh, H.; Naderi-Manesh, H.; Gharibi, R.; Hassan, Z.M. Polyurethane/siloxane membranes containing graphene oxide nanoplatelets as antimicrobial wound dressings: In vitro and in vivo evaluations. J. Mater. Sci. Mater. Med. 2017, 28, 75. [Google Scholar] [CrossRef]
- Mitra, T.; Manna, P.J.; Raja, S.T.K.; Gnanamani, A.; Kundu, P.P. Curcumin loaded nano graphene oxide reinforced fish scale collagen—A 3D scaffold biomaterial for wound healing applications. RSC Adv. 2015, 5, 98653–98665. [Google Scholar] [CrossRef]
- Mahmoudi, N.; Eslahi, N.; Mehdipour, A.; Mohammadi, M.; Akbari, M.; Samadikuchaksaraei, A.; Simchi, A. Temporary skin grafts based on hybrid graphene oxide-natural biopolymer nanofibers as effective wound healing substitutes: Pre-clinical and pathological studies in animal models. J. Mater. Sci. Mater. Med. 2017, 28, 73. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.; Wang, H.; Liu, M.; Chen, S.; Wang, Z.; Qian, W.; Luo, G.; Xia, H. Polyurethane-modified graphene oxide composite bilayer wound dressing with long-lasting antibacterial effect. Mater. Sci. Eng. C 2020, 111, 110833. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Deng, B.; Lv, M.; Li, J.; Zhang, Y.; Jiang, H.; Peng, C.; Li, J.; Shi, J.; Huang, Q.; et al. Graphene oxide-based antibacterial cotton fabrics. Adv. Healthc. Mater. 2013, 2, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Q.; Zhou, N.; Tan, J.; Ashley, J.; Wang, W.; Wu, F.; Shen, J.; Zhang, M. Study on a novel poly (vinyl alcohol)/graphene oxide-citicoline sodium-lanthanum wound dressing: Biocompatibility, bioactivity, antimicrobial activity, and wound healing effect. Chem. Eng. J. 2020, 395, 125059. [Google Scholar] [CrossRef]
- Xu, L.Q.; Liao, Y.B.; Li, N.N.; Li, Y.J.; Zhang, J.Y.; Wang, Y.B.; Hu, X.F.; Li, C.M. Vancomycin-assisted green synthesis of reduced graphene oxide for antimicrobial applications. J. Colloid Interface Sci. 2018, 514, 733–739. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, K.; Jiao, T.; Xing, R.; Shen, G.; Yan, X. Water-insoluble photosensitizer nanocolloids stabilized by supramolecular interfacial assembly towards photodynamic therapy. Sci. Rep. 2017, 7, 42978. [Google Scholar] [CrossRef]
- Han, W.; Wu, Z.; Li, Y.; Wang, Y. Graphene family nanomaterials (GFNs)—Promising materials for antimicrobial coating and film: A review. Chem. Eng. J. 2019, 358, 1022–1037. [Google Scholar] [CrossRef]
- Santos, C.M.; Tria, M.C.R.; Vergara, R.A.M.V.; Ahmed, F.; Advincula, R.C.; Rodrigues, D.F. Antimicrobial graphene polymer (PVK-GO) nanocomposite films. Chem. Commun. 2011, 47, 8892. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Deng, B.; Chen, G.; Lei, B.; Hua, H.; Peng, H.; Yan, Z. Large-area chemical vapor deposition-grown monolayer graphene-wrapped silver nanowires for broad-spectrum and robust antimicrobial coating. Nano Res. 2016, 9, 963–973. [Google Scholar] [CrossRef]
- Xie, X.; Mao, C.; Liu, X.; Zhang, Y.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Chu, P.K.; Wu, S. Synergistic bacteria killing through photodynamic and physical actions of graphene oxide/Ag/collagen coating. ACS Appl. Mater. Interfaces 2017, 9, 26417–26428. [Google Scholar] [CrossRef] [PubMed]
- Janković, A.; Eraković, S.; Vukašinović-Sekulić, M.; Mišković-Stanković, V.; Park, S.J.; Rhee, K.Y. Graphene-based antibacterial composite coatings electrodeposited on titanium for biomedical applications. Prog. Org. Coat. 2015, 83, 1–10. [Google Scholar] [CrossRef]
- Mohammed, H.; Kumar, A.; Bekyarova, E.; Al-Hadeethi, Y.; Zhang, X.; Chen, M.; Ansari, M.S.; Cochis, A.; Rimondini, L. Antimicrobial mechanisms and effectiveness of graphene and graphene-functionalized biomaterials. A scope review. Front. Bioeng. Biotechnol. 2020, 8, 465. [Google Scholar] [CrossRef] [PubMed]
- Fatima, N.; Qazi, U.Y.; Mansha, A.; Bhatti, I.A.; Javaid, R.; Abbas, Q.; Bhatti, I.A.; Javaid, R.; Abbas, Q.; Nadeem, N.; et al. Recent developments for antimicrobial applications of graphene-based polymeric composites: A review. J. Ind. Eng. Chem. 2021, 100, 40–58. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, R.; He, T.; Xu, K.; Du, D.; Zhao, N.; Cheng, X.; Yang, J.; Shi, H.; Lin, Y. Biomedical potential of ultrafine Ag/AgCl nanoparticles coated on graphene with special reference to antimicrobial performances and burn wound healing. ACS Appl. Mater. Interfaces 2016, 8, 15067–15075. [Google Scholar] [CrossRef]
- He, C.; Shi, Z.-Q.; Cheng, C.; Lu, H.-Q.; Zhou, M.; Sun, S.-D.; Zhao, C.-S. Graphene oxide and sulfonated polyanion co-doped hydrogel films for dual-layered membranes with superior hemocompatibility and antibacterial activity. Biomater. Sci. 2016, 4, 1431–1440. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Photocatalytic reduction of graphene oxide nanosheets on TiO2 thin film for photoinactivation of bacteria in solar light irradiation. J. Phys. Chem. C 2009, 113, 20214–20220. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zhang, Y.; Uliana, A.; Zhu, J.; Liu, J.; van der Bruggen, B. Zeolitic imidazolate framework/graphene oxide hybrid nanosheets functionalized thin film nanocomposite membrane for enhanced antimicrobial performance. ACS Appl. Mater. Interfaces 2016, 8, 25508–25519. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Park, S.; Kim, H.; Park, S.; Ruoff, R.S.; Hwang, S.-J. Strongly-coupled freestanding hybrid films of graphene and layered titanate nanosheets: An effective way to tailor the physicochemical and antibacterial properties of graphene film. Adv Funct. Mater. 2014, 24, 2288–2294. [Google Scholar] [CrossRef]
- Duan, L.; Wang, Y.; Zhang, Y.; Liu, J. Graphene immobilized enzyme/polyethersulfone mixed matrix membrane: Enhanced antibacterial, permeable and mechanical properties. Appl. Surf. Sci. 2015, 355, 436–445. [Google Scholar] [CrossRef]




| Material | Inference | Antibacterial Ability | Reference |
|---|---|---|---|
| Benzalkonium bromide/GO | Commercial preservative based benzalkonium bromide/GO hydrogel | Strong antibacterial action against gram positive (91%) and gram negative (99%) | [16] |
| Rose Bengal/GO/Poly vinyl alcohol (PVA) | This hydrogel can be used in photothermal therapy and photodynamic therapy | Sustainable activity against S. aureus and E. coli | [84] |
| Tannic acid/rGO | Plant polyphenol (tannic acid) was used for green one-step strategy is developed to fabricate three-dimensional (3D) hydrogel | 99.99% activity against S. aureus and 58.12% against E. coli | [85] |
| Ag/rGO hydrogel | Gravity-driven 3D hydrogel for water disinfection applications | 97% against E. coli | [86] |
| Electroresponsive Supramolecular GO Hydrogels | Electroresponsive hydrogel, electric field at 15 V was used to inactivate bacteria | 100% against S. aureus and E. coli | [87] |
| GO−silver/bacterial cellulose hydrogel | Wearable Hydrogel Microfibers with sustainable antibacterial property | Sustainable activity aginst S. aureus and E. coli | [88] |
| Gr/PVA/Ag | Polymer based hydrogel | Good antimicrobial activity (90%) | [82] |
| Gr/Ag | Hybrid hydrogel | Excelnet antibacterial activity (>98%) | [83] |
| Material | Inference | Antibacterial Ability | Reference |
|---|---|---|---|
| Chitosan (CS)/GO composite | Green composite for good mechanical and barrier properties | Sustainable effect against S. aureus and E. coli. | [93] |
| CS/crosslinked GO | Thermally stable and suitable for food packaging | Against E. coli (90%) and gram positive B | [94] |
| GO with polystyrene | High mechanical strength and low water permeability | biocide effect on pathogenic bacteria | [95] |
| GO/PLA composite | High flexibility and lowers the oxygen permeability | Excellent antibacterial activity (>95%) against S. aureus and E. coli. | [90] |
| PVA/GO | Good mechanical and barrier properties | Efficient against E. coli (90%) | [89] |
| LLDPE/EVA/Gr | Excellent barrier properties | Satisfactory aginast against S. aureus and E. coli. | [89] |
| MTAC/rGO/EVOH | Potential food packaging | Sustainable effect on all pathogens | [91] |
| PHBV/rGO/ZnO | Good mechanical and barrier properties | Sustainable effect aginst S. aureus and E. coli. | [92] |
| Material | Inference | Antibacterial Ability | Reference |
|---|---|---|---|
| GO/cotton fabric | Excellent wound healer | Good antibacterial activity against S. aureus and E. coli | [100] |
| GO/CS/PVP | Increases the wound healing rate | Excellent antibacterial ability (>95%) against S. aureus and E. coli | [90] |
| GO/β-cylcodextrin aldehyde/PVA | biocompatible and antibacterial material for wound dressing applications | Sustainable activity against S. aureus and E. coli | [84] |
| GO-Polyurethane-siloxane | Good mechaincal stability with effective wound healing | Efficient against S. aureus and E. coli (>90%) | [96] |
| Ag/GO/acrylic acid/acrylamide | Efficient biocompatibility with promising mechanical properties | Excellent against S. aureus and E. coli (>95%) | [96] |
| PVA/GO-citicoline sodium lanthanum(PVA/GO-CDPC-La) | Excellent wound dressing material | Active against S. aureus and E. coli (>90%) | [101] |
| rGO/Vancomycin | Better wound healing effeiciency | Sustainable activity against S. aureus and E. coli | [102] |
| silver/reduced graphene/sodium-alginate (AGSA) | Effective wound healer | Significant activity against S. aureus and E. coli (>90%) | [103] |
| Material | Inference | Antibacterial Ability | Reference |
|---|---|---|---|
| Graphene with silver nanowires coated on poly ethylene vinyl acetate/poly ethylene terephthalate | Good antimicrobial coating with high disinfection capability | Excellent antibacterial activity against C. albicans, S. aureus and E. coli | [111] |
| GO/Ag/Collagen | Composite exhibited quick and effectual disinfection | Good against S. aureus and E. coli | [107] |
| Graphene/hydroxyapatite/Ag | Outstanding corrosion stability | Remarkable antibacterial activity without any side effects | [108] |
| Go/sulfonated polyanion/polyethersulfone coated on glass surface | Good coatings with high disinfection capability | Excellent antimicrobial activity against pathogens | [112] |
| RGO/TiO2 film | This film is prepared through photoreduction of GO on TiO2 | Excellent activity against E. coli@100% | [113] |
| GO/Zeolitic imidazolate framework film | The composite was used as bactericidal agent to fabricate antimicrobial thin film through interfacial polymerization | Activity against E. coli@84.3% | [114] |
| Graphene and layered titanate nanosheets film | Freestanding hybrid films consisting of strongly-coupled rGO and titanate nanosheets | Activity against E. coli@99.98% | [115] |
| Graphene immobilized lysozyme/polyethersulfone mixed matrix composite | Lysozyme materials were blended into polyethersulfone (PES) casting solution to fabricate PES membrane through phase inversion method | Activity against E. coli@71% | [116] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaragalla, S.; Bhavitha, K.B.; Athanassiou, A. A Review on Graphene Based Materials and Their Antimicrobial Properties. Coatings 2021, 11, 1197. https://doi.org/10.3390/coatings11101197
Yaragalla S, Bhavitha KB, Athanassiou A. A Review on Graphene Based Materials and Their Antimicrobial Properties. Coatings. 2021; 11(10):1197. https://doi.org/10.3390/coatings11101197
Chicago/Turabian StyleYaragalla, Srinivasarao, Karanath Balendran Bhavitha, and Athanassia Athanassiou. 2021. "A Review on Graphene Based Materials and Their Antimicrobial Properties" Coatings 11, no. 10: 1197. https://doi.org/10.3390/coatings11101197
APA StyleYaragalla, S., Bhavitha, K. B., & Athanassiou, A. (2021). A Review on Graphene Based Materials and Their Antimicrobial Properties. Coatings, 11(10), 1197. https://doi.org/10.3390/coatings11101197






