Excellent Properties of Ni-15 wt.% W Alloy Electrodeposited from a Low-Temperature Pyrophosphate System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrodeposition of Ni-W Alloys
2.2. Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
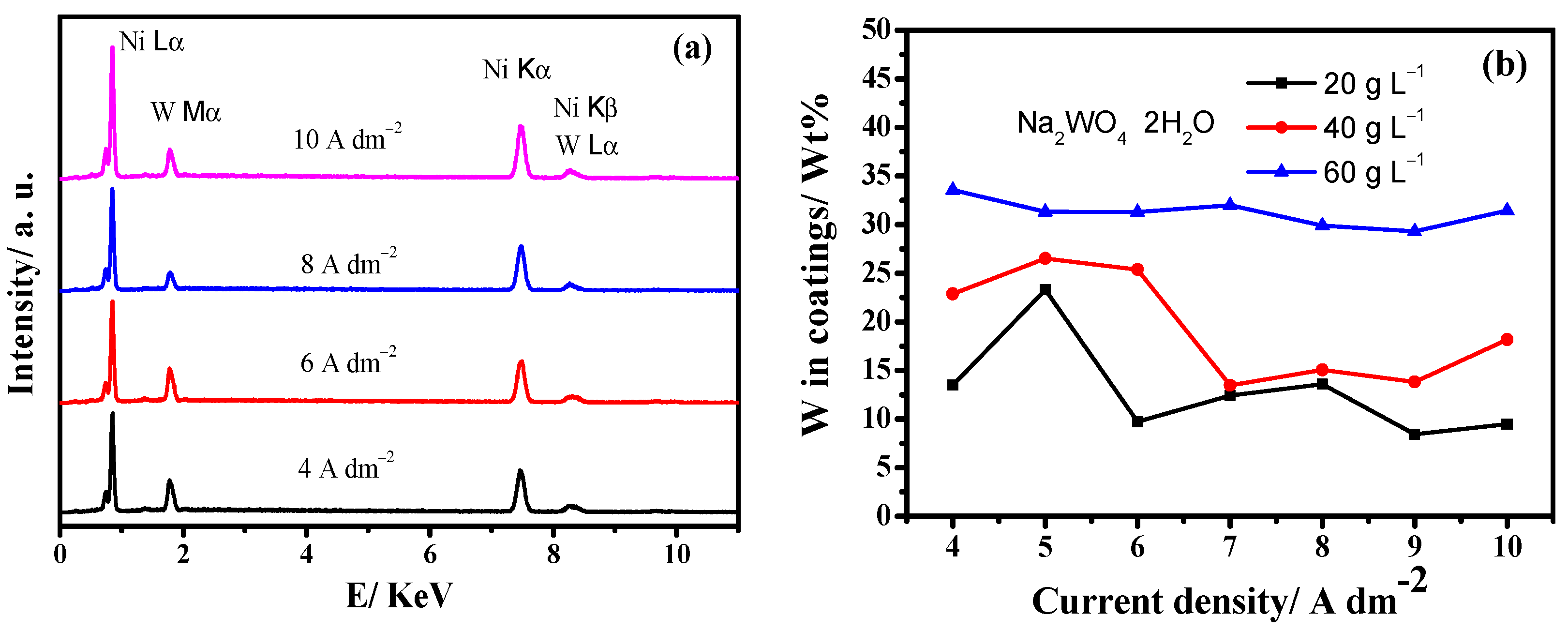
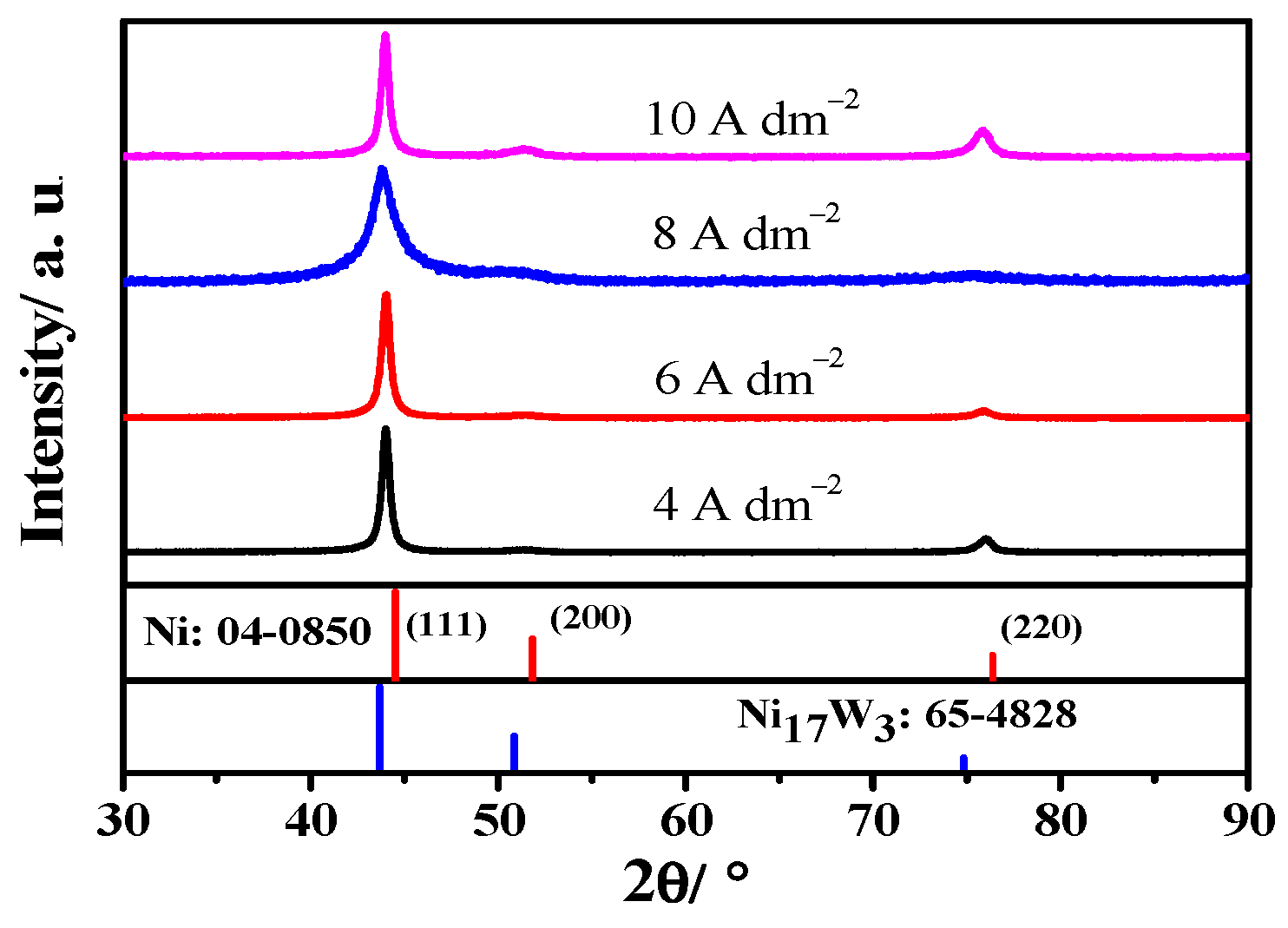
3.1. Electrodeposition of Ni-W Alloys
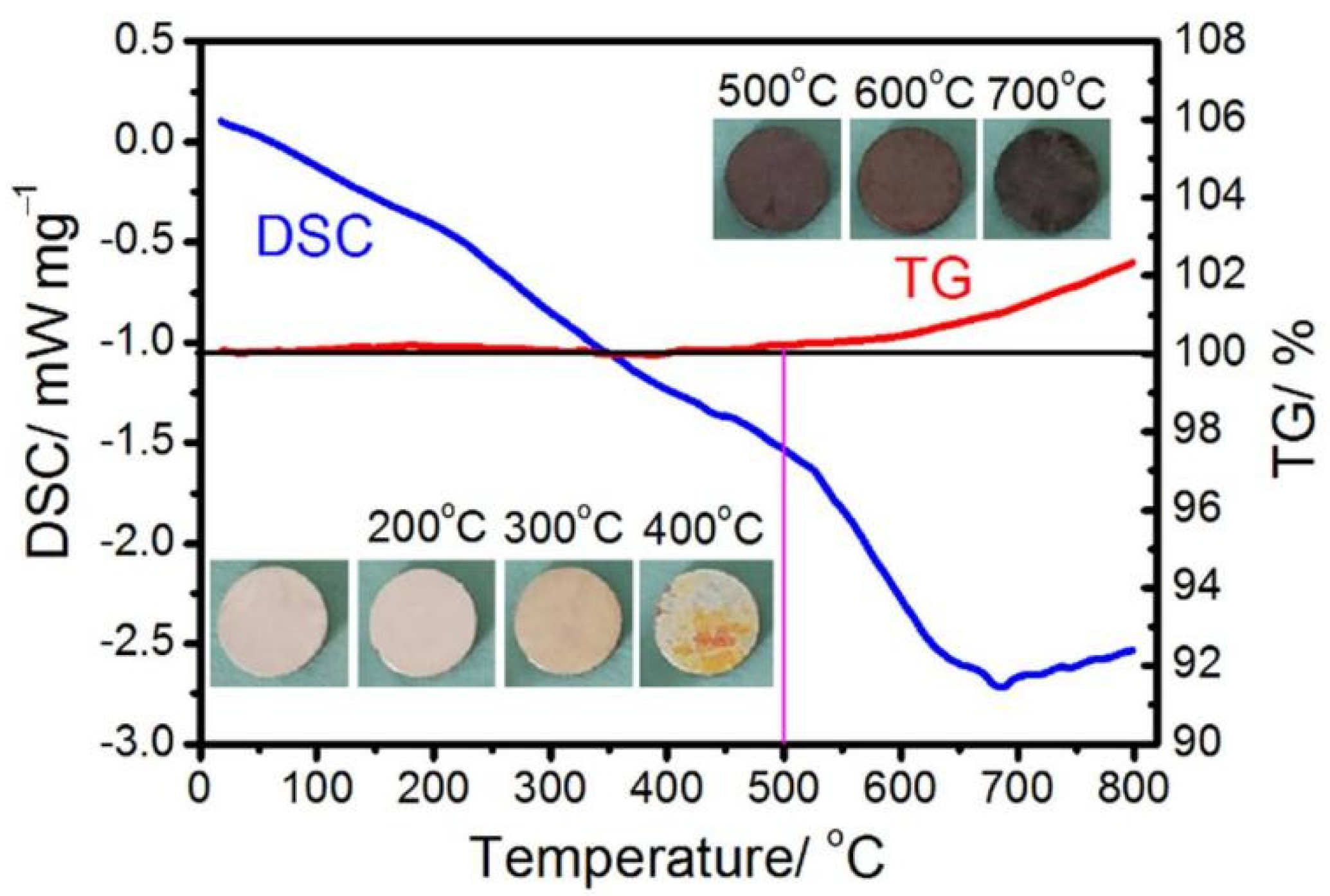
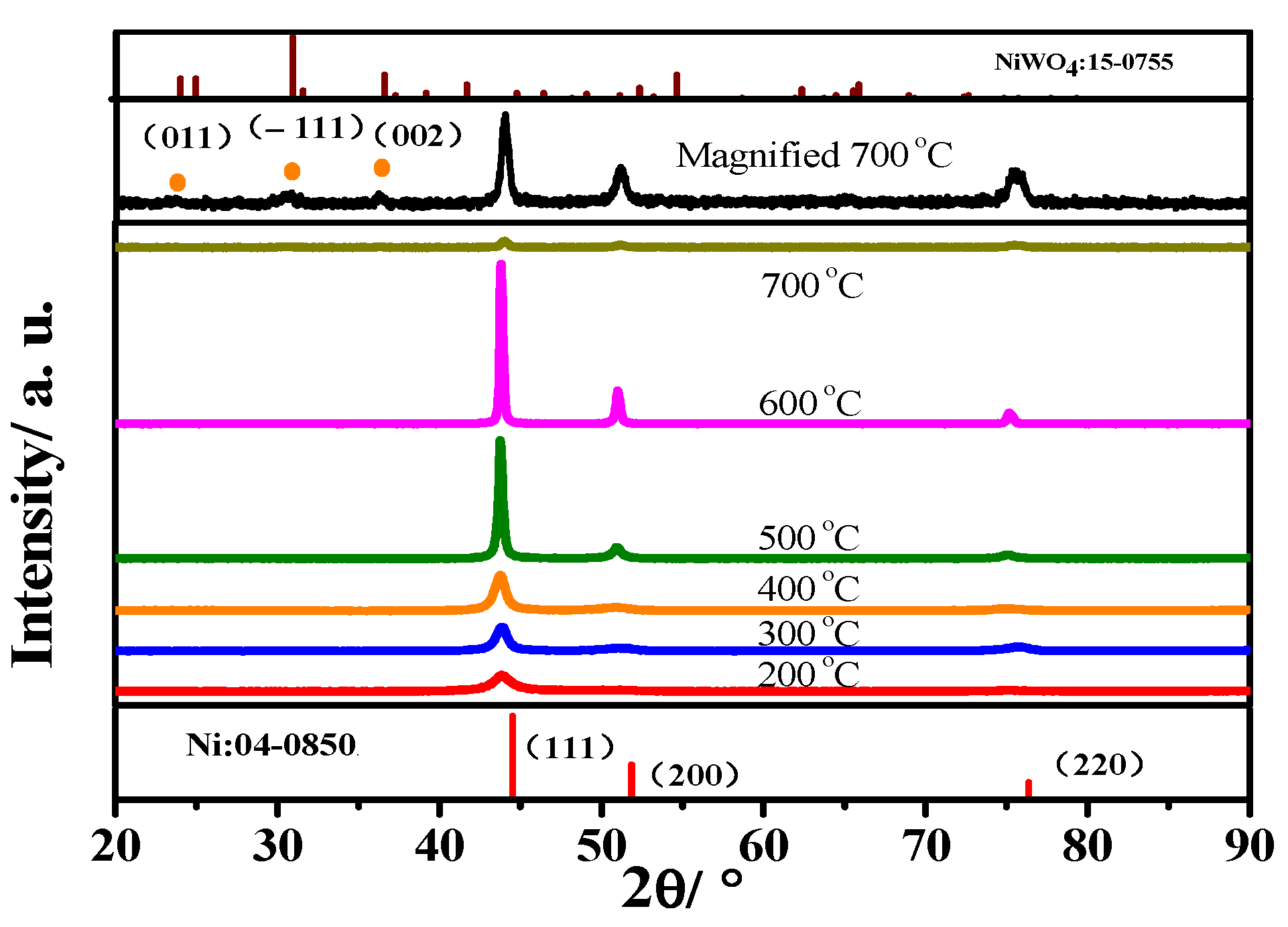
3.2. Heat Treatment of Ni-15 wt.% W Alloy
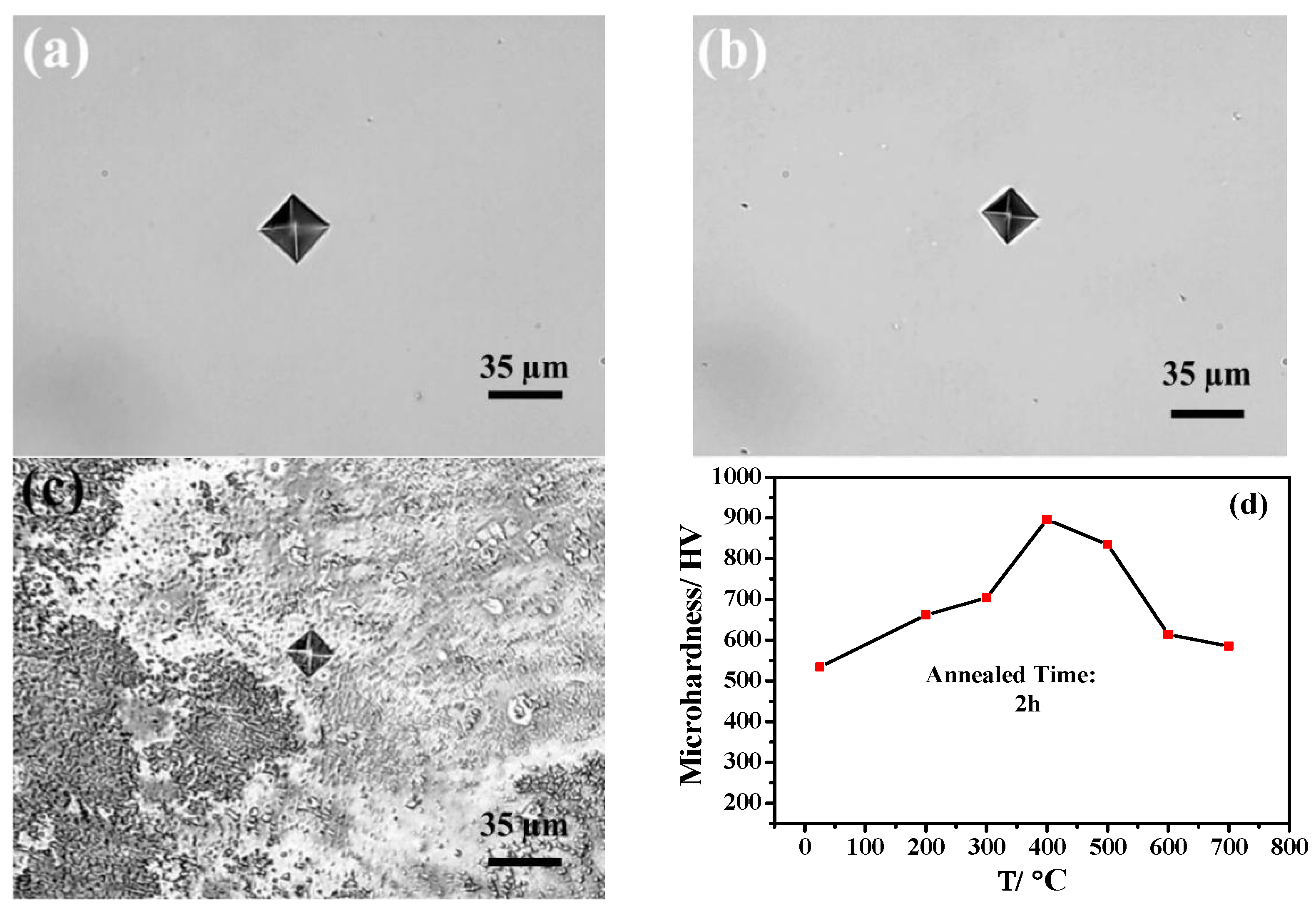
3.3. Microhardness of Ni-15 wt.% W Alloy
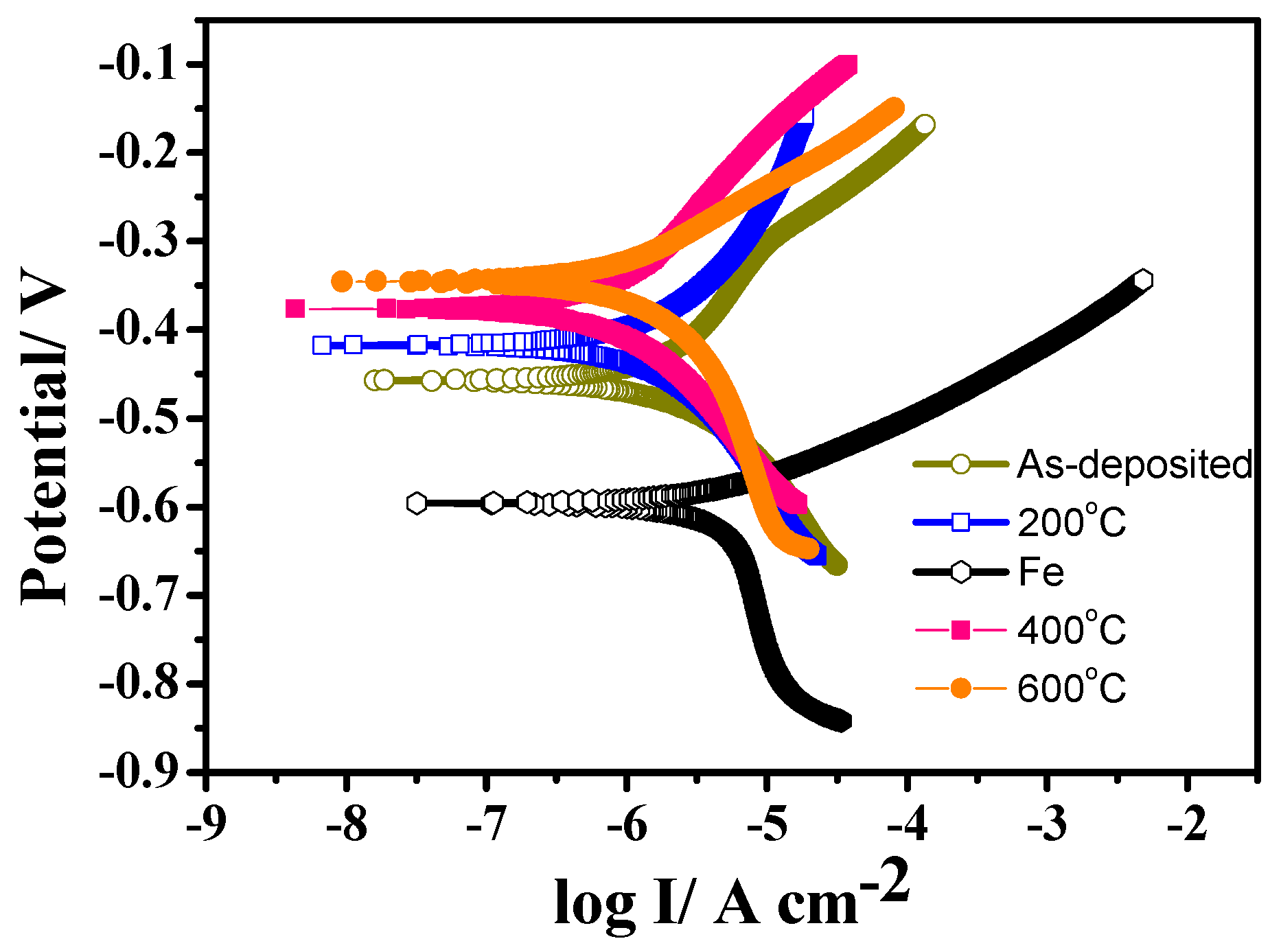
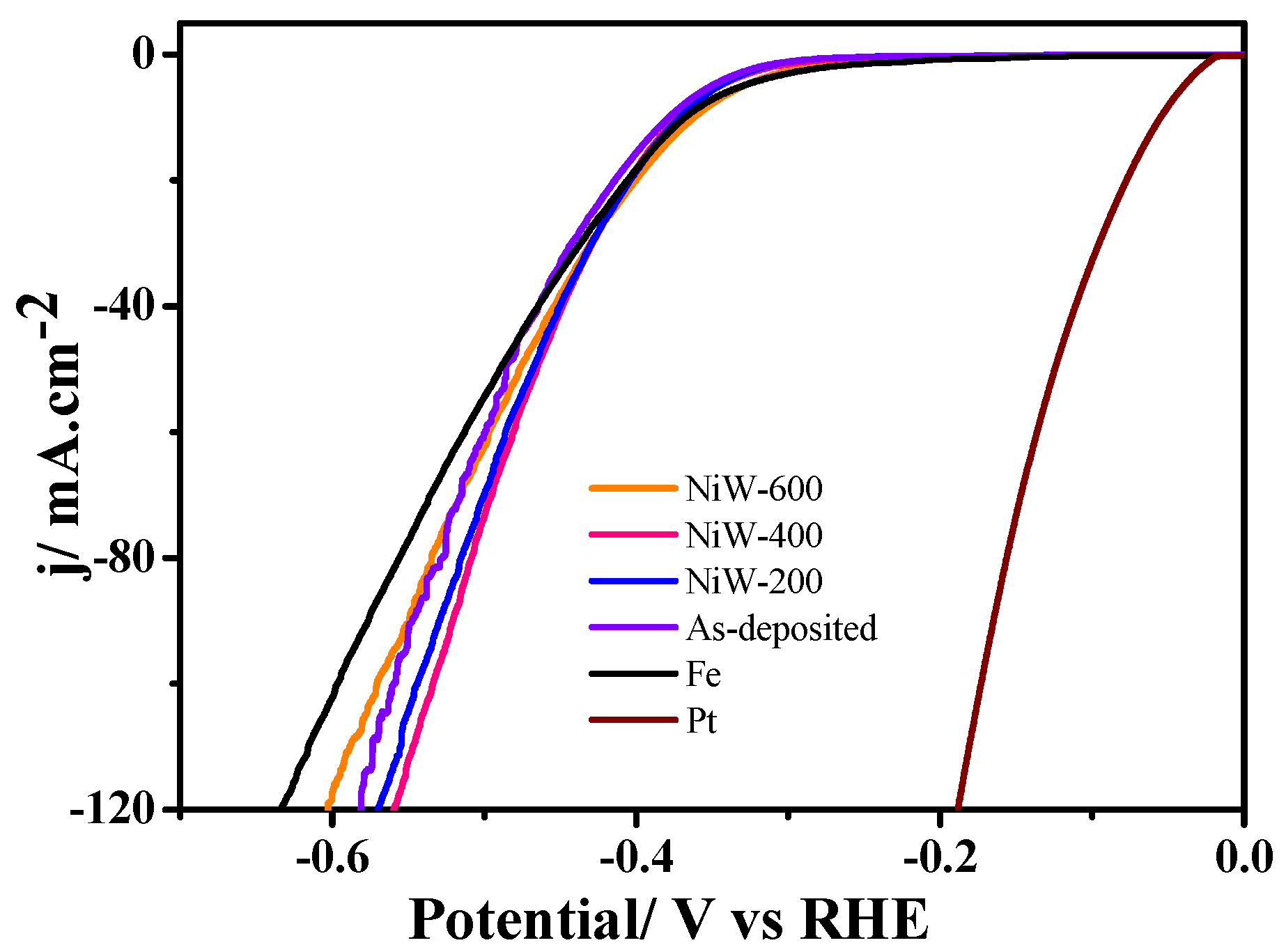
3.4. Electrochemical Properties of Ni-15 wt.% W Alloy Coating
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Zhou, Q.; Li, K.; Zhong, Q.; Bui, Q.B. Preparation of Ni–W–SiO2 nanocomposite coating and valuation of its hardness and corrosion resistance. Ceram. Int. 2015, 41, 79–84. [Google Scholar] [CrossRef]
- Määttä, A.; Kanerva, U.; Vuoristo, P. Structure and Tribological Characteristics of HVOF Coatings Sprayed from Powder Blends of Cr3C2-25NiCr and NiCrBSi Alloy. J. Therm. Spray Technol. 2010, 20, 366–371. [Google Scholar] [CrossRef]
- Huang, B.; Gao, Y.; Chen, P.; Xiong, W.; Tang, J. Microstructure and properties of Ni + B4C/Ti coatings by high-frequency induction cladding. Surf. Innov. 2019, 7, 59–67. [Google Scholar] [CrossRef]
- Zikin, A.; Antonov, M.; Hussainova, I.; Katona, L.; Gavrilović, A. High temperature wear of cermet particle reinforced NiCrBSi hardfacings. Tribol. Int. 2013, 68, 45–55. [Google Scholar] [CrossRef]
- Garrido, A.H.; González, R.; Cadenas, M.; Battez, A.H. Tribological behavior of laser-textured NiCrBSi coatings. Wear 2011, 271, 925–933. [Google Scholar] [CrossRef]
- Gyftou, P.; Stroumbouli, M.; Pavlatou, E.A.; Asimidis, P.; Spyrellis, N. Tribological study of Ni matrix composite coatings containing nano and micro SiC particles. Electrochim. Acta 2005, 50, 4544–4550. [Google Scholar] [CrossRef]
- Allahyarzadeh, M.H.; Aliofkhazraei, M.; Rezvanian, A.R.; Torabinejad, V.; Sabour Rouhaghdam, A.R. Ni-W electrodeposited coatings: Characterization, properties and applications. Surf. Coat. Technol. 2016, 307, 978–1010. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Abdolmaleki, M.; Sadjadi, S.A.S. Electrodeposition and mechanical properties of Ni-W-B composites from tartrate bath. Prot. Met. Phys. Chem. Surf. 2010, 46, 117–122. [Google Scholar] [CrossRef]
- Kirihara, S.; Umeda, Y.; Tashiro, K.; Honma, H.; Takai, O. Development of Ni-W alloy plating as a substitution of hard chromium plating. Trans. Mater. Res. Soc. Jpn. 2016, 41, 35–39. [Google Scholar] [CrossRef] [Green Version]
- Donten, M.; Cesiulis, H.; Stojek, Z. Electrodeposition and properties of Ni-W, Fe-W and Fe-Ni-W amorphous alloys. A comparative study. Electrochim. Acta 2000, 45, 3389–3396. [Google Scholar] [CrossRef]
- Fang, M.; Dong, G.; Wei, R.; Ho, J.C. Hierarchical Nanostructures: Hierarchical Nanostructures: Design for Sustainable Water Splitting. Adv. Energy Mater. 2017, 7, 1770135. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Qi, J.; Liu, K.; Cao, R. A Nickel-Based Integrated Electrode from an Autologous Growth Strategy for Highly Efficient Water Oxidation. Adv. Energy Mater. 2016, 6, 1502489. [Google Scholar] [CrossRef]
- Pletcher, D.; Li, X. Prospects for alkaline zero gap water electrolysers for hydrogen production. Int. J. Hydrogen Energy 2011, 36, 15089–15104. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Li, Z.; Liu, Y.; Yang, Y.; Wang, L. Nickel-iron phosphides nanorods derived from bimetallic-organic frameworks for hydrogen evolution reaction. Appl. Surf. Sci. 2018, 457, 1081–1086. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Zhu, Y.; Li, L.H.; Han, Y.; Chen, Y.; Du, A.; Jaroniec, M.; Qiao, S.Z. Hydrogen evolution by a metal-free electrocatalyst. Nat. Commun. 2014, 5, 15729–15735. [Google Scholar] [CrossRef] [Green Version]
- McKone, J.R.; Marinescu, S.C.; Brunschwig, B.S.; Winkler, J.R.; Gray, H.B. Earth-abundant hydrogen evolution electrocatalysts. Chem. Sci. 2014, 5, 865–878. [Google Scholar] [CrossRef] [Green Version]
- Karaguiozova, Z.; Stavrev, S.; Babul, T.; Ciski, A. Influence of cubic nanostructure additions on the properties of electroless coatings. Int. J. Nanomanufacturing 2010, 5, 129–138. [Google Scholar] [CrossRef]
- Wang, H.-T.; Sheu, H.-H.; Ger, M.-D.; Hou, K.-H. The effect of heat treatment on the microstructure and mechanical properties of electrodeposited nanocrystalline Ni–W/diamond composite coatings. Surf. Coat. Technol. 2014, 259, 268–273. [Google Scholar] [CrossRef]
- Yamasaki, T.; Oda, N.; Matsuoka, H.; Fukami, T. Tensile strength of electrodeposited nanocrystalline Ni–W alloys with finely dispersed micrometer-sized array through-holes. Mater. Sci. Eng. A 2007, 449–451, 833–835. [Google Scholar] [CrossRef]
- Suvorov, D.; Gololobov, G.; Tarabrin, D.; Slivkin, E.; Karabanov, S.; Tolstoguzov, A. Electrochemical Deposition of Ni–W Crack-Free Coatings. Coatings 2018, 8, 233. [Google Scholar] [CrossRef] [Green Version]
- Ghorbanian, B.; Tajally, M.; Khoie, S.M.M.; Tavakoli, H. Corrosion Behavior of MoS2-Incorporated PEO Coatings Prepared on Al Alloy. Surf. Innov. 2020, 8, 252–262. [Google Scholar] [CrossRef]
- Jameei Rad, P.; Aliofkhazraei, M.; Barati Darband, G. Ni-W nanostructure well-marked by Ni selective etching for enhanced hydrogen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 880–894. [Google Scholar] [CrossRef]
- Krasikov, A.V.; Krasikov, V.L. Mechanism of Nickel-Tungsten Alloy Electrodeposition from Pyrophosphate Electrolyte. Bull. SPbSTI 2016, 36, 12–23. [Google Scholar] [CrossRef]
- Panagopoulos, C.N.; Plainakis, G.D.; Lagaris, D.A. Nanocrystalline Ni–W coatings on copper. Mater. Sci. Eng B 2011, 176, 477–479. [Google Scholar] [CrossRef]
- Yin, D.; Marvel, C.J.; Cui, F.Y.; Vinci, R.P.; Harmer, M.P. Microstructure and fracture toughness of electrodeposited Ni-21 at.% W alloy thick films. Acta Mater. 2018, 143, 272–280. [Google Scholar] [CrossRef]
- Haj-Taieb, M.; Haseeb, A.S.M.A.; Caulfield, J.; Bade, K.; Aktaa, J.; Hemker, K.J. Thermal stability of electrodeposited LIGA Ni–W alloys for high temperature MEMS applications. Microsyst. Technol. 2008, 14, 1531–1536. [Google Scholar] [CrossRef]
- Hwang, W.S.; Lee, J.J. Effects of Heat Treatment on Hardness of Nanocrystalline Ni-W Electrodeposits. Mater. Sci. Forum 2006, 510–511, 1126–1129. [Google Scholar] [CrossRef]
- Hernn, D.M.V.; Aida, M.E.; Gilberto, B.G. Detailed Study of the Electrochemical Behavior of Low Reflectivity TiAlN Coatings. Surf. Innov. 2021, 9, 293–307. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Abdolmaleki, M.; Ghahremani, J. Investigation of corrosion resistance of electrodeposited Ni–W/SiC composite coatings. Corros. Eng. Sci. Technol. 2013, 49, 247–253. [Google Scholar] [CrossRef]
- Popczyk, M.; Kubisztal, J.; Swinarew, A.S.; Waśkiewicz, Z.; Stanula, A.; Knechtle, B. Corrosion Resistance of Heat-Treated Ni-W Alloy Coatings. Materials 2020, 13, 1172. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Yu, L.B.; Zhong, Q.J.; Gu, J.F.; Zhou, Q.Y.; Zhong, Q.D. The Effect of Cl- Concentration on the Corrosion Behavior of Electroplated Cu-Ni-W Alloy Coating. Adv. Mater. Res. 2013, 785–786, 953–956. [Google Scholar] [CrossRef]
- Jinlong, L.; Zhuqing, W.; Tongxiang, L.; Suzuki, K.; Hideo, M. Effect of tungsten on microstructures of annealed electrodeposited Ni-W alloy and its corrosion resistance. Surf. Coat. Technol. 2018, 337, 516–524. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, Y.; He, H.; Tan, W. Potentiostatic electrodeposition of Ni-Se-Cu on nickel foam as an electrocatalyst for hydrogen evolution reaction. J. Colloid Interface Sci. 2020, 578, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Elias, L.; Scott, K.; Hegde, A.C. Electrolytic Synthesis and Characterization of Electrocatalytic Ni-W Alloy. J. Mater. Eng. Perform. 2015, 24, 4182–4191. [Google Scholar] [CrossRef]
- Elias, L.; Cao, P.; Hegde, A.C. Magnetoelectrodeposition of Ni–W alloy coatings for enhanced hydrogen evolution reaction. RSC Adv. 2016, 6, 111358–111365. [Google Scholar] [CrossRef]
- Elias, L.; Hegde, A.C. Electrolytic Synthesis of Ni-W-MWCNT Composite Coating for Alkaline Hydrogen Evolution Reaction. J. Mater. Eng. Perform. 2018, 27, 1033–1039. [Google Scholar] [CrossRef]
- Ábel, M.; Záchenská, J.; Dobročka, E.; Zemanová, M. Electrocatalytic properties of pulse plated Ni-W alloy coatings in alkaline electrolytes. Trans. IMF 2021, 99, 23–28. [Google Scholar] [CrossRef]
- Ahn, S.H.; Choi, I.; Park, H.Y.; Hwang, S.J.; Yoo, S.J.; Cho, E.; Kim, H.J.; Henkensmeier, D.; Nam, S.W.; Kim, S.K.; et al. Effect of morphology of electrodeposited Ni catalysts on the behavior of bubbles generated during the oxygen evolution reaction in alkaline water electrolysis. Chem. Commun. 2013, 49, 9323–9325. [Google Scholar] [CrossRef]

| Chemicals/Parameters | Values (g/L) |
|---|---|
| NiSO4·6H2O | 12 |
| NaWO4·2H2O | 20, 30, 40, 50, 60 |
| Na4P2O7·10H2O | 40 |
| H3PO4 (85%) | 10 |
| NH3·H2O (25–28%) | ~20 |
| Additions | 20 |
| Bath temperature | 40 °C |
| Current density | 4–10 A/dm2 |
| pH | 8.5–9.0 |
| T/°C | 2θ/° | β/° | d/nm |
|---|---|---|---|
| As-deposited | 43.849 | 0.629 | 13.46 |
| 200 | 43.89 | 0.828 | 10.23 |
| 300 | 43.849 | 0.689 | 12.29 |
| 400 | 43.747 | 0.653 | 12.96 |
| 500 | 43.748 | 0.323 | 26.21 |
| 600 | 43.789 | 0.235 | 36.03 |
| 700 | 44.034 | 0.451 | - |
| Sample | T/°C | Icorr/μA.cm−2 | Ecorr (vs. SCE)/mV | ΔEcorr/mV |
|---|---|---|---|---|
| Fe | - | 5.71 | -611 | 0 |
| Ni-W alloy coating | As-deposited | 4.45 | -447 | 164 |
| 200 | 2.33 | -440 | 171 | |
| 400 | 2.02 | -386 | 225 | |
| 600 | 1.91 | -349 | 262 |
| Sample | T/(°C) | Rs/ Ω cm2 | CPE1-T/ F cm−2 | CPE1-P | Rf/ Ω cm2 | CPE2-T/ F cm−2 | CPE2-P | Rct/ Ω cm2 |
|---|---|---|---|---|---|---|---|---|
| Fe | - | 5.42 | 0.00059 | 0.76 | 27.61 | 0.0022 | 0.90 | 958 |
| Ni-W alloy coating | - | 5.39 | 0.000039 | 0.84 | 12.25 | 0.0000050 | 1.09 | 21,468 |
| 200 | 5.74 | 0.000033 | 0.85 | 5.17 | 0.0000086 | 1.05 | 22,547 | |
| 400 | 5.79 | 0.000026 | 0.86 | 12.91 | 0.0000018 | 1.10 | 20,561 | |
| 600 | 5.87 | 0.000044 | 0.86 | 20.62 | 0.0000015 | 1.16 | 37,552 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, C.; Sa, Z.; Liu, Y.; Zhao, L.; Wu, F.; Bai, W. Excellent Properties of Ni-15 wt.% W Alloy Electrodeposited from a Low-Temperature Pyrophosphate System. Coatings 2021, 11, 1262. https://doi.org/10.3390/coatings11101262
Su C, Sa Z, Liu Y, Zhao L, Wu F, Bai W. Excellent Properties of Ni-15 wt.% W Alloy Electrodeposited from a Low-Temperature Pyrophosphate System. Coatings. 2021; 11(10):1262. https://doi.org/10.3390/coatings11101262
Chicago/Turabian StyleSu, Changwei, Zhaoyao Sa, Yangyang Liu, Linfu Zhao, Fengjing Wu, and Wei Bai. 2021. "Excellent Properties of Ni-15 wt.% W Alloy Electrodeposited from a Low-Temperature Pyrophosphate System" Coatings 11, no. 10: 1262. https://doi.org/10.3390/coatings11101262






