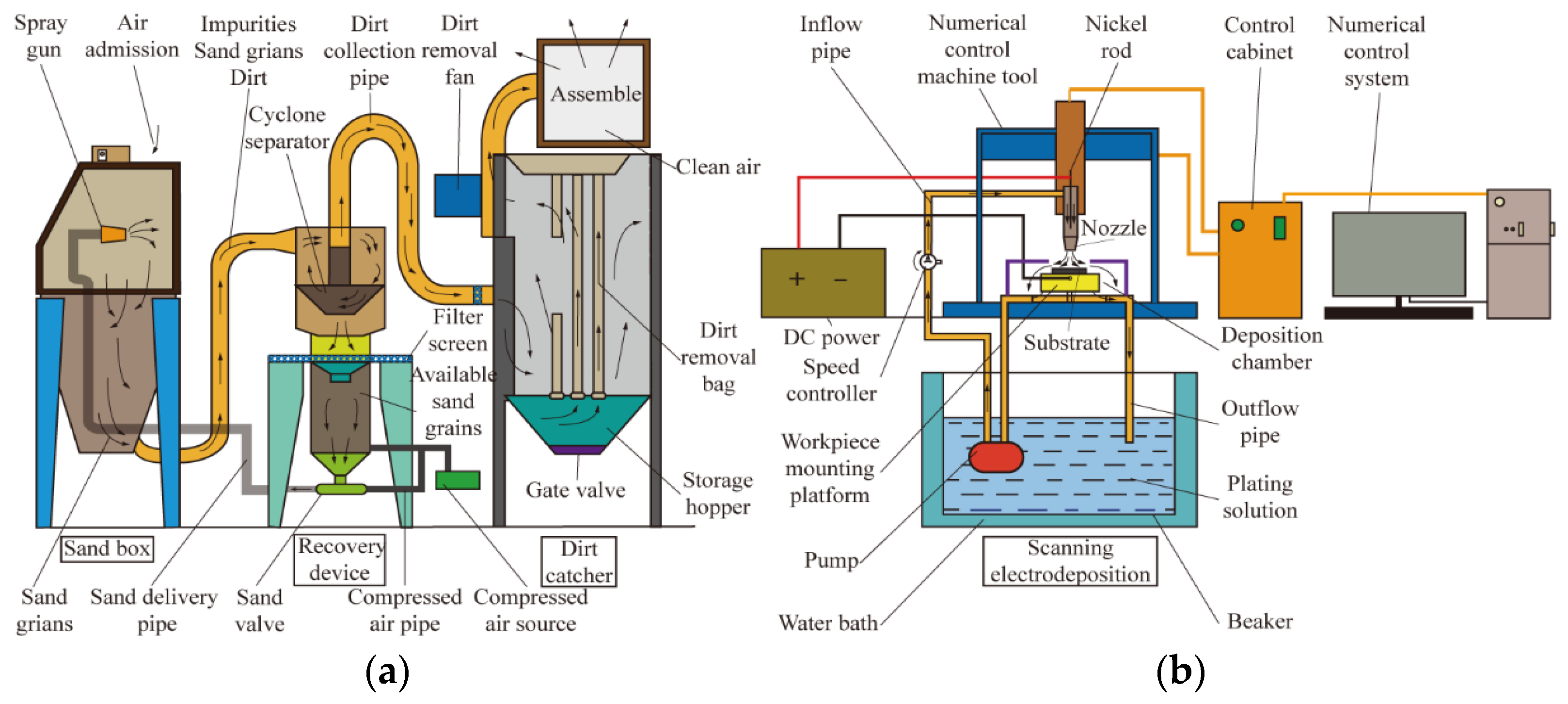
3.2.1. Adhesion Performance
Figure 7 shows the adhesion of composite coatings with different particle concentrations prepared on 45 steel substrates through scanning electrodeposition, and
Figure 8 illustrates their scratch morphologies.
Figure 8a shows that when the particle concentration is 0 g·L
−1, slight cracks appeared on the scratched surface and the coating adhesion was 33.8 N.
Figure 8b shows that when the concentration of particles is 1 g·L
−1, the scratch surface showed cracks, dense cracks, and peeling, and the coating adhesion was 22.55 N. The addition of nanoparticles increased the nucleation point of the coating and enlarged the elemental penetration region between the coating and substrate. However, because the particles were not evenly dispersed in the composite coating, the internal stress distribution was uneven, and the coating thickness was reduced. When the coating was scratched and squeezed, the adhesion degree between the coating and substrate was reduced, and the coating adhesion was reduced. When the load increased, the quality of the coating surface deposition decreased, and cracks and coating peeling occurred.
Figure 8c shows that when the particle concentration is 2 g·L
−1, the number of cracks on the scratched surface decreased, but coating peeling still existed, and the coating adhesion was 31.9 N. The gradual increase in nanoparticles refined the cell size, increased the nucleation point and coating thickness, expanded the region of elemental penetration, and increased the adhesion of the coating to the substrate. In addition, the distribution in the coating becomes uniform, the surface quality of the coating is improved, and the coating breaks less after being extruded.
Figure 8d shows that when the concentration of particles is 3 g·L
−1, the number of cracks on the scratched surface was reduced, no peeling of the coating occurred, and the coating adhesion was 36.5 N. When the concentration of added nanoparticles was 3 g·L
−1, the surface quality of the coating was the best, and the coating was less likely to crack when compressed downward by the thimble squeeze. In addition, the nanoparticles were evenly distributed in the coating, and the adhesion degree between the substrate and coating, the coating thickness, the region of elemental penetration between the coating and substrate, and the adhesion force were the largest. Hence, the coating was not easily separated from the substrate when subjected to extrusion.
Figure 8e shows that when the concentration of particles is 4 g·L
−1, cracks and coating peeling appeared again on the scratched surface, and the coating adhesion was 32.8 N. When the particle concentration continues to increase, the high concentration leads to grain precipitation, which reduces the surface and cross-sectional microstructure and makes the coating susceptible to cracking and peeling when squeezed [
35]. Excessive nanoparticles reduce the current efficiency, resulting in a decrease in the coating thickness and the range of elemental penetration. Hence, the coating failed to adhere well to the substrate, and the adhesion decreased.
Figure 8f shows that when the particle concentration is 5 g·L
−1, the surface cracking and coating peeling on the scratched surface increased, cracks started to appear at the less loaded locations, and the coating adhesion was 29.15 N. When the particle concentration further increased, the surface quality of the coating further deteriorated, and defects such as pores and cracks appeared, making the coating highly susceptible to damage when squeezed. Particle agglomeration slows down the growth rate of the coating and further reduces the coating thickness and the extent of elemental penetration, resulting in the low adhesion of the coating to the substrate.
Figure 9 shows the schematic of the adhesion mechanism based on element penetration for the prepared composite coatings. When the particle concentration is 0 g·L
−1, slight cracks appeared on the scratched surface. When the particle concentration is 1 g·L
−1, the nanoparticles enhanced the penetration and diffusion ability of the elements at the interface between the plating layer and substrate and their adhesion performance to a certain extent. However, due to the low particle concentration, the deposition distribution in the composite plating layer was not uniform, and the thickness, adhesion ability, and adhesion force of the plating layer decreased. In addition, the scratched surface showed cracks and peeling of the plating. When the concentration of particles increased, the nucleation point of the cell increased, prompting the coating thickness to increase and the elemental penetration region between the coating and substrate to expand, thus increasing the adhesion of the coating. The uniform distribution of nanoparticles in the plating layer improves the surface quality of the plating layer and prevents the formation of defects such as chipping and peeling on the scratched surface when subjected to thimble extrusion. When the concentration of nanoparticles was 3 g·L
−1, the coating adhesion was the greatest, the surface quality of the scratch was the best, and only some micro cracks appeared on the scratch surface after being squeezed. When the concentration of nanoparticles continued to increase, the agglomerated particles created excessive internal stress, which reduced the surface quality and caused defects such as cracking and flaking when the plating was extruded. Hence, the thickness of the plating and the range of elemental penetration were reduced, which eventually lead to a decreased plating adhesion.
3.2.2. Corrosion Resistance
Figure 10 shows the potential polarization curves of the prepared coatings, and
Table 5 lists each corrosion electrochemical parameter.
Figure 10 and
Table 5 show that when the concentration of SiC nanoparticles was 0 g·L
−1, the self-corrosion potential of the Ni-P coating was −0.41 V, the self-corrosion current density was 6.06 × 10
−6 A·cm
−2, and the corrosion rate was 0.073395. As a protective film, the Ni-P coating has a corrosion protection effect on the 45-gauge steel substrate. When the concentration of SiC nanoparticles was 1 g·L
−1, the self-corrosion potential of Ni-P-SiC composite coating was −0.62 V, the self-corrosion current density was 2.20 × 10
−5 A·cm
−2, and the corrosion rate was 0.19241. These findings indicate that the corrosion resistance of the coating decreased, and the corrosion rate accelerated. When the nanoparticle concentration was low, the internal stress distribution was uneven, resulting in a small coating thickness. Although the elemental penetration region could enhance the corrosion resistance, the effect was minimal. The adhesion degree of the coating was low, and the corrosive medium easily penetrated the thin coating to erode into the substrate, thereby reducing the corrosion resistance of the coating. When the concentration of SiC nanoparticles was 2 g·L
−1, the self-corrosion potential of the Ni-P-SiC composite coating was −0.44 V, the self-corrosion current density was 4.55 × 10
−6 A·cm
−2, and the corrosion rate slowed down. When the nanoparticles gradually increased, the nanoparticles were gradually distributed evenly in the coating, which promoted the growth of the coating, widened the elemental penetration region, and enhanced the coating adhesion performance. Hence, the coating protection against corrosive media enhanced the corrosion resistance of the coating. When the concentration of SiC nanoparticles was 3 g·L
−1, the self-corrosion potential of the Ni-P-SiC composite coating was −0.30 V, the self-corrosion current density was 8.45 × 10
−7 A·cm
−2, the corrosion rate was the slowest, and the corrosion resistance was the best. At this time, the concentration of nanoparticles was the best, the degree of grain refinement was the highest, the distribution inside the coating was uniform, and the coating thickness and the elemental penetration region were the largest. The coating adhered to the substrate to the best extent, thus effectively blocking the invasion of corrosive media and showed the best corrosion resistance. When the concentration of SiC nanoparticles was 4 g·L
−1, the self-corrosion potential of the Ni-P-SiC composite coating was −0.38 V, the self-corrosion current density was 1.47 × 10
−6 A·cm
−2, and the corrosion rate was accelerated. When the concentration of nanoparticles further increased, the denseness of the coating decreased, grain precipitation occurred on the surface, and the coating thickness, the range of elemental penetration, and the adhesion degree of the coating were reduced. Hence, the corrosion resistance of the coating decreased. When the concentration of SiC nanoparticles was 5 g·L
−1, the self-corrosion potential of the Ni-P-SiC composite coating was −0.53 V, the self-corrosion current density was 1.65 × 10
−5 A·cm
−2, and the corrosion rate was accelerated. The extremely high concentration of nanoparticles leads to agglomeration [
34], creates defects such as pores on the surface of the coating, accelerates the corrosion of the composite coating, and reduces the coating thickness and the range of elemental penetration. Hence, the substrate protection range and corrosion resistance of the coating decreased.
Figure 11 shows the Nyquist plots of the prepared coatings,
Figure 12 displays their corresponding equivalent circuit diagrams, and
Table 6 lists the fitted and calculated equivalent circuit parameters.
When the concentration of SiC nanoparticles was 0 g·L−1, the equivalent impedance value R2 corresponding to the impedance arc radius of the coating was 963 Ω, which is larger than that for the 45-gauge steel substrate after sandblasting. This finding indicates that scanning electrodeposition effectively improves the corrosion resistance of the metal material. When the concentration of SiC nanoparticles was 1 g·L−1, the equivalent impedance value R2 of the composite coating corresponded to an impedance arc radius of 443 Ω, which was lower than that for the coating without SiC nanoparticles. The adhesion performance decreased, which led to a decrease in the corrosion resistance of the coating and its equivalent impedance value. When the concentration of SiC nanoparticles was 2 g·L−1, the equivalent impedance value R2 corresponding to the impedance arc radius of the composite coating was 700 Ω. When the number of nanoparticles increased, the coating thickness and the elemental penetration region also increased, and the deposition quality and adhesion properties of the coating were improved. Hence, the equivalent impedance value of the coating increased. When the concentration of SiC nanoparticles was 3 g·L−1, the impedance arc radius of the composite coating corresponded to an equivalent impedance value of R2 of 3108 Ω. At the optimal nanoparticle concentration, the scanning deposition effect was the best, the coating had the greatest range of thickness and elemental penetration regions, the surface and section were dense and defect-free, and the adhesion properties were optimal, giving it the greatest equivalent impedance value. When the concentration of SiC nanoparticles was 4 g·L−1, the equivalent impedance value R2 corresponding to the impedance arc radius of the composite coating was 1505 Ω. Further increase in the concentration of nanoparticles causes agglomeration, which decreases the deposition quality, the coating thickness, elemental penetration region, and the adhesion degree of the coating to the substrate, thus contributing to the reduction in the equivalent impedance value of the composite coating. When the concentration of SiC nanoparticles was 5 g·L−1, the equivalent impedance value R2 corresponding to the impedance arc radius of the composite coating was 649 Ω. When the concentration of nanoparticles was extremely high, the deposition quality of the coating decreased again, defects such as grain precipitation and pores appeared on the surface, the coating thickness and the range of elemental penetration regions were again reduced, and the coating adhered poorly to the substrate, thus contributing to a further decrease in the equivalent impedance value of the composite coating.
A scanning electron microscope was used to observe the surface micro morphology of the composite coating after electrochemical corrosion in 3.5 wt.% NaCl solution, and the results are shown in
Figure 13.
Figure 13a shows that when the particle concentration was 0 g·L
−1, serious corrosion occurred on the surface, a large number of corrosion holes and products were formed and accumulated. No composite deposition of nanoparticles occurred in the coating, and the nanoparticles did not play a role in refining the cell structure, and the element penetration region was small and easily corroded by corrosive media.
Figure 13b shows that when the particle concentration was 1 g·L
−1, microcracks appeared on the surface and local corrosion occurred. The reason is that the addition of nanoparticles promotes the formation of crystal nuclei, refines the cellular structure, increases the element penetration region, and slows down the corrosion of the coating surface. However, the internal stress was not concentrated due to the low concentration of nanoparticles, resulting in a decrease in the coating thickness. The adhesion performance was reduced, micro-cracks appeared on the surface, and the corrosion preferentially occurred in the micro-cracks in the corrosive solution.
Figure 13c shows that when the particle concentration was 2 g·L
−1, surface corrosion was weakened, and the local corrosion area was reduced. With the increase in nanoparticles, the surface deposition quality was improved, the unit cell structure was refined, the coating thickness and element penetration region were increased, the adhesion performance and corrosion resistance were improved, and defects such as surface cracks were reduced. Hence, only local corrosion occurred in corrosive media.
Figure 13d shows that when the particle concentration was 3 g·L
−1, the corrosion on the surface was significantly reduced, and slight corrosion occurred at the boundary of the protrusion structure. When the concentration of nanoparticles was the best, the surface quality of the coating was also the best. Many small-sized unit cell structures were found at the cellular protrusion structure. During corrosion, the protruding unit cell structure will be the first to contact the corrosive medium, thus effectively blocking the corrosion of the corrosive medium. At the same time, the element penetration region was the largest, and the corrosive medium did not easily penetrate the coating.
Figure 13e shows that when the particle concentration was 4 g·L
−1, the corrosion on the surface was aggravated and even occurred at the boundary of the cell. In addition, corrosion products were precipitated. When the concentration of nanoparticles exceeded the composite degree of the coating, crystal grains that are easily eroded and infiltrated by corrosive media will appear at the boundary of the cell structure. At the same time, the quality of the coating deposition and the element penetration region decrease, which accelerates the corrosion diffusion of the coating.
Figure 13f shows that when the particle concentration was 5 g·L
−1, corrosion pits and agglomeration of corrosion products appeared on the surface. When the concentration of nanoparticles was extremely high, the cell structure of the coating surface will exhibit grain precipitation, agglomeration, and porosity. Hence, the quality of the coating section was poor, the nanoparticles were not uniformly dispersed in the coating, and the element penetration region was small. During corrosion, the pores are easily penetrated by chloride ions, which accelerate corrosion diffusion to form corrosion pits that cause the agglomeration of corrosion products and aggravate this phenomenon.