The Effects of Graphene on the Biocompatibility of a 3D-Printed Porous Titanium Alloy
Abstract
:1. Introduction
2. Materials and Methods
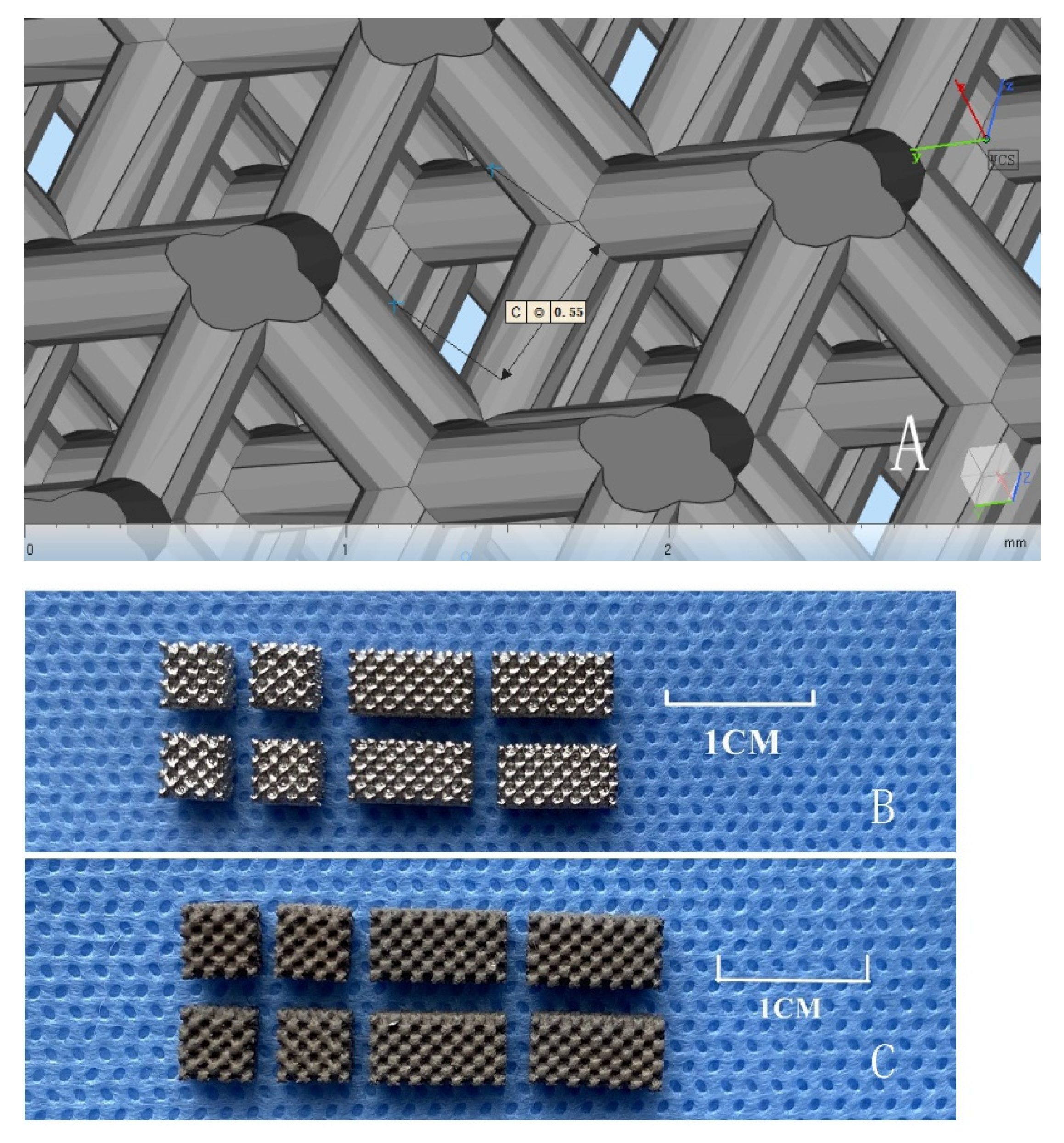
2.1. Material Preparation
2.2. Determination of the Physical and Chemical Properties of the Scaffolds
2.3. Compound Culture and Biocompatibility of ADSCs and Scaffolds In Vitro
2.4. In Vivo Experimental Study of Porous Titanium Alloy Scaffolds
3. Results
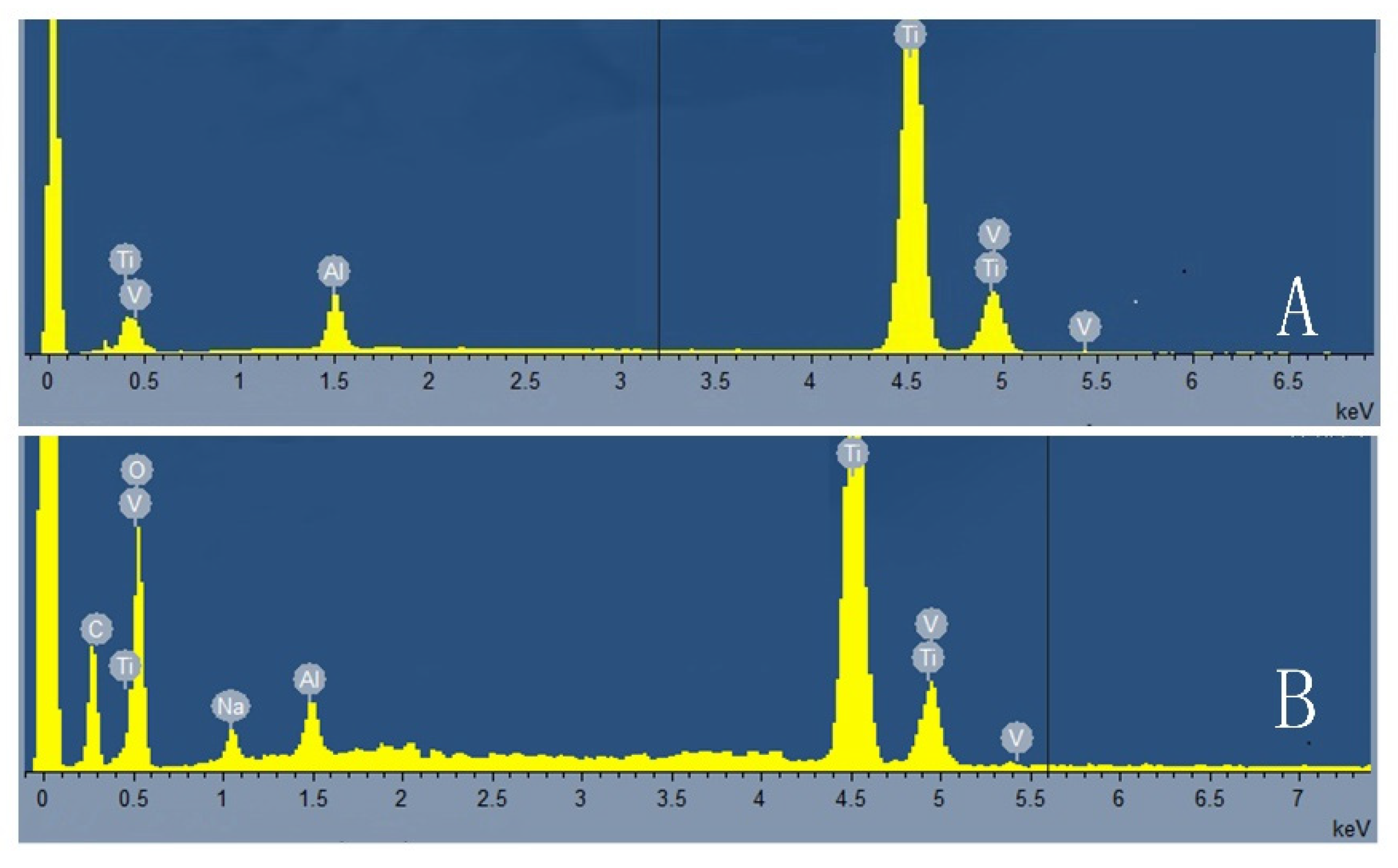
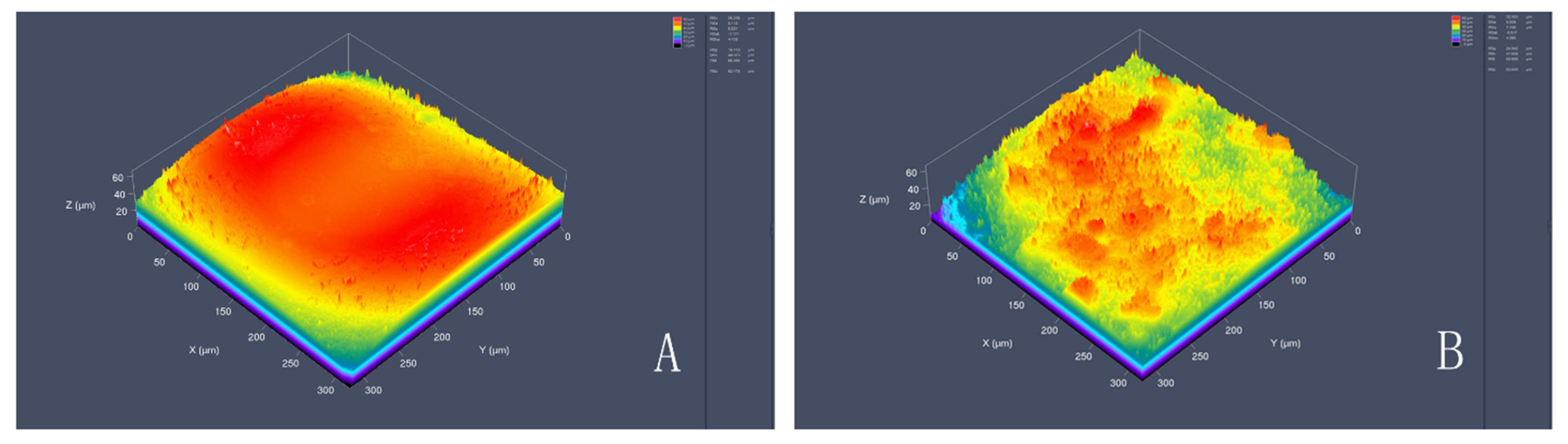
3.1. Physical and Chemical Properties of Porous Titanium Alloy Scaffolds
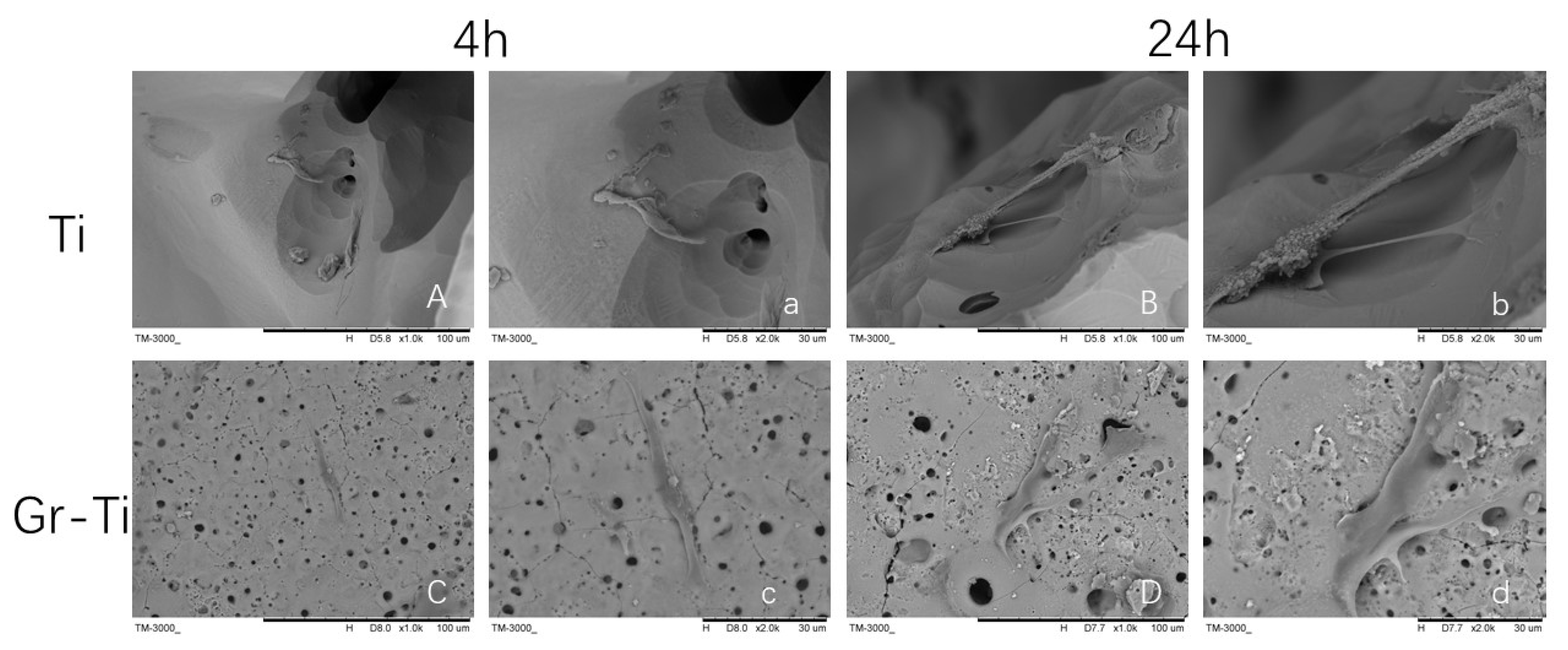
3.2. Adhesion of ADSCs
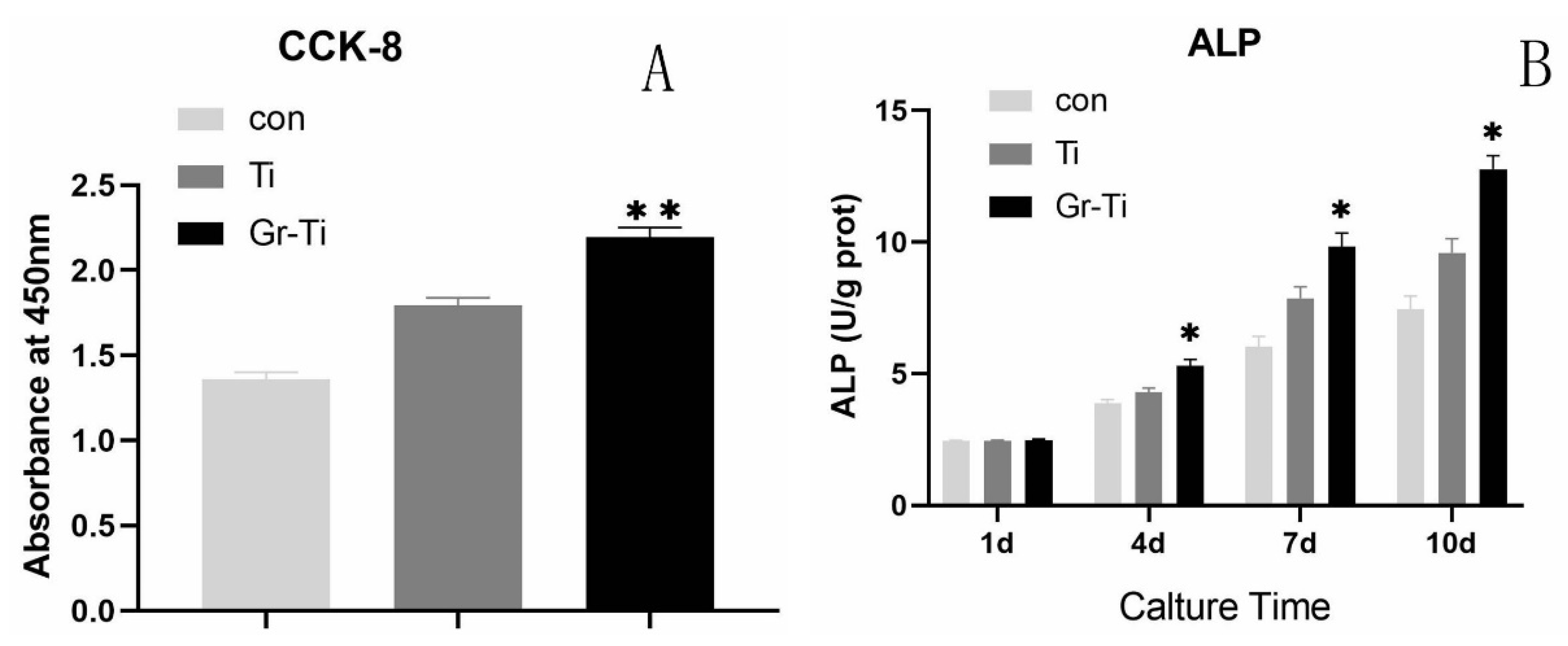
3.3. Cell Proliferation and Cytotoxicity
3.4. ALP Activity Detection
3.5. General Observation of Rabbit Mandible Model
3.6. Imaging Analysis (3D-CT)
4. Discussion
5. Conclusions
- SLM technology was used to design and prepare porous titanium alloy scaffolds with qualified porosity, pore size, and mechanical properties matching with bone tissue.
- Compared with its performance on unmodified surfaces, coating surface composite graphene significantly increases the roughness of titanium alloy.
- Graphene-coated porous titanium alloy scaffolds have a good biocompatibility and low toxicity for the growth, proliferation, and adhesion of human ADSCs.
- The composite graphene-coated porous titanium alloy stent successfully repaired the rabbit mandibular defect, which laid a foundation for the clinical application of tissue engineering in the field of oral and maxillofacial bone defect repair.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alraei, K.; Shrqawi, J.; Alarusi, K. Application of Recombinant Human BMP-2 with Bone Marrow Aspirate Concentrate and Platelet-Rich Fibrin in Titanium Mesh for Vertical Maxillary Defect Reconstruction prior to Implant Placement. Case Rep. Dent. 2021, 7, 2021. [Google Scholar] [CrossRef] [PubMed]
- Adamička, M.; Adamičková, A.; Danišovič, L.; Gažová, A.; Kyselovič, J. Pharmacological Approaches and Regeneration of Bone Defects with Dental Pulp Stem Cells. Stem Cells Int. 2021, 2021, 4593322. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J.; Wei, Y.; Xu, N.; Li, J.; Pu, X.; Wang, J.; Huang, Z.; Liao, X.; Yin, G. Ion release behavior of vanadium-doped mesoporous bioactive glass particles and the effect of the released ions on osteogenic differentiation of BMSCs via the FAK/MAPK signaling pathway. J. Mater. Chem. B 2021, 9, 7848–7865. [Google Scholar] [CrossRef] [PubMed]
- Prabha, R.D.; Kraft, D.C.E.; Harkness, L.; Melsen, B.; Varma, H.; Nair, P.D.; Kjems, J.; Kassem, M. Bioactive nano-fibrous scaffold for vascularized craniofacial bone regeneration. J. Tissue Eng. Regen. Med. 2018, 12, e1537–e1548. [Google Scholar] [CrossRef] [PubMed]
- Mitra, I.; Bose, S.; Dernell, W.S.; Dasgupta, N.; Eckstrand, C.; Herrick, J.; Yaszemski, M.J.; Goodman, S.B.; Bandyopadhyay, A. 3D Printing in alloy design to improve biocompatibility in metallic implants. Mater. Today 2021, 45, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.N.; Su, Y.; Lu, X.; Kuo, P.; Du, J.; Zhu, D. Bioactive glass coatings on metallic implants for biomedical applications. Bioact. Mater. 2019, 4, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Gruenwald, W.; Bhattacharrya, M.; Jansen, D.; Reindl, L. Electromagnetic Analysis, Characterization and Discussion of Inductive Transmission Parameters for Titanium Based Housing Materials in Active Medical Implantable Devices. Materials 2018, 11, 2089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.; Luo, C.; Zhang, Z.; Hermawan, H.; Zhu, D.; Huang, J.; Liang, Y.; Li, G.; Ren, L. Bioinspired surface functionalization of metallic biomaterials. J. Mech. Behav. Biomed. Mater. 2018, 77, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Shen, S.; Zhao, L.; Xu, Y.; Li, Y.; Zhuo, N. 3D printing of dual-cell delivery titanium alloy scaffolds for improving osseointegration through enhancing angiogenesis and osteogenesis. BMC Musculoskelet. Disord. 2021, 22, 734. [Google Scholar] [CrossRef] [PubMed]
- Mehboob, H.; Tarlochan, F.; Mehboob, A.; Chang, S.; Ramesh, S.; Harun, W.S.W.; Kadirgama, K. A novel design, analysis and 3D printing of Ti-6Al-4V alloy bio-inspired porous femoral stem. J. Mater. Sci. Mater. Med. 2020, 31, 78. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Su, K.; Su, L.; Liang, P.; Ji, P.; Wang, C. Comparison of 3D-printed porous tantalum and titanium scaffolds on osteointegration and osteogenesis. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109908. [Google Scholar] [CrossRef]
- Pereira, A.T.; Schneider, K.H.; Henriques, P.C.; Grasl, C.; Melo, S.F.; Fernandes, I.P.; Kiss, H.; Martins, M.C.L.; Bergmeister, H.; Gonçalves, I.C. Graphene Oxide Coating Improves the Mechanical and Biological Properties of Decellularized Umbilical Cord Arteries. ACS Appl. Mater. Interfaces 2021, 13, 32662–32672. [Google Scholar] [CrossRef]
- Lu, J.; Sun, J.; Zou, D.; Song, J.; Yang, S. Graphene-Modified Titanium Surface Enhances Local Growth Factor Adsorption and Promotes Osteogenic Differentiation of Bone Marrow Stromal Cells. Front. Bioeng. Biotechnol. 2020, 8, 621788. [Google Scholar] [CrossRef] [PubMed]
- Azadian, E.; Arjmand, B.; Ardeshirylajimi, A.; Hosseinzadeh, S.; Omidi, M.; Khojasteh, A. Polyvinyl alcohol modified polyvinylidene fluoride-graphene oxide scaffold promotes osteogenic differentiation potential of human induced pluripotent stem cells. J. Cell. Biochem. 2020, 121, 3185–3196. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Tian, S.; Li, H.; Zhao, Y.; Jia, D.; Zhou, Y. Formation mechanism, degradation behavior, and cytocompatibility of a double-layered structural MAO/rGO-CaP coating on AZ31 Mg. Colloids Surf. B Biointerfaces 2020, 190, 110901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Chen, Y.; Yuan, L.; Liu, H.; Wang, J.; Liu, Q.; Zhang, Y. Adipose-Derived Stem Cells: Current Applications and Future Directions in the Regeneration of Multiple Tissues. Stem Cells Int. 2020, 2020, 8810813. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Han, L.; Sun, T.; Wang, W.; Li, X.; Wu, B. Osteogenic and angiogenic lineage differentiated adipose-derived stem cells for bone regeneration of calvarial defects in rabbits. J. Biomed. Mater. Res. A 2021, 109, 538–550. [Google Scholar] [CrossRef]
- Singh, A.V.; Rosenkranz, D.; Ansari, M.H.D.; Singh, R.; Kanase, A.; Singh, S.P.; Johnston, B.; Tentschert, J.; Laux, P.; Luch, A. Artificial Intelligence and Machine Learning Empower Advanced Biomedical Material Design to Toxicity Prediction. Adv. Intell. Syst. 2020, 2, 2000084. [Google Scholar] [CrossRef]
- Arjunan, A.; Demetriou, M.; Baroutaji, A.; Wang, C. Mechanical performance of highly permeable laser melted Ti6Al4V bone scaffolds. J. Mech. Behav. Biomed. Mater. 2020, 102, 103517. [Google Scholar] [CrossRef]
- Tamashiro, N.S.M.; Souza, R.Q.; Gonçalves, C.R.; Ikeda, T.I.; Luz, R.A.; Cruz, A.S.; Padoveze, M.C.; Graziano, K.U. Cytotoxicity of cannulas for ophthalmic surgery after cleaning and sterilization: Evaluation of the use of enzymatic detergent to remove residual ophthalmic viscosurgical device material. J. Cataract. Refract. Surg. 2013, 39, 937–941. [Google Scholar] [CrossRef]
- Xiong, Y.Z.; Gao, R.N.; Zhang, H.; Dong, L.L.; Li, J.T.; Li, X. Rationally designed functionally graded porous Ti6Al4V scaffolds with high strength and toughness built via selective laser melting for load-bearing orthopedic applications. J. Mech. Behav. Biomed. Mater. 2020, 104, 103673. [Google Scholar] [CrossRef]
- Ran, Q.; Yang, W.; Hu, Y.; Shen, X.; Yu, Y.; Xiang, Y.; Cai, K. Osteogenesis of 3D printed porous Ti6Al4V implants with different pore sizes. J. Mech. Behav. Biomed. Mater. 2018, 84, 1–11. [Google Scholar] [CrossRef]
- Luo, C.; Wang, C.; Wu, X.; Xie, X.; Wang, C.; Zhao, C.; Zou, C.; Lv, F.; Huang, W.; Liao, J. Influence of porous tantalum scaffold pore size on osteogenesis and osteointegration: A comprehensive study based on 3D-printing technology. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 129, 112382. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Su, K.; Su, L.; Liang, P.; Ji, P.; Wang, C. The effect of 3D-printed Ti6Al4V scaffolds with various macropore structures on osteointegration and osteogenesis: A biomechanical evaluation. J. Mech. Behav. Biomed. Mater. 2018, 88, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 569. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Xie, H.; Cao, T.; Franco-Obregón, A.; Rosa, V. Graphene-Induced Osteogenic Differentiation Is Mediated by the Integrin/FAK Axis. Int. J. Mol. Sci. 2019, 20, 574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.Q.; Li, B.; Xu, M.; Liu, R.; Xia, T.; Zhang, Z.H.; Xu, Y.; Liu, S.J. Graphene Oxide Promotes Cancer Metastasis through Associating with Plasma Membrane to Promote TGF-β Signaling-Dependent Epithelial-Mesenchymal Transition. ACS Nano 2020, 14, 818–827. [Google Scholar] [CrossRef]
- Zou, J.; Wang, W.W.; Nie, Y.; Xu, X.; Ma, N.; Lendlein, A. Microscale roughness regulates laminin-5 secretion of bone marrow mesenchymal stem cells. Clin. Hemorheol. Microcirc. 2019, 73, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, P.; Long, Y.; Huang, C.X.; Chen, D.N. Repair of bone defects in rat radii with a composite of allogeneic adipose-derived stem cells and heterogeneous deproteinized bone. Stem Cell Res. Ther. 2018, 9, 79. [Google Scholar] [CrossRef]
- Ajay, V.S.; Romi, S.M.; Harald, J.; Heike, R.; Yves, U.H.; Philipp, R.; Frank, B.; Katherina, S.; Ashish, G.; Peter, L.; et al. Evaluating Particle Emissions and Toxicity of 3D Pen Printed Filaments with Metal Nanoparticles as Additives: In Vitro and in Silico Discriminant Function Analysis. ACS Sustain. Chem. Eng. 2021, 9, 11724–11737. [Google Scholar] [CrossRef]
- Wang, Q.; Miao, Y.M.; Qian, Z.Y.; Chen, L.D.; Lu, T.; Xu, Y.; Jiang, X.W.; Shen, Y.C. MicroRNA-15a-5p plays a role in osteogenic MC3T3-E1 cells differentiation by targeting PDCD4 (programmed cell death 4) via Wnt/β-catenin dependent signaling pathway. Bioengineered 2021, 12, 8173–8185. [Google Scholar] [CrossRef] [PubMed]
- de Souza, L.P.L.; Lopes, J.H.; Ferreira, F.V.; Martin, R.A.; Bertran, C.A.; Camilli, J.A. Evaluation of effectiveness of 45S5 bioglass doped with niobium for repairing critical-sized bone defect in in vitro and in vivo models. J. Biomed. Mater. Res. A 2020, 108, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.A.; Alba-Perez, A.; Gonzalez-Garcia, C.; Donnelly, H.; Llopis-Hernandez, V.; Jayawarna, V.; Childs, P.; Shields, D.W.; Cantini, M.; Ruiz-Cantu, L.; et al. Nanoscale Coatings for Ultralow Dose BMP-2-Driven Regeneration of Critical-Sized Bone Defects. Adv. Sci. 2019, 6, 1800361. [Google Scholar] [CrossRef] [PubMed]
- Neuber, C.; Schulze, S.; Förster, Y.; Hofheinz, F.; Wodke, J.; Möller, S.; Schnabelrauch, M.; Hintze, V.; Scharnweber, D.; Rammelt, S.; et al. Biomaterials in repairing rat femoral defects: In vivo insights from small animal positron emission tomography/computed tomography (PET/CT) studies. Clin. Hemorheol. Microcirc. 2019, 73, 177–194. [Google Scholar] [CrossRef]



| 24 h | 48 h | 72 h | ||||
|---|---|---|---|---|---|---|
| RGR | Grade | RGR | Grade | RGR | Grade | |
| Ti | 97.63 ± 1.83 | 1 | 110.54 ± 6.87 | 0 | 101.46 ± 8.25 | 1 |
| Gr–Ti | 112.36 ± 2.89 | 0 | 118.43 ± 7.22 | 0 | 102.76 ± 94.32 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Tong, S.; Yang, S.; Guo, S. The Effects of Graphene on the Biocompatibility of a 3D-Printed Porous Titanium Alloy. Coatings 2021, 11, 1509. https://doi.org/10.3390/coatings11121509
Sun X, Tong S, Yang S, Guo S. The Effects of Graphene on the Biocompatibility of a 3D-Printed Porous Titanium Alloy. Coatings. 2021; 11(12):1509. https://doi.org/10.3390/coatings11121509
Chicago/Turabian StyleSun, Xu, Shuang Tong, Shude Yang, and Shu Guo. 2021. "The Effects of Graphene on the Biocompatibility of a 3D-Printed Porous Titanium Alloy" Coatings 11, no. 12: 1509. https://doi.org/10.3390/coatings11121509





