Te-Embedded Nanocrystalline PbTe Thick Films: Structure and Thermoelectric Properties Relationship
Abstract
:1. Introduction
2. Materials and Method
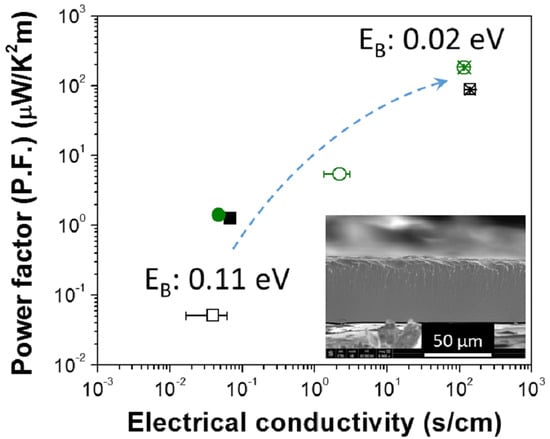
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Curzon, F.L.; Ahlborn, B. Efficiency of a Carnot engine at maximum power output. Am. J. Phys. 1975, 43, 22–24. [Google Scholar] [CrossRef]
- Vining, C.B. An inconvenient truth about thermoelectrics. Nat. Mater. 2009, 8, 83–85. [Google Scholar] [CrossRef]
- Shakouri, A. Recent developments in semiconductor thermoelectric physics and materials. Annu. Rev. Mater. Res. 2011, 41, 399–431. [Google Scholar] [CrossRef]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef]
- Poudel, B.; Hao, Q.; Ma, Y.; Lan, Y.; Minnich, A.; Yu, B.; Yan, X.; Wang, D.; Muto, A.; Vashaee, D.; et al. High-thermoelectric performance of nanostructured bismuth antimony telluride bulk alloys. Science 2008, 320, 634–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faleev, S.V.; Léonard, F. Theory of enhancement of thermoelectric properties of materials with nanoinclusions. Phys. Rev. B 2008, 77. [Google Scholar] [CrossRef] [Green Version]
- Sumithra, S.; Takas, N.J.; Misra, D.K.; Nolting, W.M.; Poudeu, P.; Stokes, K.L. Enhancement in thermoelectric figure of merit in nanostructured Bi2Te3 with semimetal nanoinclusions. Adv. Energy Mater. 2011, 1, 1141–1147. [Google Scholar] [CrossRef]
- Chen, J.; Sun, T.; Sim, D.; Peng, H.; Wang, H.; Fan, S.; Hng, H.H.; Ma, J.; Boey, F.Y.C.; Li, S.; et al. Sb2Te3Nanoparticles with enhanced seebeck coefficient and low thermal conductivity. Chem. Mater. 2010, 22, 3086–3092. [Google Scholar] [CrossRef]
- Han, L.; Fang, H.; Du, C.; Sun, J.; Li, Y.; Ma, W. Synthesis of ultra-narrow PbTe nanorods with extremely strong quantum confinement. J. Mater. Sci. Technol. 2019, 35, 703–710. [Google Scholar] [CrossRef]
- Rojas-Chávez, H.; Juárez-García, J.; Herrera-Rivera, R.; Flores-Rojas, E.; González-Domínguez, J.; Cruz-Orea, A.; Cayetano-Castro, N.; Ávila-García, A.; Mondragón-Sánchez, M. The high-energy milling process as a synergistic approach to minimize the thermal conductivity of PbTe nanostructures. J. Alloys Compd. 2020, 820, 153167. [Google Scholar] [CrossRef]
- Hsieh, H.-C.; Wang, C.-H.; Lan, T.-W.; Lee, T.-H.; Chen, Y.-Y.; Chu, H.-S.; Wu, A.T. Joint properties enhancement for PbTe thermoelectric materials by addition of diffusion barrier. Mater. Chem. Phys. 2020, 246, 122848. [Google Scholar] [CrossRef]
- Ohta, M.; Jood, P.; Murata, M.; Lee, C.-H.; Yamamoto, A.; Obara, H. An integrated approach to thermoelectrics: Combining phonon dynamics, nanoengineering, novel materials development, module fabrication, and metrology. Adv. Energy Mater. 2018, 9, 1–29. [Google Scholar] [CrossRef]
- Novak, T.G.; Kim, K.; Jeon, S. 2D and 3D nanostructuring strategies for thermoelectric materials. Nanoscale 2019, 11, 19684–19699. [Google Scholar] [CrossRef]
- Fleurial, J.-P.; Snyder, G.J.; Herman, J.A.; Giauque, P.H. Thick-film thermoelectric microdevices. In Proceedings of the Eighteenth International Conference on Thermoelectrics, Baltimore, MD, USA, 29 August–2 September 1999. [Google Scholar]
- Semeniouk, V.; Fleurial, J.-P. Modeling and minimization of intercascade thermal resistance in multi-stage thermoelectric cooler. In Proceedings of the XVI International Conference on Thermoelectrics, Dresden, Germany, 26–29 August 1997. [Google Scholar]
- Anatychuk, L.; Luste, O.; Vikhor, L. Optimal functions as an effective method for thermoelectric devices design. In Proceedings of the Fifteenth International Conference on Thermoelectrics, Pasadena, CA, USA, 26–29 March 1996; pp. 223–226. [Google Scholar]
- Anatychuk, L.I.; Luste, O.J. Physical principles of microminiaturization in thermoelectricity. In Proceedings of the Fifteenth International Conference on Thermoelectrics, Pasadena, CA, USA, 26–29 March 1996. [Google Scholar]
- Snyder, G.J.; Lim, J.R.; Huang, C.-K.; Fleurial, J.-P. Thermoelectric microdevice fabricated by a MEMS-like electrochemical process. Nat. Mater. 2003, 2, 528–531. [Google Scholar] [CrossRef]
- Dini, J.W. Electrodeposition—The Materials Science of Coating and Substrates; Noyes Publications: Westwood, NJ, USA, 1993. [Google Scholar]
- Rostek, R.; Stein, N.; Boulanger, C. A review of electroplating for V–VI thermoelectric films: From synthesis to device integration. J. Mater. Res. 2015, 30, 2518–2543. [Google Scholar] [CrossRef]
- Xiao, F.; Hangarter, C.; Yoo, B.; Rheem, Y.; Lee, K.-H.; Myung, N.V. Recent progress in electrodeposition of thermoelectric thin films and nanostructures. Electrochim. Acta 2008, 53, 8103–8117. [Google Scholar] [CrossRef]
- Beaunier, L.; Cachet, H.; Cortes, R.; Froment, M. Epitaxial electrodeposition of lead telluride films on indium phosphide single crystals. J. Electroanal. Chem. 2002, 532, 215–218. [Google Scholar] [CrossRef]
- Li, G.-R.; Yao, C.-Z.; Lu, X.-H.; Zheng, F.-L.; Feng, Z.-P.; Yu, X.-L.; Su, C.-Y.; Tong, Y.-X. Facile and efficient electrochemical synthesis of PbTe dendritic structures. Chem. Mater. 2008, 20, 3306–3314. [Google Scholar] [CrossRef]
- Ni, Y.; Zhang, Y.; Hong, J. Potentiostatic electrodeposition route for quick synthesis of featherlike PbTe dendrites: Influencing factors and shape evolution. Cryst. Growth Des. 2011, 11, 2142–2148. [Google Scholar] [CrossRef]
- Mondal, A.; Mukherrjee, N.; Bhar, S.; Banerjee, D. An electrochemical technique to deposit thin films of PbTe. Thin Solid Film. 2006, 515, 1255–1259. [Google Scholar] [CrossRef]
- Li, X.; Nandhakumar, I.S. Direct electrodeposition of PbTe thin films on n-type silicon. Electrochem. Commun. 2008, 10, 363–366. [Google Scholar] [CrossRef]
- Ivanova, Y.A.; Ivanou, D.K.; Streltsov, E.A. Electrochemical deposition of PbTe onto n-Si(100) wafers. Electrochem. Commun. 2007, 9, 599–604. [Google Scholar] [CrossRef]
- Xiao, F.; Yoo, B.; Ryan, M.A.; Lee, K.-H.; Myung, N.V. Electrodeposition of PbTe thin films from acidic nitrate baths. Electrochim. Acta 2006, 52, 1101–1107. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; Pergamon Press Inc.: Long Island City, NY, USA, 1966. [Google Scholar]
- Saloniemi, H.; Kanniainen, T.; Ritala, M.; Leskelä, M. Electrodeposition of PbTe thin films. Thin Solid Films 1998, 326, 78–82. [Google Scholar] [CrossRef]
- Saloniemi, H.; Kemell, M.; Ritala, M.; Leskelä, M. PbTe electrodeposition studied by combined electrochemical quartz crystal microbalance and cyclic voltammetry. J. Electroanal. Chem. 2000, 482, 139–148. [Google Scholar] [CrossRef]
- Miranda, C.R.B.; Abramof, P.G.; De Melo, F.C.L.; Ferreira, N.G. Morphology and stress study of nanostructured porous silicon as a substrate for PbTe thin films growth by electrochemical process. Mater. Res. 2004, 7, 619–623. [Google Scholar] [CrossRef] [Green Version]
- Qiu, X.; Lou, Y.; Samia, A.C.S.; Devadoss, A.; Burgess, J.D.; Dayal, S.; Burda, C. PbTe Nanorods by Sonoelectrochemistry. Angew. Chem. Int. Ed. 2005, 44, 5855–5857. [Google Scholar] [CrossRef]
- Erdoğan, İ.Y.; Ozer, T.O.; Bülbül, F.; Demir, Ü. Characterization of size-quantized PbTe thin films synthesized by an electrochemical co-deposition method. Thin Solid Films 2009, 517, 5419–5424. [Google Scholar] [CrossRef]
- Butler, I.B.; Schoonen, M.A.A.; Rickard, D.T. Removal of dissolved oxygen from water: A comparison of four common techniques. Talanta 1994, 41, 211–215. [Google Scholar] [CrossRef]
- Wu, T.; Lee, H.-K.; Myung, N.V. Electrodeposition of dense lead telluride thick films in alkaline solutions. J. Electrochem. Soc. 2016, 163, D801–D808. [Google Scholar] [CrossRef]
- Yoo, I.-J.; Song, Y.; Lim, D.C.; Myung, N.V.; Lee, K.H.; Oh, M.; Lee, D.; Kim, Y.D.; Kim, S.; Choa, Y.-H.; et al. Thermoelectric characteristics of Sb2Te3 thin films formed via surfactant-assisted electrodeposition. J. Mater. Chem. A 2013, 1, 5430–5435. [Google Scholar] [CrossRef]
- Kim, J.; Zhang, M.; Bosze, W.; Park, S.-D.; Lim, J.-H.; Myung, N.V. Maximizing thermoelectric properties by nanoinclusion of γ-SbTe in Sb2Te3 film via solid-state phase transition from amorphous Sb–Te electrodeposits. Nano Energy 2015, 13, 727–734. [Google Scholar] [CrossRef]
- Kim, J.; Lee, K.H.; Kim, S.-D.; Lim, J.-H.; Myung, N.V. Simple and effective fabrication of Sb2Te3 films embedded with Ag2Te nanoprecipitates for enhanced thermoelectric performance. J. Mater. Chem. A 2017, 6, 349–356. [Google Scholar] [CrossRef]
- Mostany, J.; Scharifker, B.R.; Saavedra, K.; Borras, C. Electrochemical nucleation and the classical theory: Overpotential and temperature dependence of the nucleation rate. Russ. J. Electrochem. 2008, 44, 652–658. [Google Scholar] [CrossRef]
- Moti’, E.; Shariat, M.H.; Bahrololoom, M.E. Influence of cathodic overpotential on grain size in nanocrystalline nickel deposition on rotating cylinder electrodes. J. Appl. Electrochem. 2008, 38, 605–612. [Google Scholar] [CrossRef]
- Milchev, A.; Lacmann, R. On the nucleation theory of electrochemical alloy formation I. overvoltage dependence of the stationary nucleation rate. J. Cryst. Growth 1991, 110, 919–924. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, M.; Lee, K.-H.; Lee, C.-M.; Lee, H.-K.; Choa, Y.; Myung, N.V. Electrodeposition of compact tellurium thick films from alkaline baths. J. Electrochem. Soc. 2016, 164, D82–D87. [Google Scholar] [CrossRef]
- Mo, M.; Zeng, J.; Liu, X.; Yu, W.; Zhang, S.; Qian, Y. Controlled hydrothermal synthesis of thin single-crystal tellurium nanobelts and nanotubes. Adv. Mater. 2002, 14, 1658–1662. [Google Scholar] [CrossRef]
- Hippel, G.A.v. Structure, conductivity in the VIb group of the periodic system. J. Chem. Phys. 1948, 16, 372–380. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, M.; Lee, K.-H.; Kim, S.-I.; Choa, Y.; Myung, N.V. Synthesis of tellurium heterostructures by galvanic displacement reaction of zinc in alkaline baths. Electrochim. Acta 2014, 150, 298–307. [Google Scholar] [CrossRef]
- Wu, T.; Myung, L.Y.; Zhang, M.; Lee, K.-H.; Lee, Y.L.; Lim, H.-R.; Kim, B.S.; Choa, Y.-H.; Myung, N.V. Size controlled synthesis of tellurium nanorices by galvanic displacement reaction of aluminum. Electrochim. Acta 2015, 176, 1382–1392. [Google Scholar] [CrossRef]
- Patterson, A.L. The scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Williamson, G.; Hall, W. X-ray line broadening from filed aluminium and wolfram. Acta Met. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Burton, A.W.; Ong, K.; Rea, T.; Chan, I.Y. On the estimation of average crystallite size of zeolites from the Scherrer equation: A critical evaluation of its application to zeolites with one-dimensional pore systems. Microporous Mesoporous Mater. 2009, 117, 75–90. [Google Scholar] [CrossRef]
- Weidmann, E.; Anderson, J. Structure and growth of oriented tellurium thin films. Thin Solid Films 1971, 7, 265–276. [Google Scholar] [CrossRef]
- Sciences, C. Large grain tellurium thin films. Thin Solid Films 1972, 11, 229–236. [Google Scholar]
- Santucci, S.; Di Nardo, S.; Lozzi, L.; Passacantando, M.; Picozzi, P. XPS, LEED and AFM investigation of the Si(100) surface after the deposition and annealing of tellurium thin films. Surf. Sci. 1996, 352–354, 1027–1032. [Google Scholar] [CrossRef]
- Bhandarkar, V.; Sen, S.; Muthe, K.; Kaur, M.; Kumar, M.S.; Deshpande, S.; Gupta, S.; Yakhmi, J.V.; Sahni, V. Effect of deposition conditions on the microstructure and gas-sensing characteristics of Te thin films. Mater. Sci. Eng. B 2006, 131, 156–161. [Google Scholar] [CrossRef]
- Kim, D.-H.; Lee, G.-H. Effect of rapid thermal annealing on thermoelectric properties of bismuth telluride films grown by co-sputtering. Mater. Sci. Eng. B 2006, 131, 106–110. [Google Scholar] [CrossRef]
- Rostek, R.; Sklyarenko, V.; Woias, P. Influence of vapor annealing on the thermoelectric properties of electrodeposited Bi2Te3. J. Mater. Res. 2011, 26, 1785–1790. [Google Scholar] [CrossRef]
- Kim, D.H.; Kwon, I.H.; Kim, C.; Han, B.; Im, H.J.; Kim, H. Tellurium-evaporation-annealing for p -type bismuth—Antimony—Telluride thermoelectric materials. J. Alloys Compd. 2013, 548, 126–132. [Google Scholar] [CrossRef]
- Zalar, S.M. High-Temperature Resistivity of the Chalcopyritic Compound CulnTe2. J. Electrochem. Soc. 1966, 113, 230. [Google Scholar] [CrossRef]
- Ostwald, W. Studies on the formation and transformation of solid bodies. Z. Phys. Chem. 1897, 22, 289–330. [Google Scholar]
- McNaught, A.D.; Wilkinson, A. Compendium of Chemical Terminology; International Union of Pure and Applied Chemistry: Raleigh, NC, USA, 1997. [Google Scholar]
- Ratke, L.; Voorhees, P.W. Growth and Coarsening Ostwald Ripening in Material Processing; Springer: Berlin, Germany, 2002. [Google Scholar]
- CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2002.
- Rojas-Chávez, H.; Cruz-Martínez, H.; Flores-Rojas, E.; Juárez-García, J.M.; Gonzalez-Dominguez, J.L.; Daneu, N.; Santoyo-Salazar, J.; Santoyo, J. The mechanochemical synthesis of PbTe nanostructures: Following the Ostwald ripening effect during milling. Phys. Chem. Chem. Phys. 2018, 20, 27082–27092. [Google Scholar] [CrossRef] [PubMed]
- Seto, J.Y.W. The electrical properties of polycrystalline silicon films. J. Appl. Phys. 1975, 46, 5247–5254. [Google Scholar] [CrossRef]
- Scheele, M.; Oeschler, N.; Veremchuk, I.; Peters, S.-O.; Littig, A.; Kornowski, A.; Klinke, C.; Weller, H. Thermoelectric properties of lead chalcogenide core–shell nanostructures. ACS Nano 2011, 5, 8541–8551. [Google Scholar] [CrossRef] [Green Version]
- Kishimoto, K.; Yamamoto, K.; Koyanagi, T. Influences of potential barrier scattering on the thermoelectric properties of sintered n-Type PbTe with a small grain size. Jpn. J. Appl. Phys. 2003, 42, 501–508. [Google Scholar] [CrossRef]
- Kishimoto, K.; Koyanagi, T. Preparation of sintered degenerate n-type PbTe with a small grain size and its thermoelectric properties. J. Appl. Phys. 2002, 92, 2544. [Google Scholar] [CrossRef]
- Schenk, M.; Berger, H.; Klimakow, A.; Mühlberg, M.; Wienecke, M. Nonstoichiometry and point defects in PbTe. Cryst. Res. Technol. 1988, 23, 77–84. [Google Scholar] [CrossRef]
- Strauss, A.J. Effect of Pb- and Te-saturation on carrier concentrations in impurity-doped PbTe. J. Electron. Mater. 1973, 2, 553–569. [Google Scholar] [CrossRef]
- Allgaier, R.S. Valence bands in lead telluride. J. Appl. Phys. 1961, 32, 2185. [Google Scholar] [CrossRef]
- Brebrick, R.F.; Gubner, E. Composition stability limits of PbTe. II. J. Chem. Phys. 1962, 36, 1283–1289. [Google Scholar] [CrossRef]
- Fritts, R.W. Thermoelectric Materials and Devices; Reinhold Publication Corporation: New York, NY, USA, 1960; pp. 143–162. [Google Scholar]
- LaLonde, A.D.; Pei, Y.; Wang, H.; Snyder, G.J. Lead telluride alloy thermoelectrics. Mater. Today 2011, 14, 526–532. [Google Scholar] [CrossRef]
- Shogenji, K.; Uchiyama, S. On electrical resistivity and hall coefficient of PbTe crystals. J. Phys. Soc. Jpn. 1957, 12, 252–258. [Google Scholar] [CrossRef]
- Allgaier, R.S.; Scanlon, W.W. Mobility of electrons and holes in PbS, PbSe, and PbTe between room temperature and 4.2 °K. Phys. Rev. 1958, 111, 1029–1037. [Google Scholar] [CrossRef]
- Miller, E.; Komarek, K.; Cadoff, I. Interrelation of electronic properties and defect equilibria in PbTe. J. Appl. Phys. 1961, 32, 2457–2465. [Google Scholar] [CrossRef]
- Scanlon, W.W. Precipitation of Te and Pb in PbTe crystals. Phys. Rev. 1962, 126, 509–513. [Google Scholar] [CrossRef]
- Crocker, A.J.; Rogers, L.M. Interpretation of the Hall coefficient, electrical resistivity and Seebeck coefficient of p-type lead telluride. Br. J. Appl. Phys. 1967, 18, 563–573. [Google Scholar] [CrossRef]
- Das, V.D.; Bhat, K.S. Anomalous temperature dependence of thermoelectric power of PbTe thin films. J. Appl. Phys. 1983, 54, 6641. [Google Scholar] [CrossRef]
- Heremans, J.P.; Thrush, C.M.; Morelli, D.T. Thermopower enhancement in lead telluride nanostructures. Phys. Rev. B 2004, 70, 115334. [Google Scholar] [CrossRef]
- Martin, J.; Wang, L.; Chen, L.; Nolas, G.S. Enhanced Seebeck coefficient through energy-barrier scattering in PbTe nanocomposites. Phys. Rev. B 2009, 79, 115311. [Google Scholar] [CrossRef] [Green Version]
- Bagiyeva, G.Z.; Mustafayev, N.B.; Abdinova, G.D.; Abdinov, D.S. Electrical properties of PbTe single crystals with excess tellurium. Semiconductors 2011, 45, 1391–1394. [Google Scholar] [CrossRef]
- Rawat, P.K.; Paul, B.; Banerji, P. Lead telluride based thermoelectrics: Approaches for higher efficiency. In Materials and Processes for Energy: Communicating Current Research and Technological Developments; Formatex Research Center: Badajoz, Spain, 2013; Volume 1, pp. 840–851. [Google Scholar]
- Wright, D.A. Materials for direct-conversion thermoelectric generators. Metallurg. Rev. 1970, 15, 147–160. [Google Scholar]
- Popescu, A.; Woods, L.M.; Martin, J.; Nolas, G.S. Model of transport properties of thermoelectric nanocomposite materials. Phys. Rev. B 2009, 79, 205302. [Google Scholar] [CrossRef] [Green Version]






| Ref. | Year | Grain Size | S (µV K−1) | σ (S cm−1) | PF (µW K−2 m−1) | κ (W m−1 K−1) | p (1019 cm−3) | µ (cm2 V−1 s−1) |
|---|---|---|---|---|---|---|---|---|
| [74] | 1957 | – | – | 25 | – | – | – | 500 |
| [75] | 1958 | – | – | 329 | – | – | 0.302 | 724 |
| [76] | 1961 | – | 260 | 40 | – | – | – | 560 |
| [70] | 1961 | – | – | – | – | – | 0.0048–1.67 | – |
| [77] | 1962 | – | 440 | 17.2 | 333 | – | 0.015 | 715 |
| – | 420 | 24.4 | 430 | – | 0.019 | 790 | ||
| – | 370 | 38.5 | 527 | – | 0.033 | 730 | ||
| – | 340 | 52.6 | 608 | – | 0.048 | 690 | ||
| – | 290 | 109.9 | 924 | – | 0.100 | 680 | ||
| [78] | 1967 | – | 400 | 62 | 992 | – | 0.043 | 900 |
| [69] | 1973 | – | – | – | – | 0.4–0.8 | – | |
| [79] | 1983 | – | 350–470 | – | – | – | – | – |
| [68] | 1988 | – | – | – | – | 0.2–1.2 | – | |
| [80] | 2004 | ≥60 nm | 406 | – | – | – | 0.038 | – |
| 44 nm | 456 | – | – | – | 0.046 | – | ||
| 42 nm | 508 | 6.7 | 173 | – | 0.021 | – | ||
| – | 494 | – | – | – | 0.014 | – | ||
| 36 nm | 189 | 22.2 | 79 | – | 0.820 | – | ||
| – | 174 | – | – | – | 0.760 | – | ||
| – | 265 | – | – | – | 0.220 | – | ||
| [81] | 2009 | 316 nm | 328 | 40.2 | 432 | 2.2 | 0.095 | – |
| 396 nm | 324 | 79.4 | 834 | 2.5 | 0.150 | – | ||
| [82] | 2011 | – | 180 | 25.1 | 81 | – | – | – |
| – | 300 | 52.5 | 473 | – | – | – | ||
| [83] | 2013 | – | 330 | 25 | 280 | – | – | – |
| – | 315 | 50 | 500 | – | – | – | ||
| – | 305 | 83 | 780 | – | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.; Lim, J.-H.; Lee, K.-H.; Kim, J.; Myung, N.V. Te-Embedded Nanocrystalline PbTe Thick Films: Structure and Thermoelectric Properties Relationship. Coatings 2021, 11, 356. https://doi.org/10.3390/coatings11030356
Wu T, Lim J-H, Lee K-H, Kim J, Myung NV. Te-Embedded Nanocrystalline PbTe Thick Films: Structure and Thermoelectric Properties Relationship. Coatings. 2021; 11(3):356. https://doi.org/10.3390/coatings11030356
Chicago/Turabian StyleWu, Tingjun, Jae-Hong Lim, Kyu-Hwan Lee, Jiwon Kim, and Nosang V. Myung. 2021. "Te-Embedded Nanocrystalline PbTe Thick Films: Structure and Thermoelectric Properties Relationship" Coatings 11, no. 3: 356. https://doi.org/10.3390/coatings11030356
APA StyleWu, T., Lim, J.-H., Lee, K.-H., Kim, J., & Myung, N. V. (2021). Te-Embedded Nanocrystalline PbTe Thick Films: Structure and Thermoelectric Properties Relationship. Coatings, 11(3), 356. https://doi.org/10.3390/coatings11030356