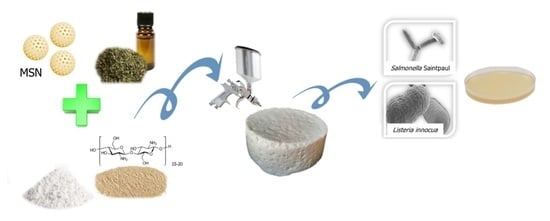
Design of an Active Edible Coating Based on Sodium Caseinate, Chitosan and Oregano Essential Oil Reinforced with Silica Particles and Its Application on Panela Cheese
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Oregano Essential Oil (OEO)
2.3. MSN-OEO Preparation
2.4. Preparation of SC:CH Film Forming Solution (FFS)
2.5. Characterization of the FFSs and Films
2.5.1. ζ-Potential and Particle Size Measurement
2.5.2. Film Preparation
2.5.3. Mechanical Properties
2.5.4. Barrier Properties
2.5.5. Solubility
2.5.6. Scanning Electron Microscopy (SEM)
2.5.7. Color and Transparency
2.5.8. Atomic Force Microscopy (AFM)
2.5.9. Antimicrobial Activity
2.6. Application as a Coating on Panela Cheese
2.6.1. Moisture Content, pH and Titratable Acidity
2.6.2. Microbiological Analysis
Molds and Yeasts
Mesophilic Aerobic Bacteria
2.7. Statistical Analysis
3. Results and Discussion
3.1. SEM of MSN and MSN-OEO
3.2. FFSs Stability
3.3. Characteristics of the SC:CH Films
3.3.1. Microstructural Properties of Films
3.3.2. Thickness
3.3.3. Solubility of the Films
3.3.4. Optical Properties of the Films
3.4. Surface Roughness of the Films
3.5. Mechanical Properties of the Films
3.6. Barrier Properties
3.7. Antimicrobial Activity
3.8. Application of FFS on Panela Cheese
3.8.1. Moisture Content
3.8.2. pH and Titratable Acidity (TA)
3.8.3. Microbiological Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costa, M.J.; Maciel, L.C.; Teixeira, J.A.; Vicente, A.A.; Cerqueira, M.A. Use of edible films and coatings in cheese preservation: Opportunities and challenges. Food Res. Int. 2018, 107, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Pavlath, A.E.; Orts, W. Edible Films and Coatings: Why, What, and How? In Edible Films and Coatings for Food Applications; Springer: New York, NY, USA, 2009; pp. 1–23. [Google Scholar] [CrossRef]
- Lotfi, M.; Tajik, H.; Moradi, M.; Forough, M.; Divsalar, E.; Kuswandi, B. Nanostructured chitosan/ monolaurin film: Preparation, characterization and antimicrobial activity against Listeria monocytogenes on ultrafiltered white cheese. LWT-Food Sci. Technol. 2018, 92, 576–583. [Google Scholar] [CrossRef]
- Belyamani, I.; Prochazka, F.; Assezat, G.; Debeaufort, F. Mechanical and barrier properties of extruded film made from sodium and calcium caseinates. Food Packag. Shelf Life 2014, 2, 65–72. [Google Scholar] [CrossRef]
- Rezvani, E.; Schleining, G.; Sümen, G.; Taherian, A.R. Assessment of physical and mechanical properties of sodium caseinate and stearic acid based film-forming emulsions and edible films. J. Food Eng. 2013, 116, 598–605. [Google Scholar] [CrossRef]
- Lin, H.C.; Wang, B.J.; Weng, Y.M. Development and characterization of sodium caseinate edible films cross-linked with genipin. LWT-Food Sci. Technol. 2020, 118, 108813. [Google Scholar] [CrossRef]
- CANILAC. Nuevo Boletín de la Leche (ene-may2021) Final. Available online: https://www.canilec.org.mx/wp-content/uploads/2021/06/Nuevo-Boletin-de-la-Leche-ene-may2021-PDF-final.pdf. (accessed on 13 August 2021).
- Reyes-Díaz, R.; Gonzáles-Córdova, A.F.; Estrada-Montoya, M.; Méndez-Romero, J.I.; Mazorra-Manzano, J.Á.; Soto-Valdez, H.; Vallejo-Cordoba, B. Volatile and sensory evaluation of Mexican Fresco cheese as affected by specific wild Lactococcus lactis strains. J. Dairy Sci. 2019, 103, 242–253. [Google Scholar] [CrossRef] [Green Version]
- Barukcic, I.; Scetar, M.; Marasovic, I.; Jakopovic, K.L.; Galic, K.; Bozanic, R. Evaluation of quality parameters and shelf life of fresh cheese packed under modified atmosphere. J. Food Sci. Technol. 2020, 57, 2722–2731. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Sayed, S.M.; El-Sayed, H.S.; Salama, H.H.; Assem, F.M.; Abd El-Salam, M.H. Novel bionanocomposite materials used for packaging skimmed milk acid coagulated cheese (Karish). Int. J. Biol. Macromol. 2018, 115, 1002–1011. [Google Scholar] [CrossRef]
- Berti, S.; Ollé Resa, C.P.; Basanta, F.; Gerschenson, L.N.; Jagus, R.J. Edible coatings on Gouda cheese as a barrier against external contamination during ripening. Food Biosci. 2019, 31, 100447. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Chen, B.; McClements, D.J. Improving the efficacy of essential oils as antimicrobials in foods: Mechanisms of action. Annu. Rev. Food Sci. Technol. 2019, 10, 365–387. [Google Scholar] [CrossRef]
- Prakash, B.; Kujur, A.; Yadav, A.; Kumar, A.; Singh, P.P.; Dubey, N.K. Nanoencapsulation: An efficient technology to boost the antimicrobial potential of plant essential oils in food system. Food Control 2018, 89, 1–11. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Use of essential oils in bioactive edible coatings: A Review. Food Eng. Rev. 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Asbahani, A.E.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; Mousadik, A.E.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 48, 220–243. [Google Scholar] [CrossRef]
- Mehmood, A.; Ghafar, H.; Yaqoob, S.; Gohar, U.F.; Ahmad, B. Mesoporous silica nanoparticles: A Review. J Dev. Drugs 2017, 6, 1000174. [Google Scholar] [CrossRef]
- Bravo, M.; Preston, G.M.; Van der Hoorn, R.A.L.; Townley, H.E.; Thompson, I.P. Species-specific antimicrobial activity of essential oils and enhancement by encapsulation in mesoporous silica nanoparticles. Ind. Crop. Prod. 2018, 122, 582–590. [Google Scholar] [CrossRef]
- Geraci, C.; Stefani, S.; Cafiso, V.; Stracquadanio, S.; Napoli, E.; Leonardi, M.; Consoli, G.M.L.; Granata, G. Essential oils encapsulated in polymer-based nanocapsules as potential candidates for application in food preservation. Food Chem. 2018, 269, 286–292. [Google Scholar] [CrossRef]
- Hernández-Hernández, E.; Regalado-González, C.; Vázquez-Landaverde, P.; Guerrero-Legarreta, I.; García-Almendárez, B.E. Microencapsulation, chemical characterization, and antimicrobial activity of Mexican (Lippia graveolens H.B.K.) and European (Origanum vulgare L.) oregano essential oils. Sci. World J. 2014, 2014, 641814. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Bats, I.; Di Pierro, P.; Villalonga-Santana, R.; Garcia-Almendarez, B.; Porta, R. Bioactive mesoporous silica nanocomposite films obtained from native and transglutaminase-crosslinked bitter vetch proteins. Food Hydrocolloid. 2018, 82, 106–115. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Yilmaz, A.; Adman, P.K.; Bozkurt, F.; Dertli, E.; Basahel, A.; Al-Sasi, B.; Taylan, O.; Sagdic, O. Electrospraying method for fabrication of essential oil loaded-chitosan nanoparticle delivery systems characterized by molecular, thermal, morphological and antifungal properties. Innov. Food Sci. Technol. 2019, 52, 166–178. [Google Scholar] [CrossRef]
- Porta, R.; Di Pierro, P.; Sabbah, M.; Regalado-Gonzales, C.; Mariniello, L.; Kadivar, M.; Arabestani, A. Blend films of pectin and bitter vetch (Vicia ervilia) proteins: Properties and effect of transglutaminase. Innov. Food Sci. Emerg. 2016, 36, 245–251. [Google Scholar] [CrossRef]
- Vahedikia, N.; Garavand, F.; Tajeddin, B.; Cacciotti, I.; Mahdi, S. Biodegradable zein film composites reinforced with chitosan nanoparticles and cinnamon essential oil: Physical, mechanical, structural and antimicrobial attributes. Colloid. Surf. B 2019, 177, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-García, M.; Calderón-Domínguez, G.; Chanona-Pérez, J.J.; Mendoza-Madrigal, A.G.; Di Pierro, P.; García-Almendárez, B.E.; Amaro-Reyes, A.; Regalado-González, C. Physical, structural, barrier, and antifungal characterization of chitosan–zein edible films with added essential oils. Int. J. Mol. Sci. 2017, 18, 2370. [Google Scholar] [CrossRef] [Green Version]
- NOM-243-SSA1-2010. NORMA Oficial Mexicana Productos y servicios. Leche, fórmula láctea, producto lácteo combinado y derivados lácteos. Disposiciones y especificaciones sanitarias. Métodos de prueba. Retrieved 15/06/2020. Available online: http://dof.gob.mx/normasOficiales/4156/salud2a/salud2a.htm (accessed on 13 August 2021).
- Bhattacharjee, S. DLS and zeta potential–what they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Anal, A.K.; Tobiassen, A.; Flanagan, J.; Singh, H. Preparation and characterization of nanoparticles formed by chitosan–caseinate interactions. Colloid Surf. B 2008, 64, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Kharlamova, A.; Nicolai, T.; Chassenieux, C. Mixtures of sodium caseinate and whey protein aggregates: Viscosity and acid- or salt-induced gelation. Int. Dairy J. 2018, 86, 110–119. [Google Scholar] [CrossRef]
- Volpe, S.; Cavella, S.; Masi, P.; Torrieri, E. Effect of solid concentration on structure and properties of chitosan-caseinate blend films. Food Packag. Shelf Life. 2017, 13, 76–84. [Google Scholar] [CrossRef]
- Navarro, R.; Arancibia, C.; Herrera, M.L.; Matiacevich, S. Effect of type of encapsulating agent on physical properties of edible films based on alginate and thyme oil. Food Bioprod. Process. 2016, 97, 63–75. [Google Scholar] [CrossRef]
- Mudalige, T.; Qu, H.; Van Haute, D.; Ansar, S.M.; Paredes, A.; Ingle, T. Characterization of Nanomaterials: Tools and Challenges. In Nanomaterials for Food Applications. Micro and Nano Technologies; López-Rubio, A., Fabra-Rovira, M.J., Martínez-Sanz, M., Gómez-Mascaraque, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 313–353. [Google Scholar]
- Fabra, M.J.; Jiménez, A.; Atarés, L.; Talens, P.; Chiralt, A. Effect of fatty acids and beeswax addition on properties of sodium caseinate dispersions and films. Biomacromolecules 2009, 10, 1500–1507. [Google Scholar] [CrossRef]
- Pereda, M.; Aranguren, M.; Marcovich, N. Characterization of chitosan/caseinate films. J. Appl. Polymer Sci. 2007, 107, 1080–1090. [Google Scholar] [CrossRef]
- Shiku, Y.; Hamaguchi, P.Y.; Tanaka, M. Effect of pH on the preparation of edible films based on fish myofibrillar proteins. Fisheries Sci. 2003, 69, 1026–1032. [Google Scholar] [CrossRef]
- Lu, W.; Cui, R.; Zhu, B.; Qin, Y.; Cheng, G.; Li, L.; Yuan, M. Influence of clove essential oil immobilized in mesoporous silica nanoparticles on the functional properties of poly(lactic acid) biocomposite food packaging film. J. Mater. Res. Technol. 2021, 11, 1152–1161. [Google Scholar] [CrossRef]
- Giosafatto, C.; Sabbah, M.; Al-Asmar, A.; Esposito, M.; Sanchez, A.; Villalonga Santana, R.; Cammarota, M.; Mariniello, L.; Di Pierro, P.; Porta, R. Effect of mesoporous silica nanoparticles on glycerol-plasticized anionic and cationic polysaccharide edible films. Coatings 2019, 9, 172. [Google Scholar] [CrossRef] [Green Version]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Development and evaluation of a novel biodegradable film made from chitosan and cinnamon essential oil with low affinity toward water. Food Chem. 2010, 122, 161–166. [Google Scholar] [CrossRef]
- Di Pierro, P.; Villalonga, R.; Mariniello, L.; Massi, P.; Porta, R. Chitosan—Whey protein edible films produced in the absence or presence of transglutaminase: Analysis of their mechanical and barrier properties. Biomacromolecules 2006, 7, 744–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Pierro, P.; Mariniello, L.; Giosafato, C.V.L.; Massi, E.; Porta, R. Solubility and permeability properties of edible pectin-soy flour films obtained in the absence or presence of transglutaminase. Food Biotechnol. 2005, 19, 37–49. [Google Scholar] [CrossRef]
- Carmagnini, L.; Calesini, F.; Pesavento, G.; Di Martino, M.C.; Bilia, A.R.; Bernabei, M.; Calonico, C.; Mencarelli, L.; Lo Nostro, A.; Addona, R. Antibacterial activity of oregano, rosmarinus and Thymus essential oils against Staphylococcus aureus and Listeria monocytogenes in beef meat balls. Food Control. 2015, 54, 188–199. [Google Scholar] [CrossRef]
- Iturriaga, L.; Olabarrieta, I.; de Marañón, I.M. Antimicrobial assays of natural extracts and their inhibitory effect against Listeria innocua and fish spoilage bacteria, after incorporation into biopolymer edible films. Int. J. Food Microbiol. 2012, 158, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Arredondo-Ochoa, T.; García-Almendárez, B.E.; Reyes, A.A.; Pastrana, D.M.R.; López, G.F.G.; Belloso, O.M.; Regalado-González, C. Design and characterization of corn starch edible films including beeswax and natural antimicrobials. Food Bioproc. Technol. 2017, 10, 103–114. [Google Scholar] [CrossRef]
- De Gante, A.V.; Moreno, A.S. Los Quesos Mexicanos Tradicionales; Juan Pablos Editor: Mexico City, Mexico, 2016; Volume 14, pp. 323–326. [Google Scholar]
- Zhong, Y.; Cavender, G.; Zhao, Y. Investigation of different coating application methods on the performance of edible coatings on Mozzarella cheese. LWT-Food Sci. Technol. 2014, 56, 1–8. [Google Scholar] [CrossRef]
- Di Pierro, P.; Sorrentino, A.; Mariniello, L.; Giosafatto, C.V.L.; Porta, R. Chitosan/whey protein film as active coating to extend Ricotta cheese shelf-life. LWT-Food Sci. Technol. 2010, 44, 2324–2327. [Google Scholar] [CrossRef]
- Mei, J.; Yuan, Y.; Wu, Y.; Li, Y. Characterization of edible starch-chitosan film and its application in the storage of Mongolian cheese. Int. J. Biol. Macromol. 2013, 57, 17–21. [Google Scholar] [CrossRef]
- Artiga-Artigas, M.; Acevedo-Fani, A.; Martín-Belloso, O. Improving the shelf life of low-fat cut cheese using nanoemulsion-based edible coatings containing oregano essential oil and mandarin fiber. Food Control 2017, 76, 1–12. [Google Scholar] [CrossRef] [Green Version]








| SC:CH Ratio | pH | MSN-OEO | Particle Size (nm) | Ζ Potential (mV) | Polydispersity Index |
|---|---|---|---|---|---|
| 0:1 | 5.8 | - | 1238.27 ± 25.9 d | 31.07 ± 0.8 c | 0.72 ± 0.1 c |
| 1:0 | 5.3 | - | 1800.16 ±10.2 e | 27.77 ± 0.8 d | 0.53 ± 0.1 a |
| 4:1 | 5.3 | - | 1010.64 ± 8.1 a | 34.77 ± 1.2 a | 0.54 ± 0.0 a |
| 4:1 | 5.3 | + | 1008.00 ± 4.5 a | 36.93 ± 1.2 a | 0.39 ± 0.1 b |
| 8:1 | 5.3 | - | 781.33 ± 11.7 b | 29.33 ± 1.3 b | 0.53 ± 0.0 a |
| 8:1 | 5.3 | + | 764.80 ± 23.3 c | 29.93 ± 1.1 b | 0.50 ± 0.0 a |
| SC:CH Ratio | MSN-OEO | L* | a* | b* | Transparency |
|---|---|---|---|---|---|
| 4:1 | + | 91.62 ± 0.2 a | 0.33 ± 0.0 a,b | −1.16 ± 0.2 a | 0.89 ± 0.0 a |
| 4:1 | - | 92.00 ± 0.1 a | 0.34 ± 0.0 a,b | −1.39 ± 0.1 a | 1.14 ± 0.1 b,c |
| 8:1 | + | 92.01 ± 0.3 a | 0.29 ± 0.0 b | −1.18 ± 0.4 a | 0.82 ± 0.0 c |
| 8:1 | - | 92.19 ± 0.1 a | 0.39 ± 0.0 a | −1.79 ± 0.0 a | 1.03 ± 0.0 a,b |
| Treatment | Rq (nm) | Ra (nm) |
|---|---|---|
| SC:CH 8:1 | 3.20 ± 1.0 a | 1.05 ± 0.2 a |
| SC:CH 8:1 MSN-OEO | 5.04 ± 0.6 b | 3.89 ± 0.5 b |
| SC:CH 4:1 | 3.76 ± 0.9 b | 2.88 ± 0.6 b |
| SC:CH 4:1 MSN-OEO | 4.50 ± 0.3 b | 3.44 ± 0.2 b |
| SC:CH Ratio | MSN-OEO | Tensile Stress at Break (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) |
|---|---|---|---|---|
| 4:1 | - | 7.06 ± 0.9 a,b | 103.24 ± 2.4 a | 12.48 ± 1.4 a |
| 4:1 | + | 8.71 ± 1.6 a | 99.52 ± 1.3 a,b | 15.71 ± 1.4 a |
| 8:1 | - | 4.59 ± 0.6 b | 97.81 ± 0.3 b | 7.60 ± 3.0 b |
| 8:1 | + | 9.11 ± 1.9 a | 94.12 ± 2.2 c | 13.70 ± 2.3 a |
| Material | O2 | CO2 | WVP |
|---|---|---|---|
| cm3·mm/(m2·24 h) | |||
| 4:1 SC:CH | 0.260 ± 0.0 a,b | 3.659 ± 0.3 a | 0.0806 ± 0.0 a |
| 4:1 SC:CH MSN-OEO | 0.218 ± 0.0 b | 0.214 ± 0.0 b | 0.0144 ± 0.0 b |
| 8:1 SC:CH | 0.217 ± 0.0 b | 0.514 ± 0.0 b | 0.0735 ± 0.0 a |
| 8:1 SC:CH MSN-OEO | 0.328 ± 0.0 a | 0.340 ± 0.0 b | 0.0321 ± 0.0 b |
| Mater-BI * | 22.69 ± 0.1 | 8.35 ± 0.2 | 15.68 ± 0.1 |
| Low-density polyethylene ** | 7 | 1 | 0.2 |
| High-density polyethylene ** | 1 | 0.03 | 0.04 |
| Microorganisms | Inhibition Zone Diameter (mm) | |||
|---|---|---|---|---|
| OEO | MSN-OEO | 8:1 SC:CH | 8:1 SC:CH MSN-OEO | |
| 25% | 8 mg | 0 mg | 8 mg | |
| L. innocua | 24.8 ± 2.4 a | 6.0 ± 0.2 a | 0.2 ± 0.0 a | 3.5 ± 0.5 a |
| S. Saintpaul | 8.3 ± 2.5 b | 2.7 ± 1.5 b | ND | 2.2 ± 1.0 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ríos-de-Benito, L.F.; Escamilla-García, M.; García-Almendárez, B.; Amaro-Reyes, A.; Di Pierro, P.; Regalado-González, C. Design of an Active Edible Coating Based on Sodium Caseinate, Chitosan and Oregano Essential Oil Reinforced with Silica Particles and Its Application on Panela Cheese. Coatings 2021, 11, 1212. https://doi.org/10.3390/coatings11101212
Ríos-de-Benito LF, Escamilla-García M, García-Almendárez B, Amaro-Reyes A, Di Pierro P, Regalado-González C. Design of an Active Edible Coating Based on Sodium Caseinate, Chitosan and Oregano Essential Oil Reinforced with Silica Particles and Its Application on Panela Cheese. Coatings. 2021; 11(10):1212. https://doi.org/10.3390/coatings11101212
Chicago/Turabian StyleRíos-de-Benito, Luis Fernando, Monserrat Escamilla-García, Blanca García-Almendárez, Aldo Amaro-Reyes, Prospero Di Pierro, and Carlos Regalado-González. 2021. "Design of an Active Edible Coating Based on Sodium Caseinate, Chitosan and Oregano Essential Oil Reinforced with Silica Particles and Its Application on Panela Cheese" Coatings 11, no. 10: 1212. https://doi.org/10.3390/coatings11101212
APA StyleRíos-de-Benito, L. F., Escamilla-García, M., García-Almendárez, B., Amaro-Reyes, A., Di Pierro, P., & Regalado-González, C. (2021). Design of an Active Edible Coating Based on Sodium Caseinate, Chitosan and Oregano Essential Oil Reinforced with Silica Particles and Its Application on Panela Cheese. Coatings, 11(10), 1212. https://doi.org/10.3390/coatings11101212