Bonding Mechanism of Cold-Sprayed TiO2 Coatings on Copper and Aluminum Substrates
Abstract
:1. Introduction
2. Materials and Process
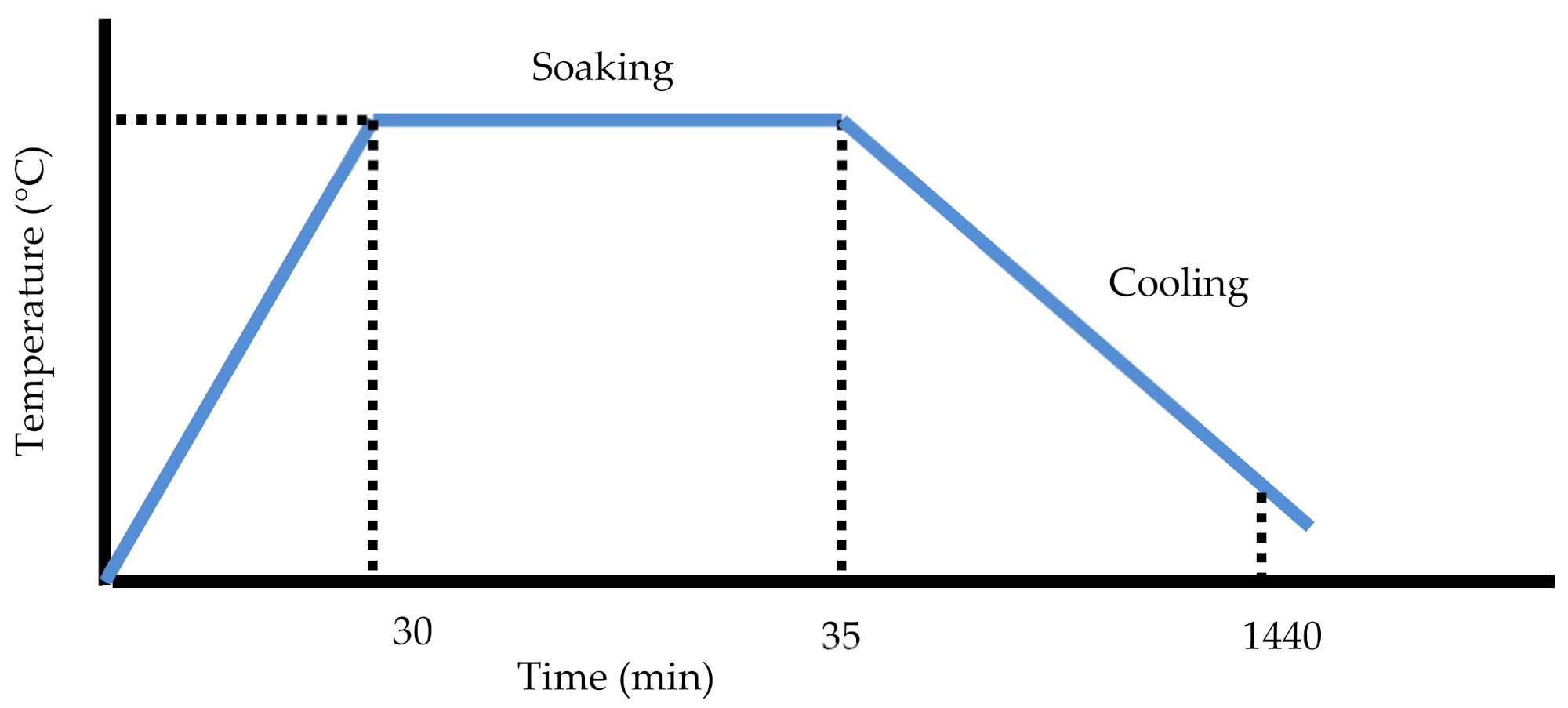
2.1. Process
2.2. Materials
2.3. Characterization
2.3.1. Strength Testing
2.3.2. Coatings Evaluation
2.3.3. Micro Vickers Hardness
2.3.4. Substrate Oxide Layer Evaluations
2.3.5. Oxide Surface Roughness
2.3.6. Single-Particle Deposition
2.3.7. Transmission Electron Microscopy Testing
3. Results
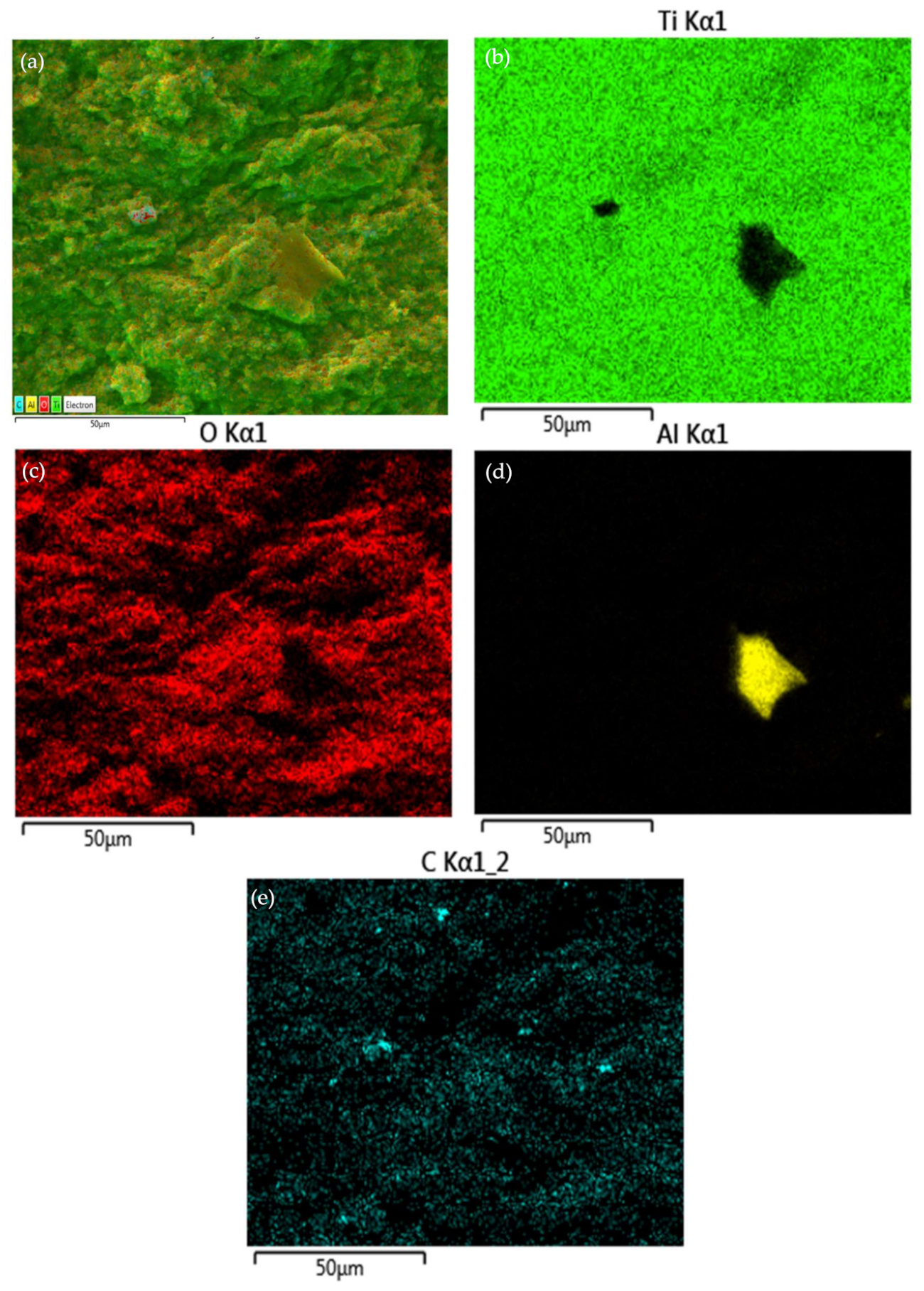
3.1. Titanium Dioxide Coating Microstructure on Annealed Pure Metals
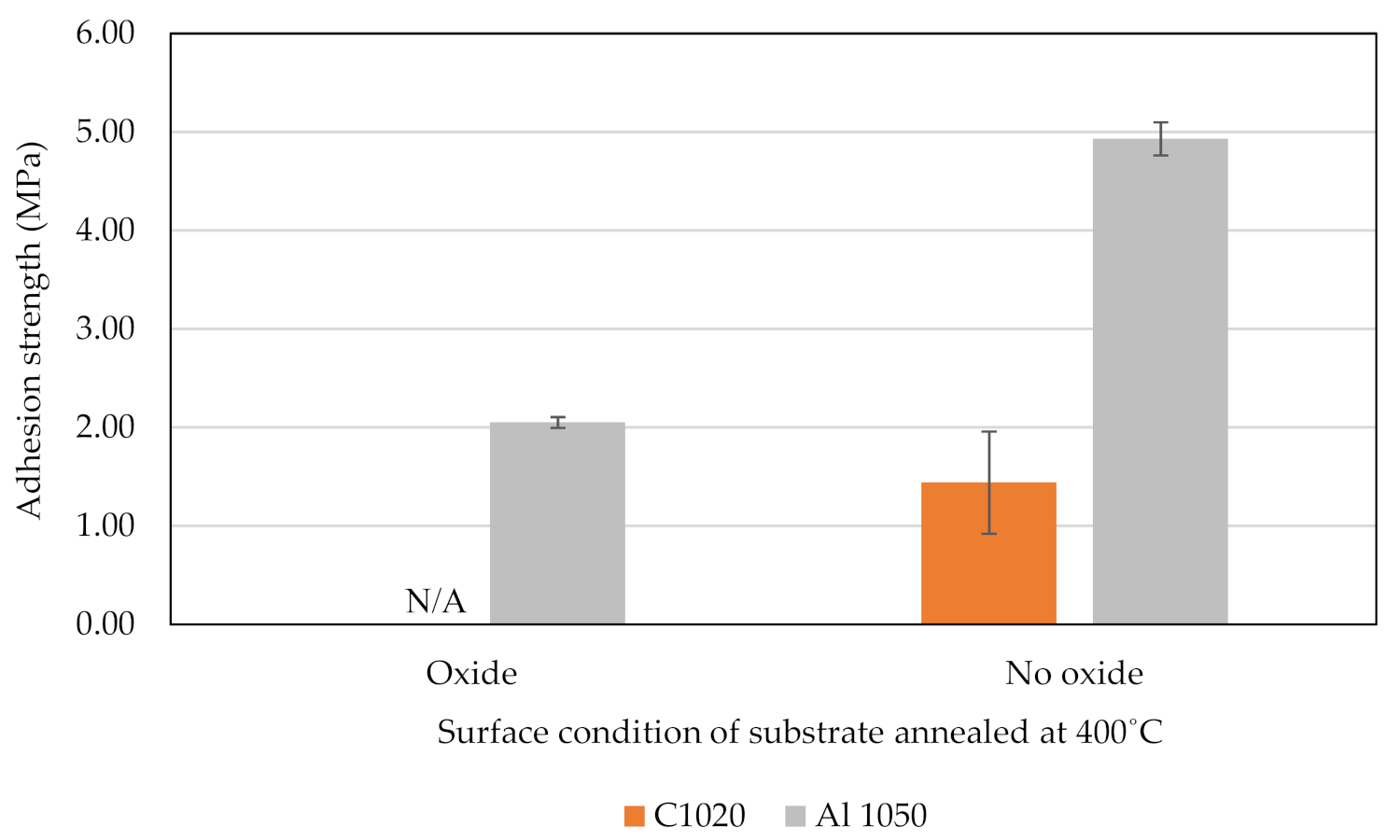
3.2. Strength of Adhesion
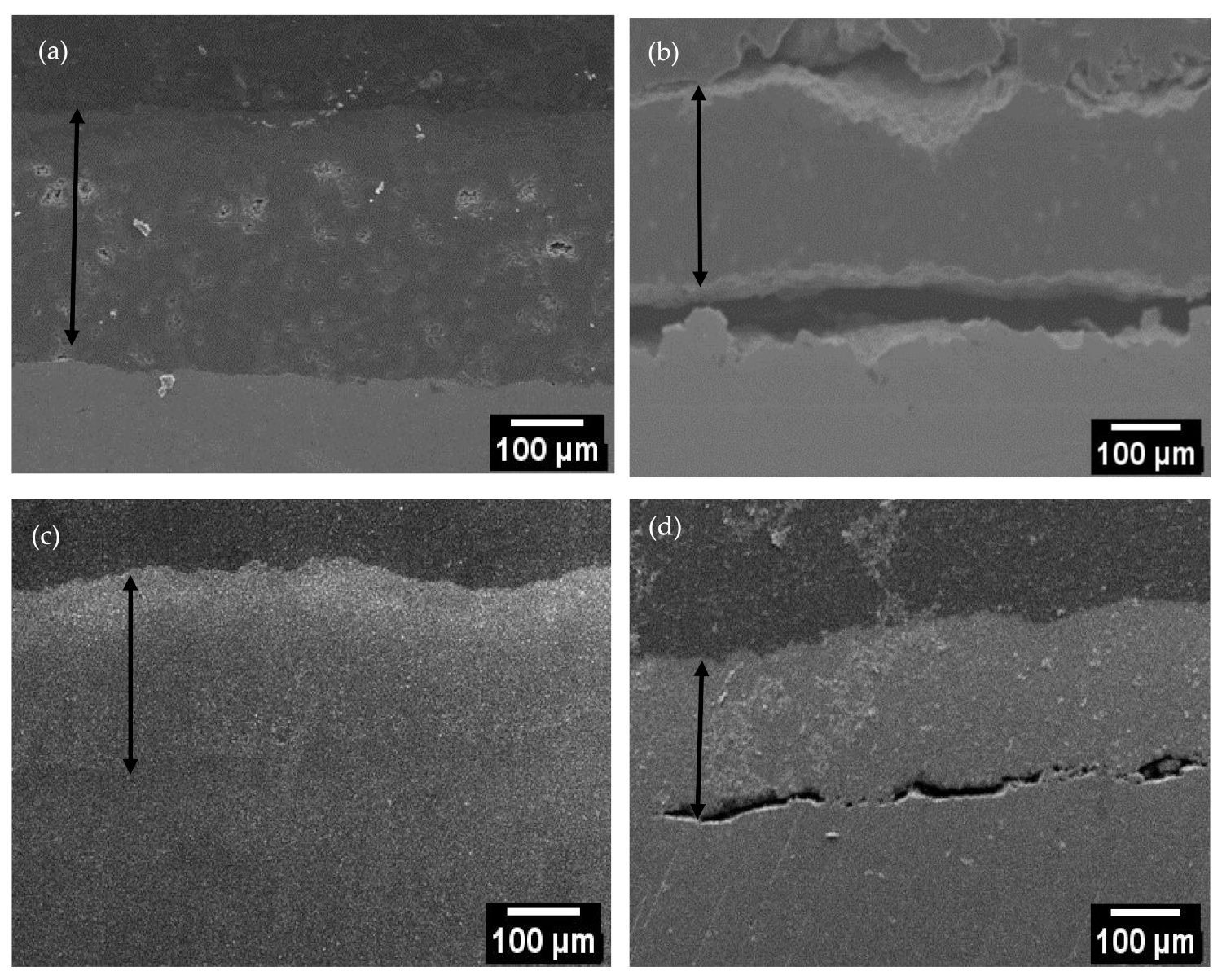
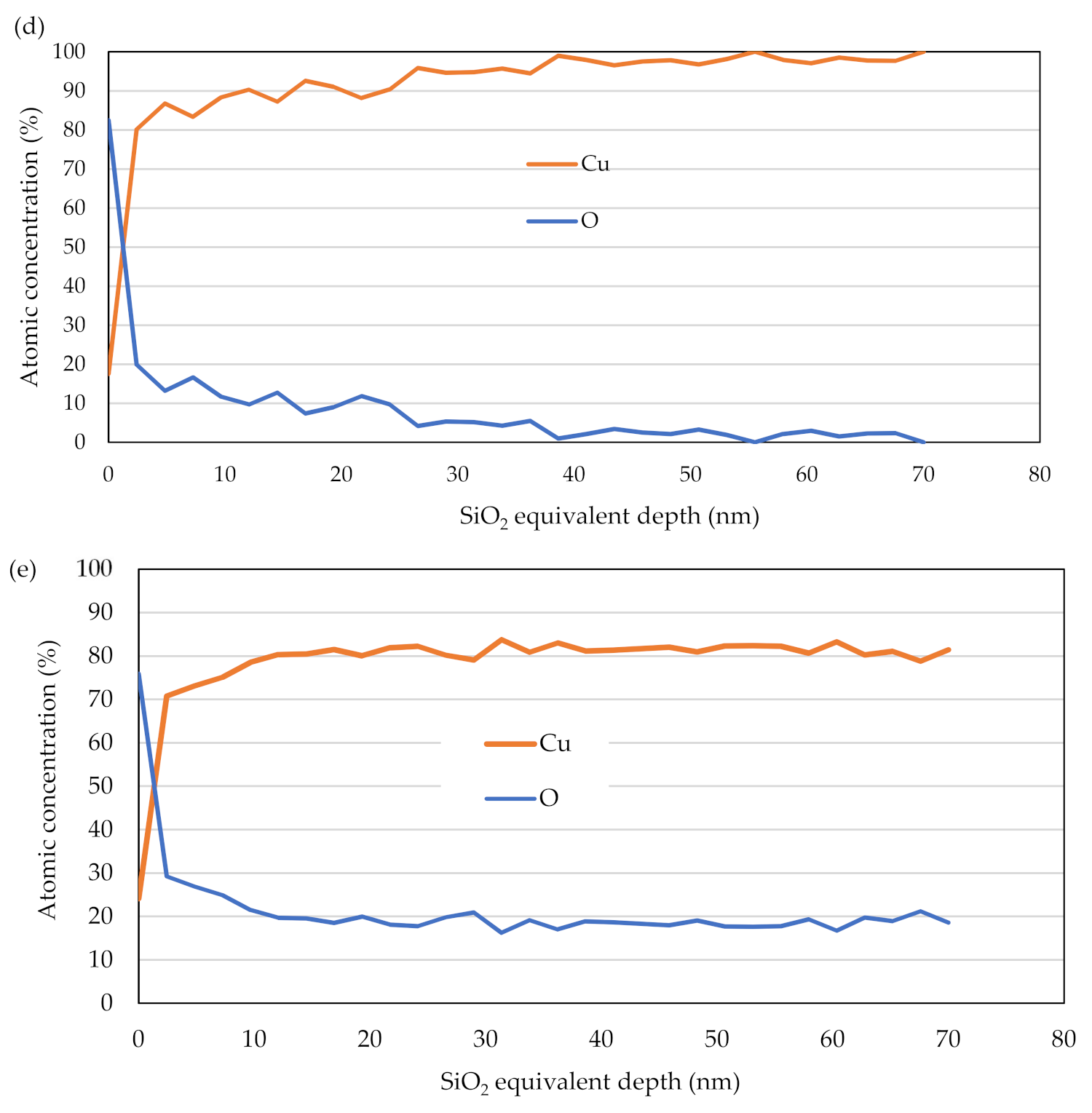
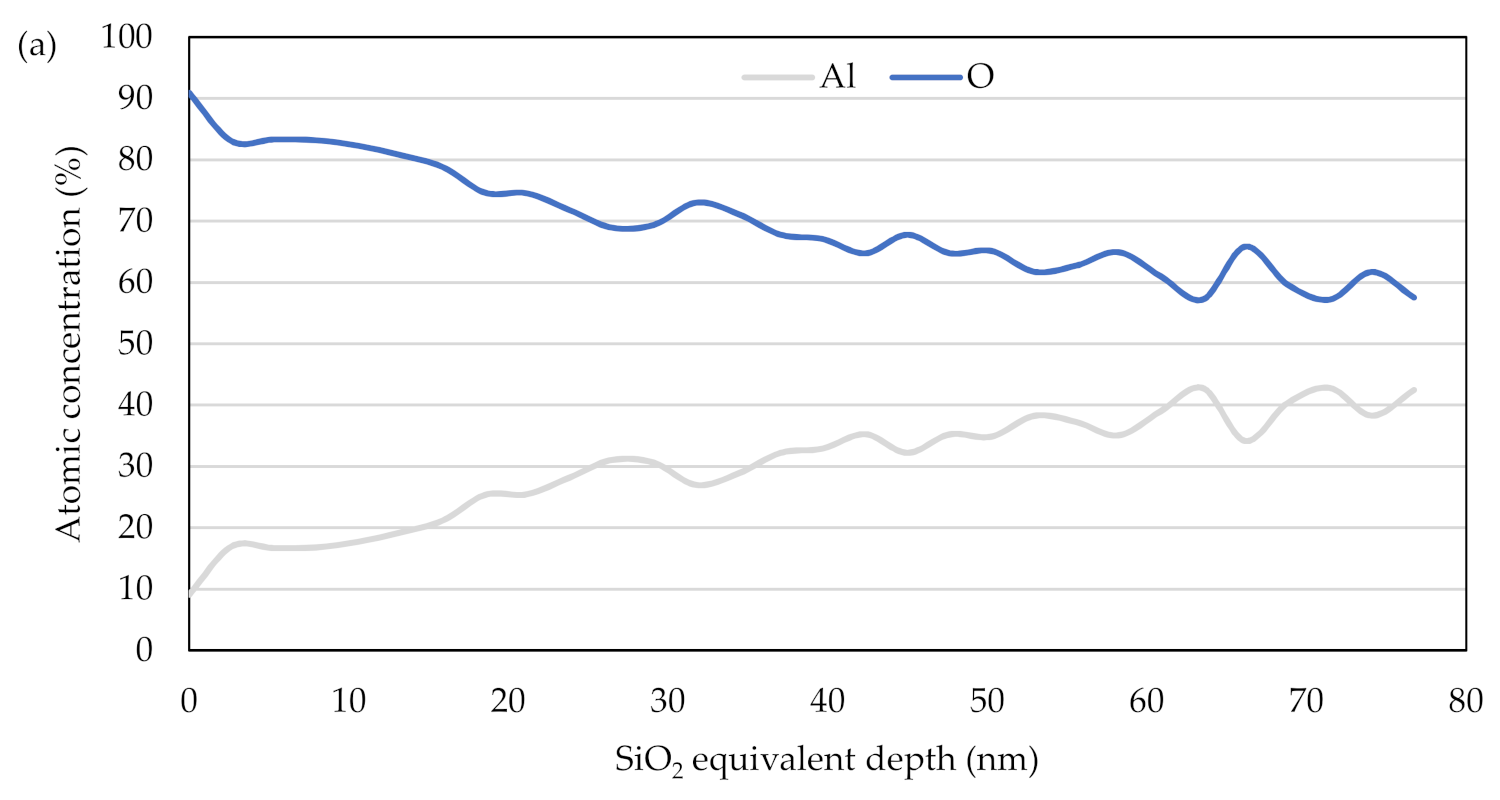
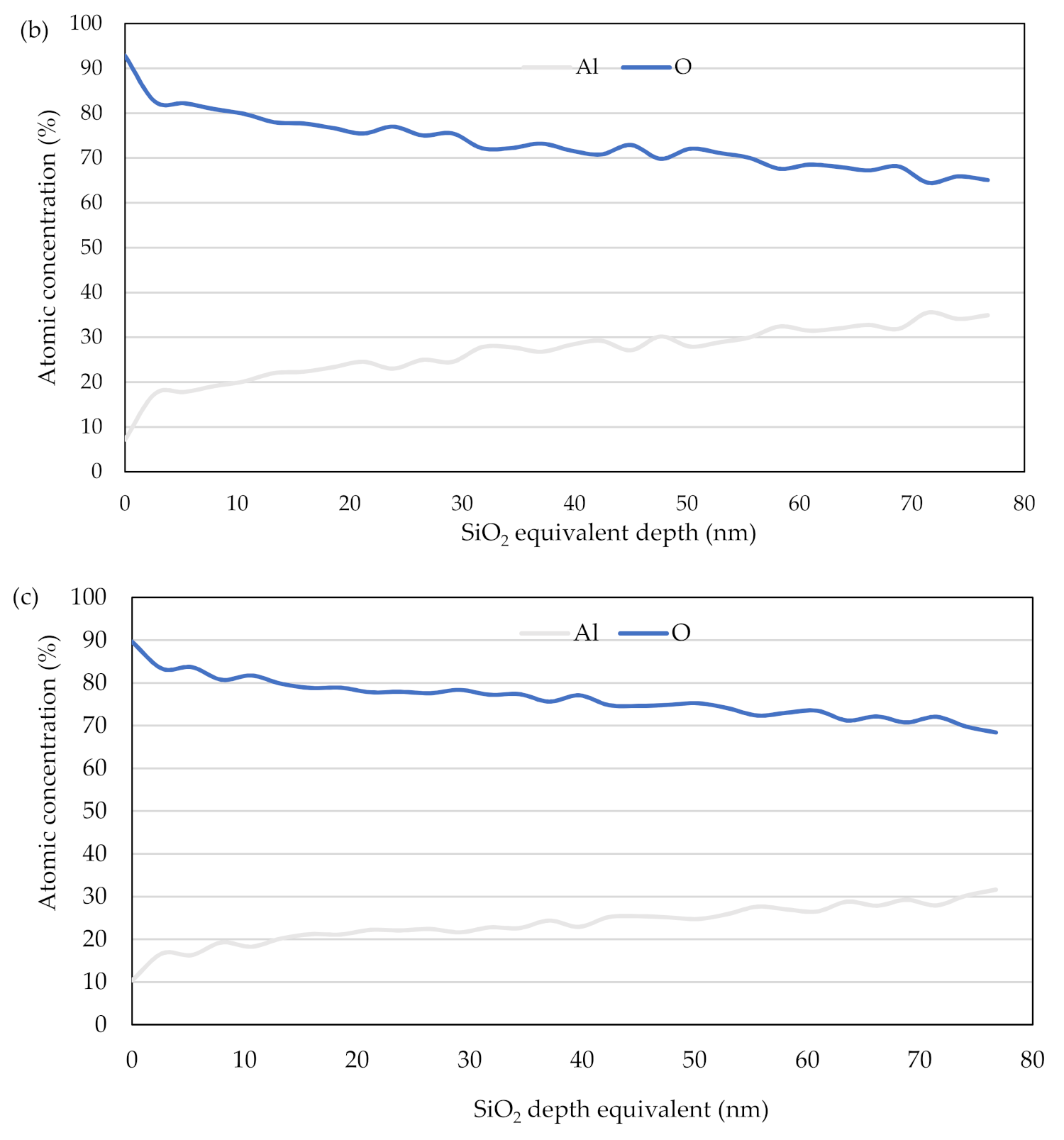
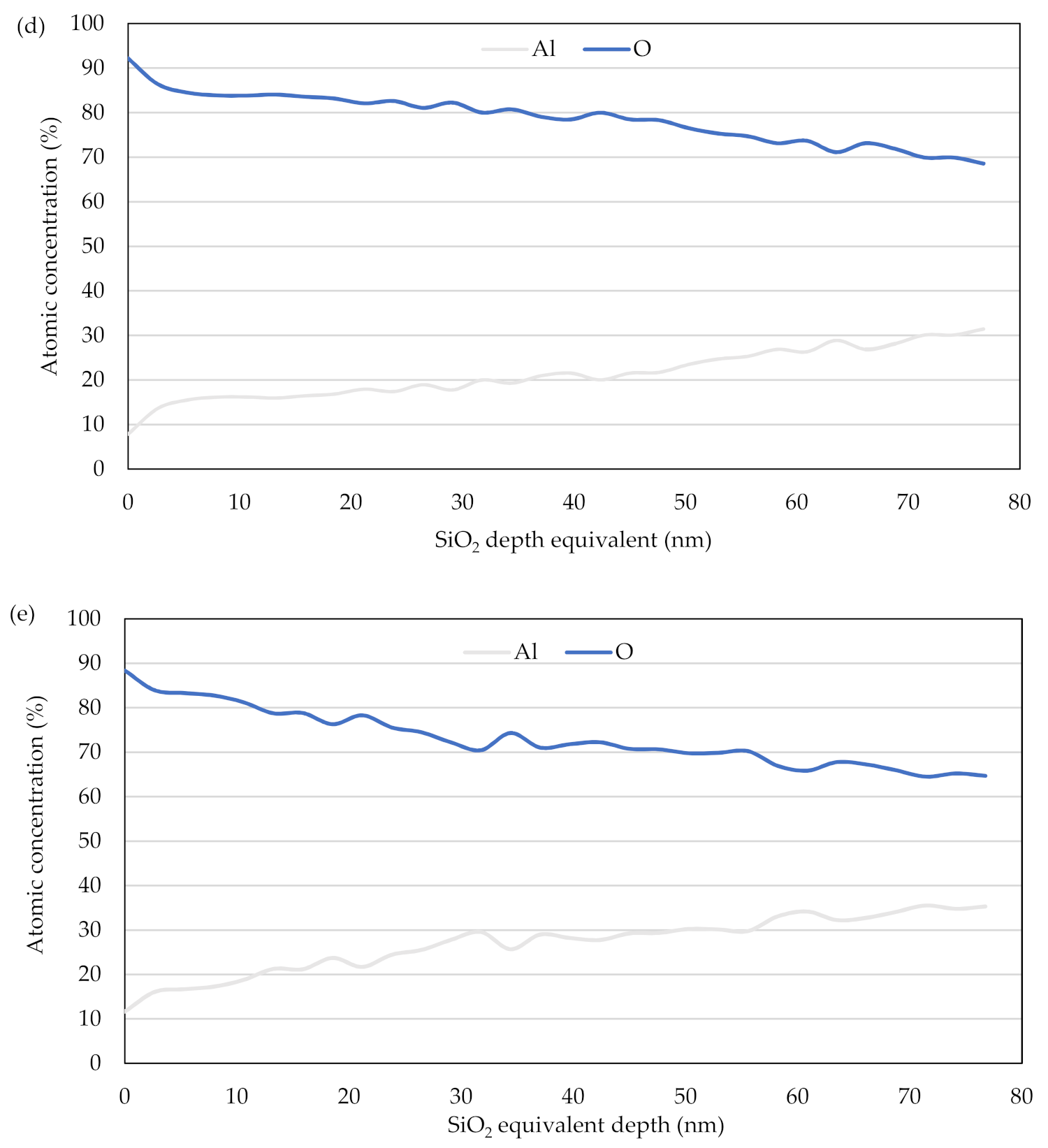
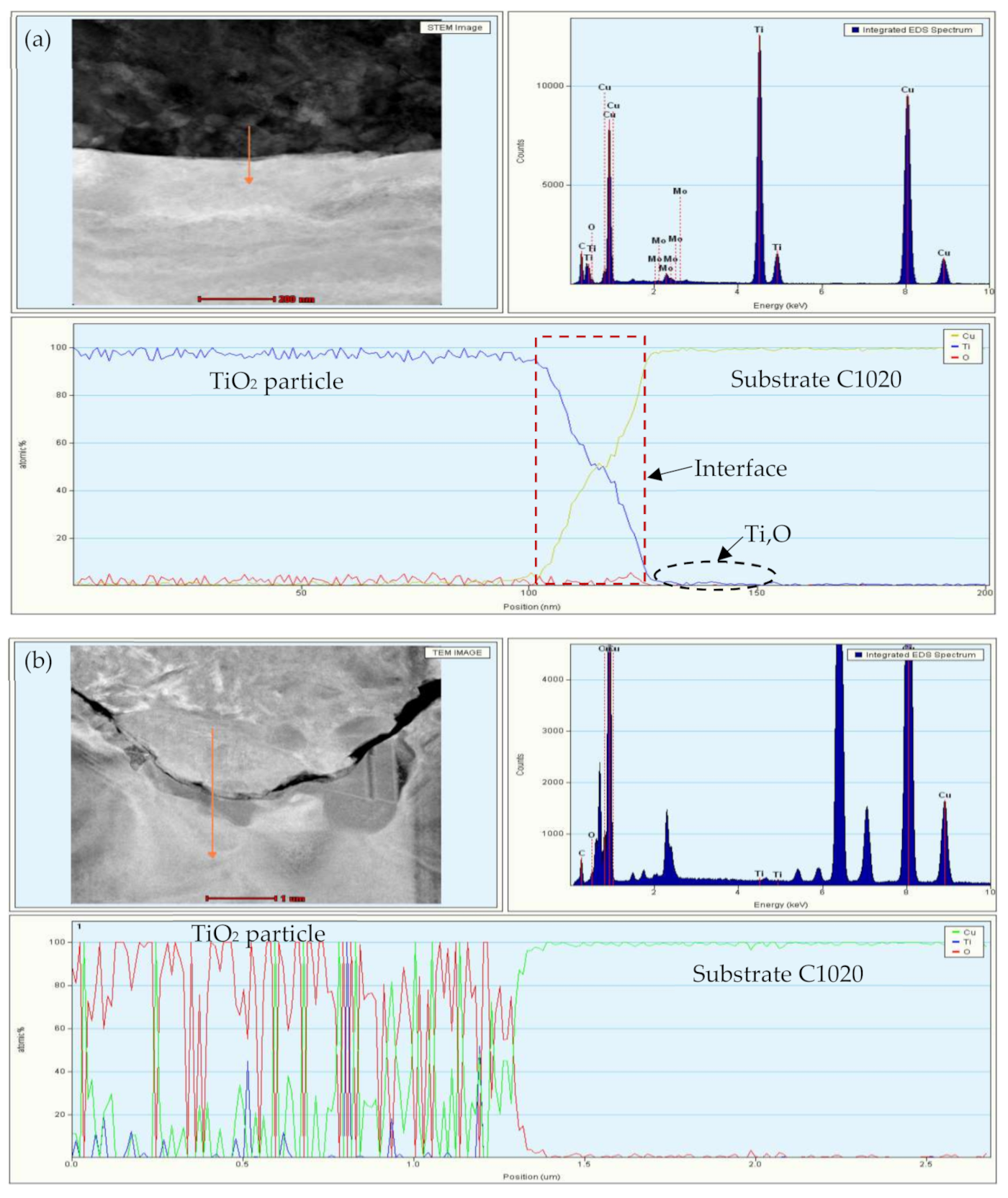
3.3. Depth Profile of the Oxide Layer
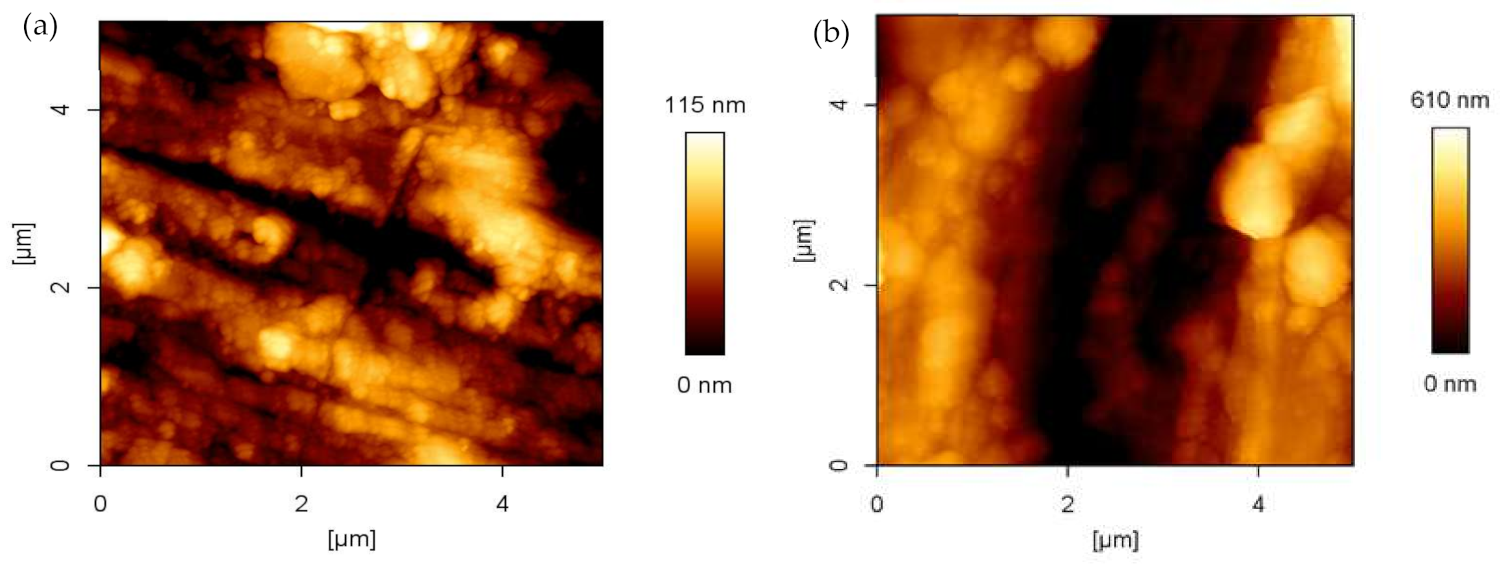
3.4. Oxide Surface Roughness
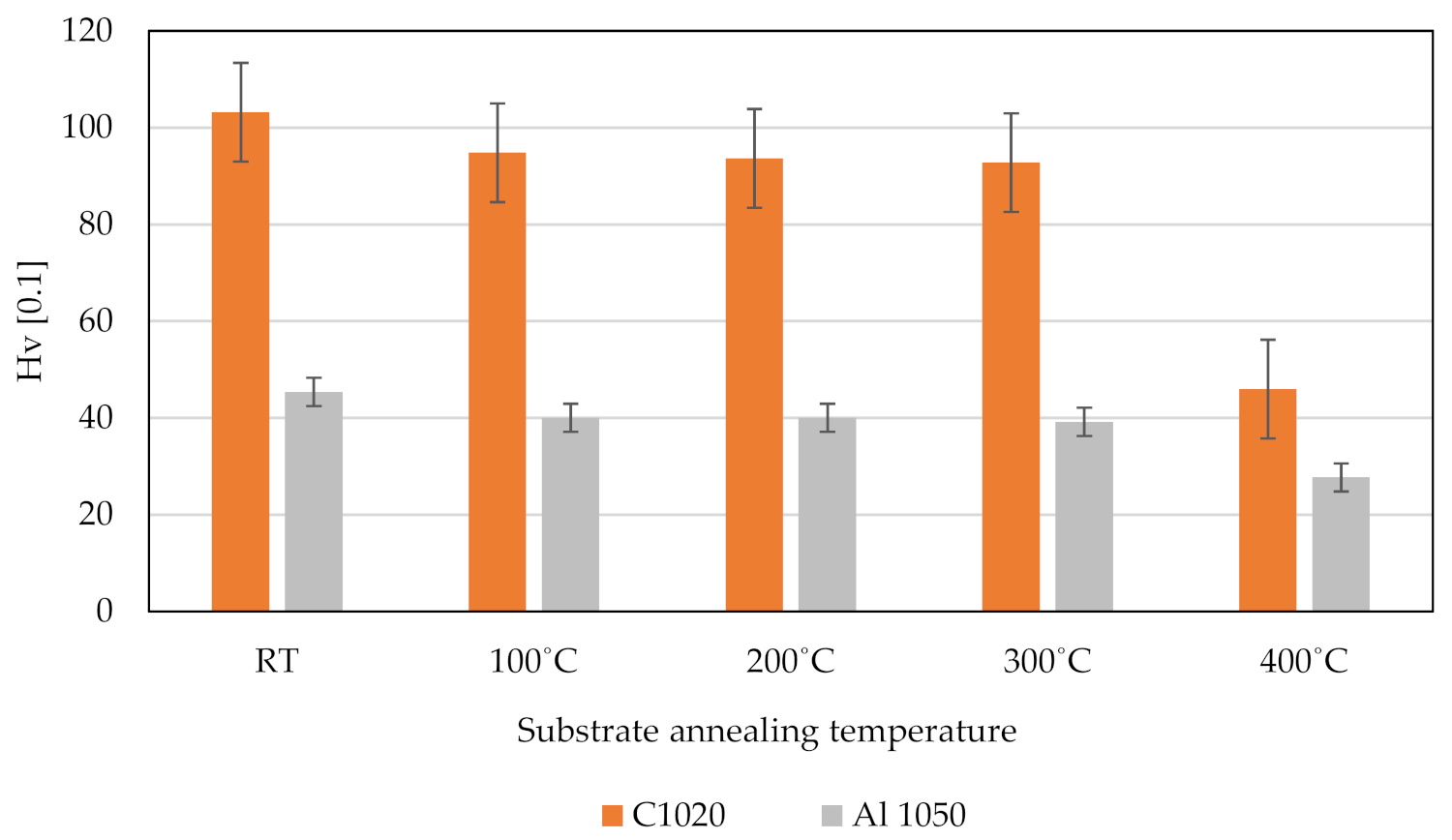
3.5. Pure Metal Vickers Microhardness
3.6. Focus Ion Beam Single Particle Titanium Dioxide on Pure Metals Annealed at 400 °C
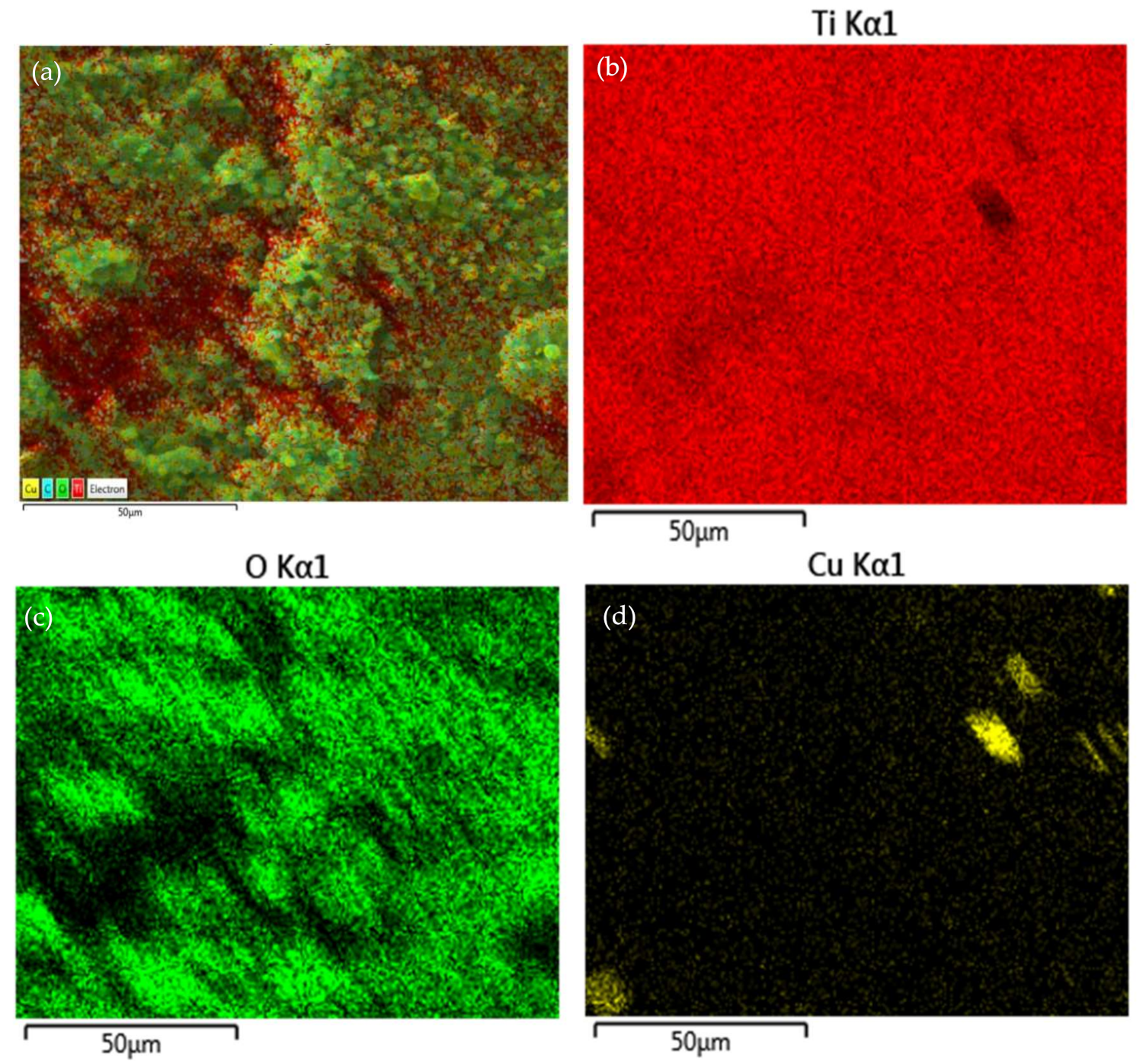
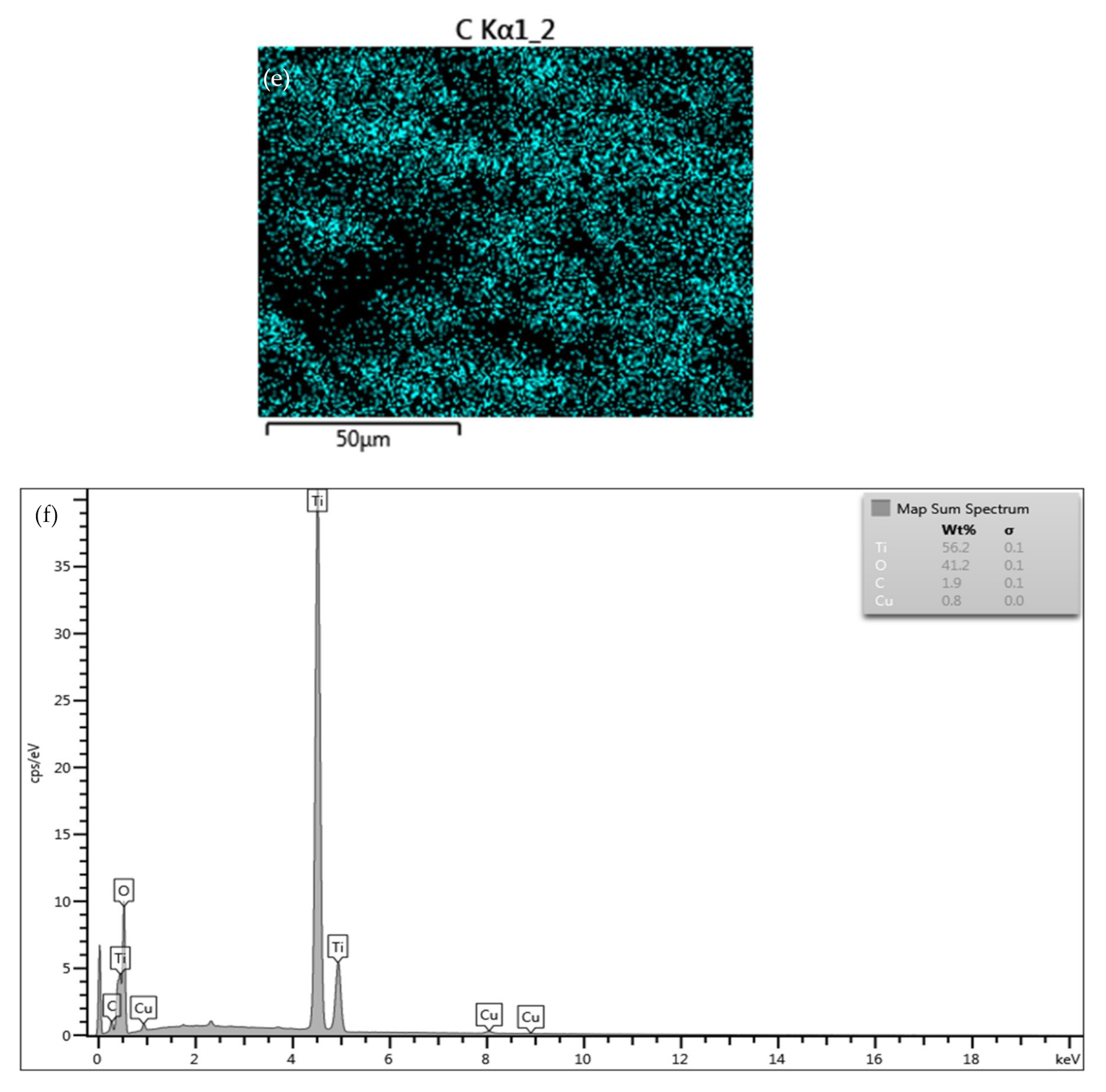
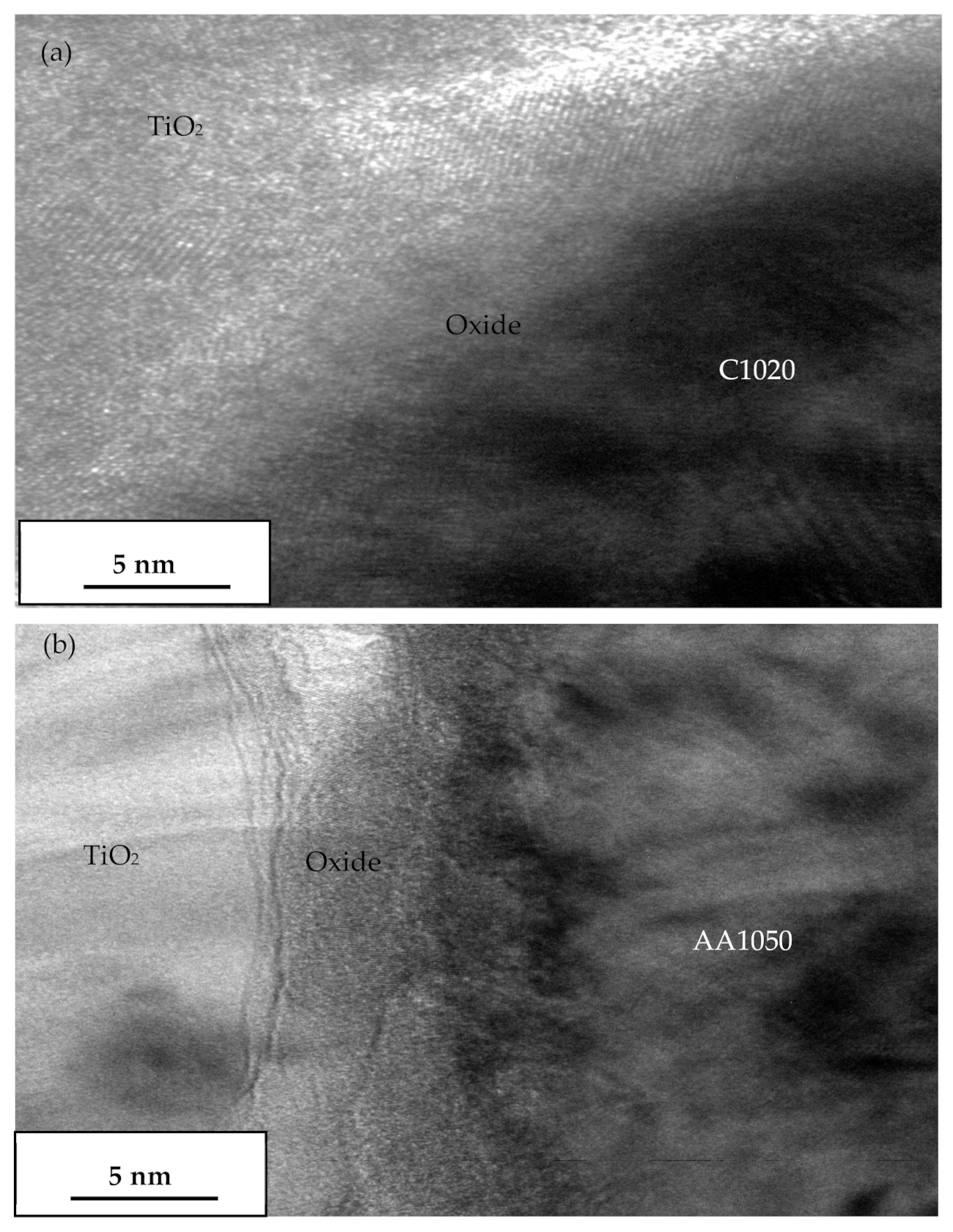
3.7. Transmission Electron Microscopy Investigation of the Oxide Film Interface between TiO2 Particles and Substrates at Room Temperature and Annealed at 400 °C
3.8. Adhesion Strength on Substrate Annealed at 400 °C Surface Condition
4. Conclusions
- The annealing process contributed to the induced ductility of C1020 and AA1050, especially when annealed at a recrystallization temperature of 400 °C. The substrate’s hardness was reduced as a result, and it became softer. When the high-velocity cold-sprayed TiO2 particle struck the pure metals annealed at 400 °C with an oxide film, plastic deformation of the substrate is present, but some of the oxide layer remained, as shown by the TEM results, which prevented metallurgical bonding from occurring in between the TiO2 coating and pure metals;
- Metallurgical bonding of cold-sprayed TiO2 particles and newly created oxide-free pure metal surfaces is the primary bonding mechanism.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Papyrin, A.; Kosarev, V.; Klinkov, S.; Alkhimov, A.; Fomin, V.M. Cold Spray Technology; Elsevier: Amsterdam, The Netherlands, 2006; pp. 70–96. [Google Scholar]
- Champagne, V.K. The Cold Spray Materials Deposition Process. Fundamentals and Applications, 1st ed.; Victor, K.C., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2007; pp. 132–149. [Google Scholar]
- Assadi, H.; Gärtner, F.; Stoltenho, T.; Kreye, H. Bonding mechanism in cold gas spraying. Acta Mater. 2003, 51, 4379–4394. [Google Scholar] [CrossRef]
- Assadi, H.; Schmidt, T.; Richter, H.; Kliemann, J.-O.; Binder, K.; Klassen, F.; Kreye, H. On Parameter Selection in Cold Spraying. J. Therm. Spray Technol. 2011, 20, 1161–1176. [Google Scholar] [CrossRef]
- Grujicic, M.; Zhao, C.I.; De Rosset, W.S.; Helfritch, D. Adabatic shear instability-based mechanism for article/substrate bonding in the cold-gas dynamic-spray process. Mater. Des. 2004, 25, 681–688. [Google Scholar] [CrossRef]
- Villafuerte, J. Modern Cold Spray: Materials, Process, and Applications, 1st ed.; Villafuerte, J., Ed.; Springer: Windsor, ON, Canada, 2015; pp. 1–6. [Google Scholar]
- Moridi, A.; Hassani-Gangaraj, S.M.; Guagliano, M.; Dao, M. Cold spray coating: Review of material systems and future perspectives. Surf. Eng. 2014, 30, 369–395. [Google Scholar] [CrossRef]
- Chromik, R.R.; Alidokht, S.A.; Shockley, J.M.; Zhang, Y. Tribological Coatings Prepared by Cold Spray. In Cold-Spray Coatings: Recent Trends and Future Perspectives; Cavaliere, P., Ed.; Springer: Lecce, Italy, 2018; pp. 321–348. [Google Scholar]
- Hench, L.L.; Best, S.M. Ceramics, Glasses, and Glass Ceramics: Basic Principles. In Biomaterials Science; Academic Press: Lodon, UK, 2013; pp. 128–151. [Google Scholar]
- Shahid, H.; Xiaoyong, Y.; Muhammad Kashif, A.; Asma, S.; Muhammad Sufyan, J.; Nimra, A.; Bilal, A.; Guiwu, L.; Guanjun, Q. Robust TiN Nanoparticles Polysulfide Anchor for Li–S Storage and Diffusion Pathways Using First Principle Calculations. Chem. Eng. J. 2020, 391, 123595. [Google Scholar]
- Shahid, H.; Abdul Jabbar, K.; Muhammad, A.; Muhammad Sufyan, J.; Awais, A.; Syed Shoaib, A.S.; Mohammad Rizwan, K.; Shakeel, A.; Zulfiqar; Shafaqat, A.; et al. Charge storage in binder-free 2D-hexagonal CoMoO4 nanosheets as a redox active material for pseudocapacitors. Ceram. Intern. 2021, 47, 8659–8667. [Google Scholar]
- Shahid, H.; Mobashar, H.; Muhammad Sufyan, J.; Asma, S.; Syed Shoaib, A.S.; Muhammad Tariq, N.; Tayyba, N.; Abdul Jabbar, K.; Xiangzhao, Z.; Guiwu, L. Distinctive flower-like CoNi2S4 nanoneedle arrays (CNS–NAs) for superior supercapacitor electrode performances. Ceram. Intern. 2020, 46, 25942–25948. [Google Scholar]
- Yamada, M.; Kandori, Y.; Sato, K.; Fukumoto, M. Fabrication of Titanium Dioxide Photocatalyst Coatings by Cold Spray. J. Solid Mech. Mater. Eng. 2009, 3, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Yamada, M.; Isago, H.; Nakano, H.; Fukumoto, M. Cold spraying of TiO2 photocatalyst coating with nitrogen process gas. J. Therm. Spray Technol. 2010, 19, 1218–1223. [Google Scholar] [CrossRef]
- Salim, N.T.; Yamada, M.; Nakano, H.; Shima, K.; Isago, H.; Fukumoto, M. The effect of post-treatments on the powder morphology of titanium dioxide (TiO2) powders synthesized for cold spray. Surf. Coat. Technol. 2011, 206, 366–371. [Google Scholar] [CrossRef]
- Toibah, A.R.; Sato, M.; Yamada, M.; Fukumoto, M. Cold-Sprayed TiO2 Coatings from Nanostructured Ceramic Agglomerated Powders. Mater. Manuf. Process. 2016, 31, 1527–1534. [Google Scholar] [CrossRef]
- Toibah, A.R.; Takahashi, K.; Yamada, M.; Fukumoto, M. Effect of Powder Calcination on the Cold Spray Titanium Dioxide Coating. Mater. Trans. 2016, 57–58, 1345–1350. [Google Scholar]
- Winnicki, M.; Latka, L.; Jasiorski, M.; Baszczuk, A. Mechanical properties of TiO2 coatings deposited by low pressure cold spraying. Surf. Coat. Technol. 2020, 405, 126516. [Google Scholar] [CrossRef]
- Schmidt, K.; Buhl, S.; Davoudi, N.; Godard, C.; Merz, R.; Raid, I.; Kerscher, E.; Kopnarski, M.; Renno, C.M.; Ripperger, S.; et al. Ti surface modification by cold spraying with TiO2 mircoparticles. Surf. Coat. Technol. 2017, 309, 749–758. [Google Scholar] [CrossRef]
- Kliemann, J.-O.; Gutzmann, H.; Gärtner, F.; Hübner, H.; Borchers, C.; Klassen, T. Formation of cold-sprayed ceramic titanium dioxide layers on metal surfaces. J. Therm. Spray Technol. 2010, 20, 292–298. [Google Scholar] [CrossRef] [Green Version]
- Gutzmann, H.; Freese, S.; Gartner, F.; Klassen, T. Cold gas spraying of ceramics using the example of titanium dioxide. In Proceedings of the International Thermal Spray Conference, ITSC, Hamburg, Germany, 27–29 September 2011; pp. 391–396. [Google Scholar]
- Gardon, M.; Fernández-Rodíguez, C.; Garzón Sousa, D.; Doña-RodRíguez, J.M.; Dosta, S.; Cano, I.G.; Guilemany, J.M. Photocatalytic activity of nanostructured anatase coatings obtained by cold gas spray. J. Therm. Spray Technol. 2014, 23, 1135–1140. [Google Scholar] [CrossRef] [Green Version]
- Li, W.-Y.; Li, C.-J.; Liao, H. Significant influence of particle surface oxidation on deposition efficiency, interface microstructure and adhesive strength of cold-sprayed copper coatings. Appl. Surf. Sci. 2010, 256, 4953–4958. [Google Scholar] [CrossRef]
- Kumar, S.; Bae, G.; Lee, C. Deposition characteristics of copper particles on roughened substrates through kinetic spraying. Appl. Surf. Sci. 2009, 255, 3472–3479. [Google Scholar] [CrossRef]
- Richer, P.; Jodoin, B.; Ajdelsztajn, L.; Lavernia, E.J. Substrate roughness and thickness effects on cold spray nanocrystalline Al−Mg coatings. J. Therm. Spray Technol. 2006, 15, 246–254. [Google Scholar] [CrossRef]
- Mäkinen, H.; Lagerbom, J.; Vuoristo, P. Adhesion of Cold Sprayed Coatings: Effect of Powder, Substrate, and Heat Treatment. In Proceedings of the Thermal Spray 2007: Global Coating Solutions, Beijing, China, 14–16 May 2007; Marple, B.R., Hyland, M.M., Lau, Y.-C., Li, C.-J., Lima, R.S., Montavon, G., Eds.; ASM International: Almere, The Netherlands, 2007; pp. 31–36. [Google Scholar]
- Clayton, C.R. Materials science and engineering: An introduction. Mater. Sci. Eng. 1987, 94, 266–267. [Google Scholar] [CrossRef]
- Benchabanea, G.; Boumerzouga, Z.; Thibonb, I.; Gloriantb, T. Recrystallization of pure copper investigated by calorimetry and microhardness. Mater. Charact. 2008, 59, 1425–1428. [Google Scholar] [CrossRef]
- Ernst, K.-R.; Braeutigam, J.; Gaetner, F.; Klassen, T. Effect of substrate temperature on cold-gas-sprayed coatings on ceramic substrates. J. Therm. Spray Technol. 2013, 22, 422–432. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Ogawa, K. Effect of Substrate Surface Oxide Film Thickness on Deposition Behavior and Deposition Efficiency in the Cold Spray Process. J. Therm. Spray Technol. 2015, 24, 1269–1276. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Tokoro, R.; Tanno, M.; Ogawa, K. Elucidation of cold-spray deposition mechanism by auger electron spectroscopic evaluation of bonding interface oxide film. Acta Mater. 2019, 164, 39–49. [Google Scholar] [CrossRef]
- Irissou, E.; Legoux, J.G.; Ryabinin, A.N.; Jodoin, B.; Moreau, C. Review on Cold Spray Process and Technology: Part I—Intellectual Property. J. Therm. Spray Technol. 2008, 17, 495–516. [Google Scholar] [CrossRef] [Green Version]
- Goldbaum, D.; Poirier, D.; Irissou, E.; Legoux, J.G.; Moreau, C. Review on cold spray process and technology in US patents. In Modern Cold Spray; Springer: Cham, Switzerland, 2015; pp. 403–429. [Google Scholar]
- Grigoriev, S.; Okunkova, A.; Sova, A.; Bertrand, P.; Smurov, I. Cold spraying: From process fundamentals towards advanced applications. Surf. Coats Technol. 2015, 268, 77–84. [Google Scholar] [CrossRef]
- Mostafa, H.-G.; David, V.; Keith, A.N.; Christopher, A.S. Impact-bonding with aluminum, silver, and gold microparticles: Toward understanding the role of native oxide layer. Appl. Surf. Sci. 2019, 476, 528–532. [Google Scholar]







| Cu | P | Mn | Zn | Sn | Ni | Co | Zr | Ag | |
| C1020 | Bal | 0.04 | 1.1 | 0.1 | 0.72 | 0.06 | 0.21 | 0.05 | 0.04 |
| Al | Fe | Si | Zn | Cu | Mn | Mg | Ti | Other | |
| AA1050 | Bal | 0.40 | 0.25 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.03 |
| Measured Regions | Cu2 p3/2, Al 2p, O 1s |
|---|---|
| Examined X-ray output (W) | 10 |
| Probe diameter (µm) | 50 |
| Time per step (ms) | 30 |
| Pass energy | 140 |
| Cycle | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omar, N.i.; Yamada, M.; Yasui, T.; Fukumoto, M. Bonding Mechanism of Cold-Sprayed TiO2 Coatings on Copper and Aluminum Substrates. Coatings 2021, 11, 1349. https://doi.org/10.3390/coatings11111349
Omar Ni, Yamada M, Yasui T, Fukumoto M. Bonding Mechanism of Cold-Sprayed TiO2 Coatings on Copper and Aluminum Substrates. Coatings. 2021; 11(11):1349. https://doi.org/10.3390/coatings11111349
Chicago/Turabian StyleOmar, Noor irinah, Motohiro Yamada, Toshiaki Yasui, and Masahiro Fukumoto. 2021. "Bonding Mechanism of Cold-Sprayed TiO2 Coatings on Copper and Aluminum Substrates" Coatings 11, no. 11: 1349. https://doi.org/10.3390/coatings11111349
APA StyleOmar, N. i., Yamada, M., Yasui, T., & Fukumoto, M. (2021). Bonding Mechanism of Cold-Sprayed TiO2 Coatings on Copper and Aluminum Substrates. Coatings, 11(11), 1349. https://doi.org/10.3390/coatings11111349








