1. Introduction
Currently, bone implants made of metals and their alloys are widely used in orthopedics and dentistry. Due to the combination of bioinertness, strength, and low density [
1,
2,
3], titanium and its alloys are commonly used for implant applications [
4]. Titanium alloy Ti-6Al-4V is the predominant material for implant production because it combines corrosion resistance and high mechanical properties; however, this alloy contains cytotoxic elements Al and V, which can cause allergic reactions in the body tissues or a general toxic effect on the human organism [
5]. The functional reliability of metal implants is largely determined by the elastic modulus values of the implant and bone tissue: the closer they are, the better. Since the elastic modulus for titanium and its commercial alloys are higher than that for the bone tissue, such an implant can damage the adjacent bone, promote osteoporosis, and lead to other undesirable consequences [
6].
To overcome these biological and mechanical disadvantages, experimental Ti alloys doped with non-toxic elements such as niobium (Nb), zirconium (Zr), and tantalum (Ta) have been developed [
7,
8,
9,
10]. When exposed to an oxidative environment, oxide films of the alloy constituents (TiO
2, Nb
2O
5, ZrO
2, Ta
2O
5) are formed on the surface [
11]. This oxide layer protects the substrate, provides corrosion resistance and chemical stability [
11,
12], and induces a number of biological reactions, which increases the biocompatibility of implants [
13]. Moreover, stable and metastable β titanium alloys have both higher corrosion resistance and lower elastic modulus than α-type alloys, and especially commercially pure (cp) Ti [
1,
14,
15]. Most of these ternary and quaternary alloys are based on a Ti-Nb system with additional alloying by Zr, Sn, Ta, Mo and Hf [
2,
15,
16,
17,
18]. Furthermore, metastable β titanium alloys can be designed to exhibit a superelastic behavior at human body temperature, which promotes an additional decrease in the elastic modulus [
17,
18]. It was shown that Ti-18Zr-(14-15) Nb (at. %) alloy exhibits a high value of superelastic recovery strain (5–6%) [
18], which is decisive for improving the functional fatigue resistance [
19]. Thus, the Ti-18Zr-(14-15) Nb (at. %) alloy is a promising biocompatible material, allowing a reduction in the likelihood of implant failure.
The tissue response to a metal implant depends on the properties of the implant surface that gets into direct contact with the living tissue after the implantation [
20]. To improve the osseointegration and biocompatibility of titanium and titanium alloys, various surface treatments are used [
21,
22,
23]. Such methods can be based on mechanical, electrochemical, sol–gel techniques [
24,
25], physical vapor deposition [
26], anodizing and plasma electrolytic oxidation (PEO) [
27,
28], hybrid, and other methods [
29]. It should be noted that methods based on physical or chemical vapor deposition are considered as high-cost processes compared with the PEO, and they produce the coatings with rather less thickness. Other coating methods include high energy flux (HEF) surface treatment technologies, e.g., electron or laser beam technologies. Valkov et al. [
30] have shown that electron beam machining (EBT) results in increased surface roughness compared to other HEFs. The higher surface roughness after the EBT process should be suitable to support the cell growth and adhesion to the coated surface.
Anodizing is anodic oxidation in which a metal workpiece is anodically polarized at voltages not exceeding the potential corresponding to the dielectric breakdown of the oxide layer. As a result of anodizing, relatively thin uniform oxide coatings of the barrier type are obtained [
31]. Plasma electrolytic oxidation is a progression of the anodizing into higher voltages; it is a relatively new, inexpensive, and efficient method that allows the formation of a biocompatible oxide layer on the titanium alloys to improve cell attachment and adhesion to the implant surface [
32,
33,
34]. The PEO process has a good throwing power; the resultant coatings are uniform, and they can be much thicker than conventional anodic films, with controllable porosity and good adhesion.
Numerous studies on the coating growth, biocompatibility, and corrosion resistance by variation of PEO technological parameters and/or electrolytes show that plasma electrolytic oxidation of β titanium alloys is described by different kinetics compared to that of cp-Ti because these alloys contain significant amounts of doping elements. Therefore, it is important to understand the differences between the mechanisms of PEO for cp-Ti and Ti-Zr-Nb alloys. Sowa et al. modified the surface of the Ti-13Nb-13Zr alloy using plasma electrolytic oxidation at various voltages at a fixed DC density [
31]. This treatment increased the corrosion resistance and successful incorporation of Ca and P species into the PEO coatings. The topography of the resulting surface resembled the structure of a porous bone; this biomimetic effect improves the osseointegration, as shown elsewhere [
35]. In another study, the authors produced DC PEO coatings on the gum metal Ti-36Nb-3Zr-2Ta in a calcium hypophosphite-based electrolyte system [
31]. It was shown that, at the voltages applied (438 V and below), only Ti and Nb oxides were detected in the coating, both at the highest oxidation state. Tanase et al. [
20] showed that 50 Hz AC PEO technology can be used to modify the surface of low-modulus β-titanium alloys with a porous structure that facilitates osseointegration. Songur et al. [
36] formed PEO coatings on β-titanium alloy Ti-29Nb-13Ta-4.6Zr in pulsed DC regime at various frequencies with duty cycles from 10 to 30%. Corrosion rates in all coated samples were calculated approximately 4–14 times lower than those of the uncoated sample; the highest corrosion resistance was obtained in the PEO coatings obtained at 500 Hz with 30%.
Earlier, by optimization of the PEO process parameters and the coating composition, a pulsed bipolar PEO regime was proposed; this treatment has higher efficiency compared to DC, AC and pulsed DC PEO, and it helps to obtain biocompatible coatings on cp-Ti for implant applications [
37,
38]. Therefore, the goal of this research is to conduct a comparative analysis of the coatings obtained by plasma electrolytic oxidation in the same electrolyte and same pulsed bipolar regime on cp-Ti and the novel superelastic alloy Ti-18Zr-15Nb (at. %) for prospective use on spinal implants [
18,
39]. To the best of the authors’ knowledge, the pulsed bipolar PEO technology has not yet been investigated for this type of alloy.
2. Materials and Methods
2.1. Metal Sample Preparation and PEO Coating
In this work, samples made of titanium Grade 2 and the alloy Ti-18Zr-15Nb (TZN) were used. The TZN 15 kg ingot (supplied by NUST “MISIS”, Moscow, Russia) was produced by vacuum arc remelting. The melting of the alloy provided low amounts of impurities (O < 0.05, C < 0.01, N < 0.01, H < 0.01 in wt. %). Cp-Ti and TZN specimens were cut from a solid 9 mm rod as disks 0.5 mm thick using a spark cutting machine. Then, the disks were polished on abrasive paper until the roughness Ra < 0.1 μm was reached. Before carrying out plasma electrolytic oxidation, the samples were washed ultrasonically; first, in distilled water, then, in isopropyl alcohol, both for 5 min. To achieve a good electric contact, both for the PEO and for the consequent electrochemical tests, a copper wire was attached into a small hole drilled at the sample edge. The contact place and the copper wire were coated with an epoxy resin, which was able to withstand the applied voltage.
For the plasma electrolytic oxidation, a 10-L glass vessel was used; it was equipped with a stainless steel heat exchanger arranged around its walls. The electrolyte comprised 20 g/L aqueous solution of Na3PO4·12H2O of puris grade. The electrolyte volume was 5 L. The electrolyte temperature was maintained at 20 ± 1 °C using microcontroller regulation. The PEO process was run in the pulsed bipolar regime under the voltage regulation; the positive pulse was 470 V, negative 40 V; the frequency was 300 Hz. The duty cycle of positive pulses was 51%, negative—26%. The PEO treatment duration was 5 min.
2.2. Surface Characterization
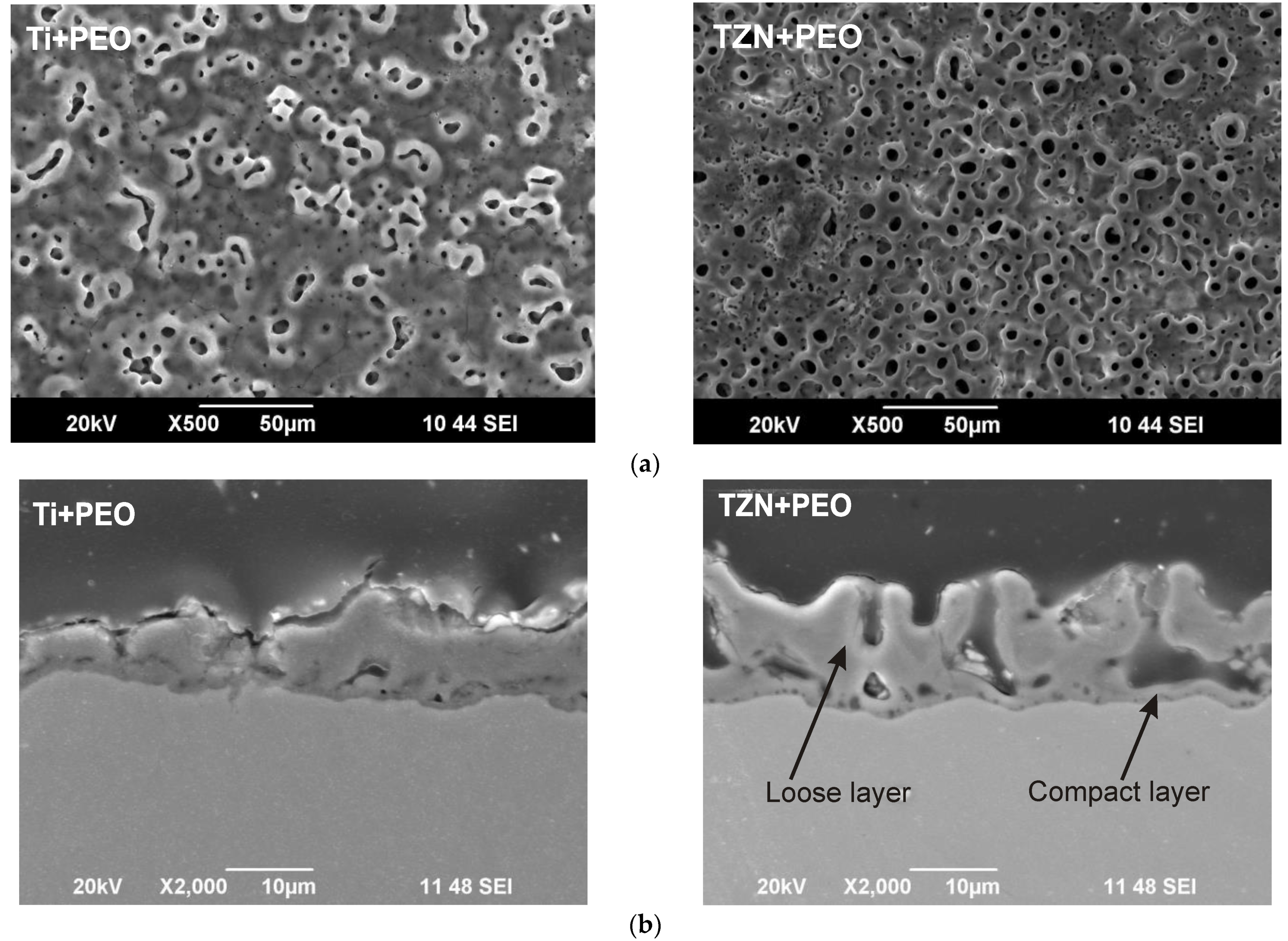
The top view of PEO coating was studied using the JEOL JSM-6490LV scanning electron microscope at an accelerating voltage of 20 kV. The coating thickness was measured using the Defelsko Positector 6000 eddy current gauge of N-type. The surface roughness was measured with the TR-220 profilometer. The coating porosity was assessed with ImageJ software from the SEM images following the ASTM E112-10. The adhesion of the coatings was evaluated using a CSM micro scratch tester. In this test, the load on a diamond Rockwell indenter with a tip radius of 200 μm was linearly increased from 0 to 20 N. After the test, the scratch on the surface of the coating was examined under an optical microscope to determine the position at which the coating was peeled off the substrate. The critical load values were determined using the data regarding acoustic emission and penetration depth. The phase composition of the surface layer was characterized using a Rigaku Ultima IV X-ray diffractometer in Cu Kα radiation at 40 kV and 40 mA using 0.02 deg. step scan with 2 s exposure, from 20 to 80 degrees 2θ. Further, the XRD spectra were processed using Philips X’Pert Highscore Plus software with the PDF2 pattern database; a built-in SemiQuant algorithm was employed to quantify the amounts of the crystalline phases in the coating. The elemental analysis of the coatings was carried out using a Shimadzu EDX-800HS energy-dispersive X-ray fluorescence spectrometer.
2.3. Electrochemical Tests
The electrochemical tests were carried out in Ringer’s solution at room temperature using the P-5X (Elins, Moscow, Russia) electrochemical system. Open circuit potential (OCP) measurements, electrochemical impedance spectroscopy (EIS) from 100 kHz to 1 mHz (20 points/decade) with the magnitude of 10 mV around OCP, and potentiodynamic polarization (PDP) tests in the range from −0.6 V to 1.5 V with respect to the OCP at 0.25 mV/s scan rate, were performed. A silver chloride electrode filled with 3.5 M KCl was used as a reference electrode. The counter electrode was a graphite rod. The polarization resistance Rp was calculated from the slope of the polarization curve at ± 10 mV around the free corrosion potential. The EIS results were analyzed using ZView software from Scribner Associates [
40].
2.4. Estimation of Kinetic Coefficients for the PEO Process
To study the kinetic aspects of the PEO process for the two different Ti alloys, we used the electrochemical relaxation method based on removing the electrochemical system from equilibrium using an input step action and further monitoring its relaxation back to equilibrium or new stationary state [
41]. In this work, a chronoamperometric method was used to quantitatively estimate the growth kinetics of the PEO coating via the amplitude of the anodic current density pulses. According to the method, when evaluating the kinetic coefficients, the transient processes of the current density in the relaxation region were analyzed. In this case, the input step was the power supply voltage, the amplitude values of which were maintained at a constant level after a soft-start ramp, and the response was the dependence of the resulting amplitude values of the current density
j. This approach was successfully applied elsewhere for the kinetic studies of plasma electrolytic oxidation of a Mg alloy [
42]. We used the kinetic model which contains three terms: (i) for the metal dissolution, (ii) for crystallization of the oxides, (iii) for the oxygen evolution. These terms follow from the Avrami theorem and Cottrell equation. The derivation of the formulae for the first two terms is described elsewhere [
41]; the third term is presented as a constant
jC [
43]:
where
j0—initial (maximum) current density;
m—the type of crystallization;
z—nuclear charge;
F—Faraday’s constant;
c—concentration of metal in the coating;
D—diffusion coefficient; τ—time constant of nucleation. The transients of the anodic current density values were approximated using Curve Fitting Toolbox in Matlab; the coefficient of determination R
2 was used to characterize the goodness of the fit.
4. Discussion
During the PEO treatment of cp-Ti and Ti-Zr-Nb alloy, the following reactions occur in the system [
49,
50]. For the PEO of cp-Ti, on the anode, metal ions are released into the electrolyte due to anodic dissolution:
This reaction is described by the first term in Equation (1) for the kinetic model of the PEO cp-Ti. The anodic oxidation reaction of Ti to titanium dioxide can be written as:
This reaction of the oxide formation during PEO of the cp-Ti alloy is described by the second term of Equation (1).
For the Ti-Zr-Nb alloy, in addition to that mentioned above, reactions for the alloying elements Zr and Nb also occur. The following reactions of the anodic dissolution also contribute to the first term of Equation (1) for Ti-Zr-Nb alloy:
The reactions of formation of the Zr and Nb oxides also influence the second term of Equation (1) for Ti-Zr-Nb alloy:
As a competing reaction on the anode, water is decomposed according to the following reaction:
The release of oxygen at the anode is described by the third term of Equation (1).
The oxides of Ti, Zr and Nb were identified in TZN+PEO coating. However, the main part of the coating consists of Ti and Nb oxides, while ZrO
2 content is less than 5%. This is consistent with the results presented elsewhere [
51], and it can be explained as follows. In this work, the voltage of positive pulses of the PEO treatment was below 500 V, which corresponds to the effective treatment of Ti and Nb [
52,
53]. At higher voltages, coatings on Ti and Nb can crack due to the action of powerful discharges. Unlike these elements, Zr is effectively oxidized at voltages above 600 V in a similar phosphate electrolyte [
54]. This is consistent with the values of the melting temperatures of the oxides (
Table 7). Since the zirconia has a much higher melting temperature compared to that of titania and niobia, the microdischarges at the voltages below 500 V are not powerful enough to contribute to the formation of ZrO
2. Therefore, after the PEO treatment of the Ti-Zr-Nb alloy, the content of ZrO
2 in the coating is insignificant, which is reflected in the XRD results.
From the calculated parameters of the kinetic model, it follows that the diffusion coefficient D is higher for the PEO process of the Ti-Zr-Nb alloy. This indicates higher mobility of the products of the electrochemical reactions in the microdischarges during the PEO process of the Ti-Zr-Nb alloy. This may be due to the difference in the melting temperatures of the oxides [
50] (
Table 7) since the temperature gradient enhances the diffusion processes.
As a result, a thicker coating with higher porosity and roughness is formed on the Ti-Zr-Nb alloy compared to the coating on the cp-Ti alloy. The TZN+PEO coating is formed faster, since the time constant of the crystallization process τ for the TZN+PEO sample is almost twice as small as that of the Ti+PEO sample. The formation of the oxides leads to an increase in the electrical resistance of the coating; as a result, the current density decreases—this is reflected in the lower value of jc of the third term in Equation (1) for the TZN alloy. An increase in the porosity and roughness of the TZN+PEO coating occurs because of processing at a lower current density compared to the Ti+PEO sample. Therefore, if the alloy is exposed to lower energy pulses, the formation of the coating with higher porosity and roughness is observed.
The morphology of the PEO coating of the TZN+PEO sample significantly influenced the corrosion behavior, which was shown in electrochemical tests. Based on the results of PDP electrochemical tests, it was found that PEO treatment significantly reduces the corrosion current of the Ti+PEO and TZN+PEO samples. The results of the electrochemical impedance spectroscopy show a significant increase in the corrosion resistance for the TZN+PEO sample.
Finally, to obtain the PEO coatings in the pulsed regime on a novel Ti-18Zr-15Nb superelastic alloy, further research based on the uncovered regularities is required to find the most efficient electrolyte composition and electrical parameters for optimal PEO treatment of this alloy for implant applications.