Interface Characteristics and Anticorrosion Performances of Cold Galvanizing Coatings Incorporated with ?-chloropropyl Triethoxysilane on Hot-Dip Galvanized Steel
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Chemicals
2.2. Preparation of Coated Steel Panels
2.3. Pull-Off Adhesion Measurements
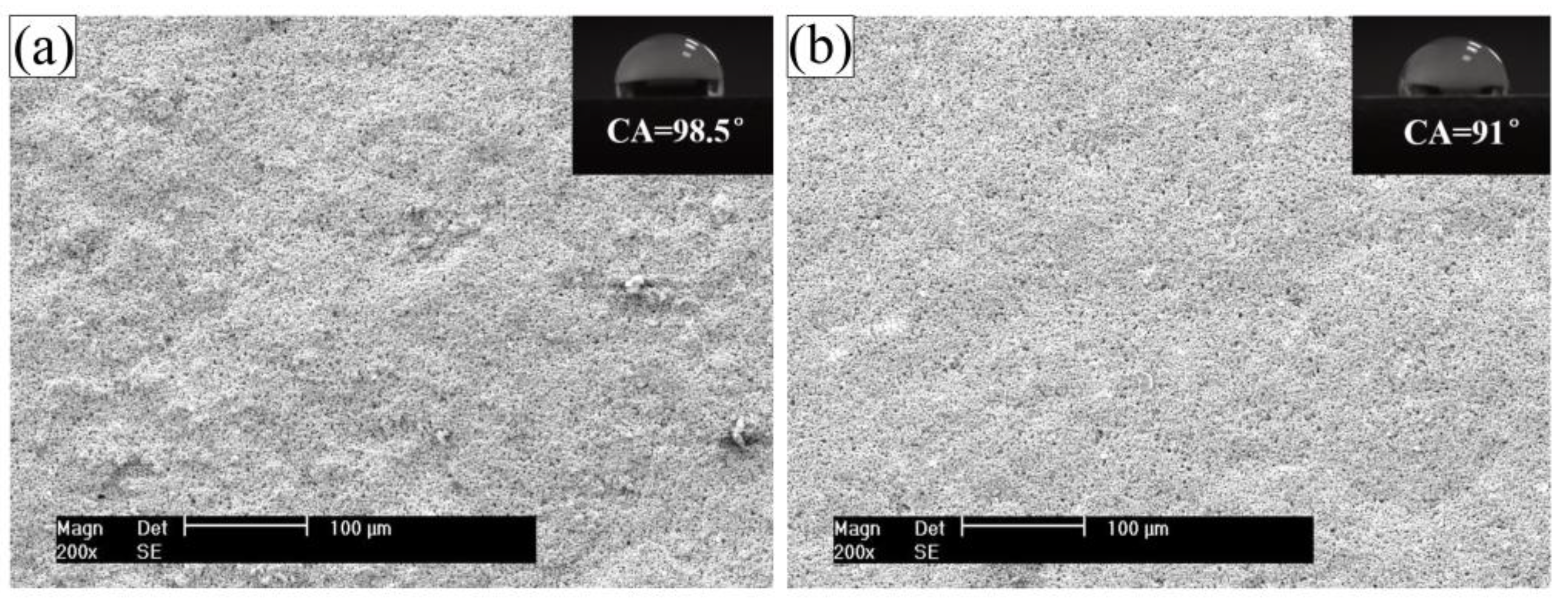
2.4. Water Contact Angle
2.5. Characterization
2.6. Electrochemical Corrosion Tests
3. Results and Discussion
3.1. Wettability
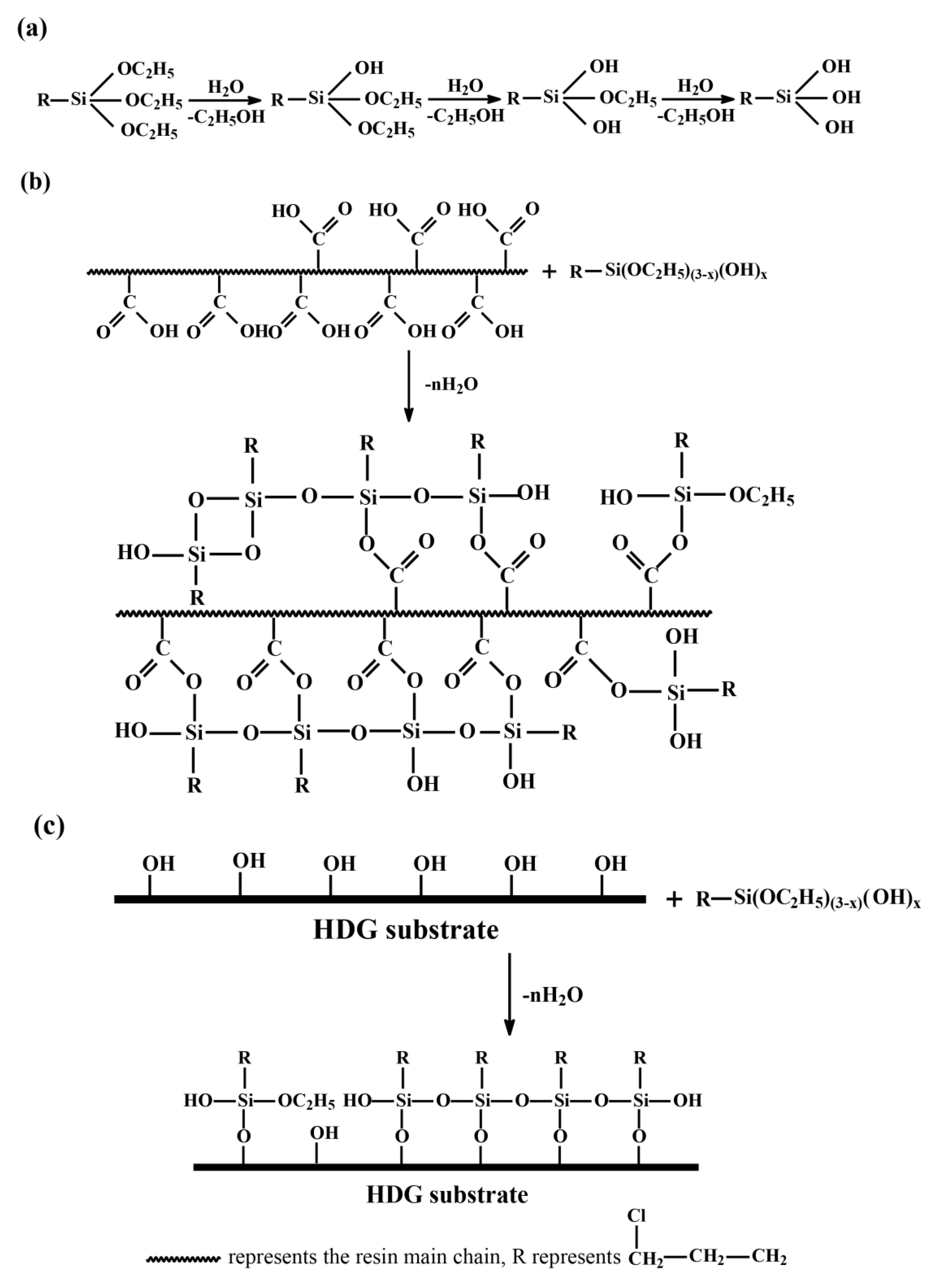
3.2. FT-IR and XPS Analysis
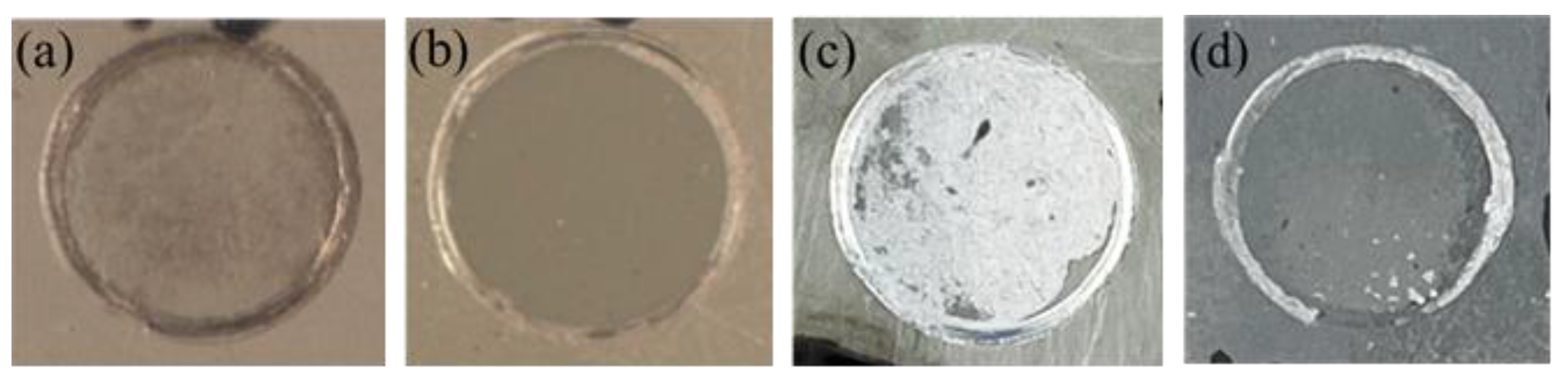
3.3. Pull-off Adhesion Measurements
3.4. Electrochemical Measurements
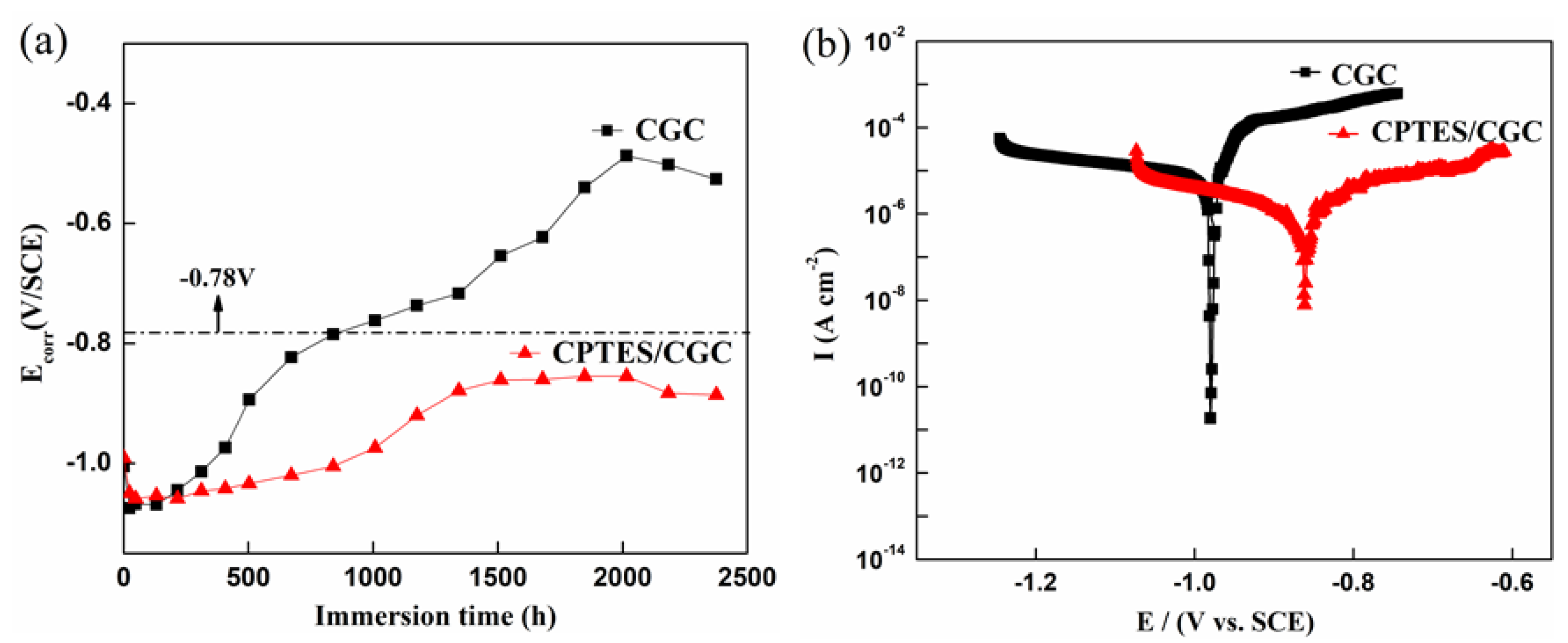
3.4.1. OCP and Potentiodynamic Polarization
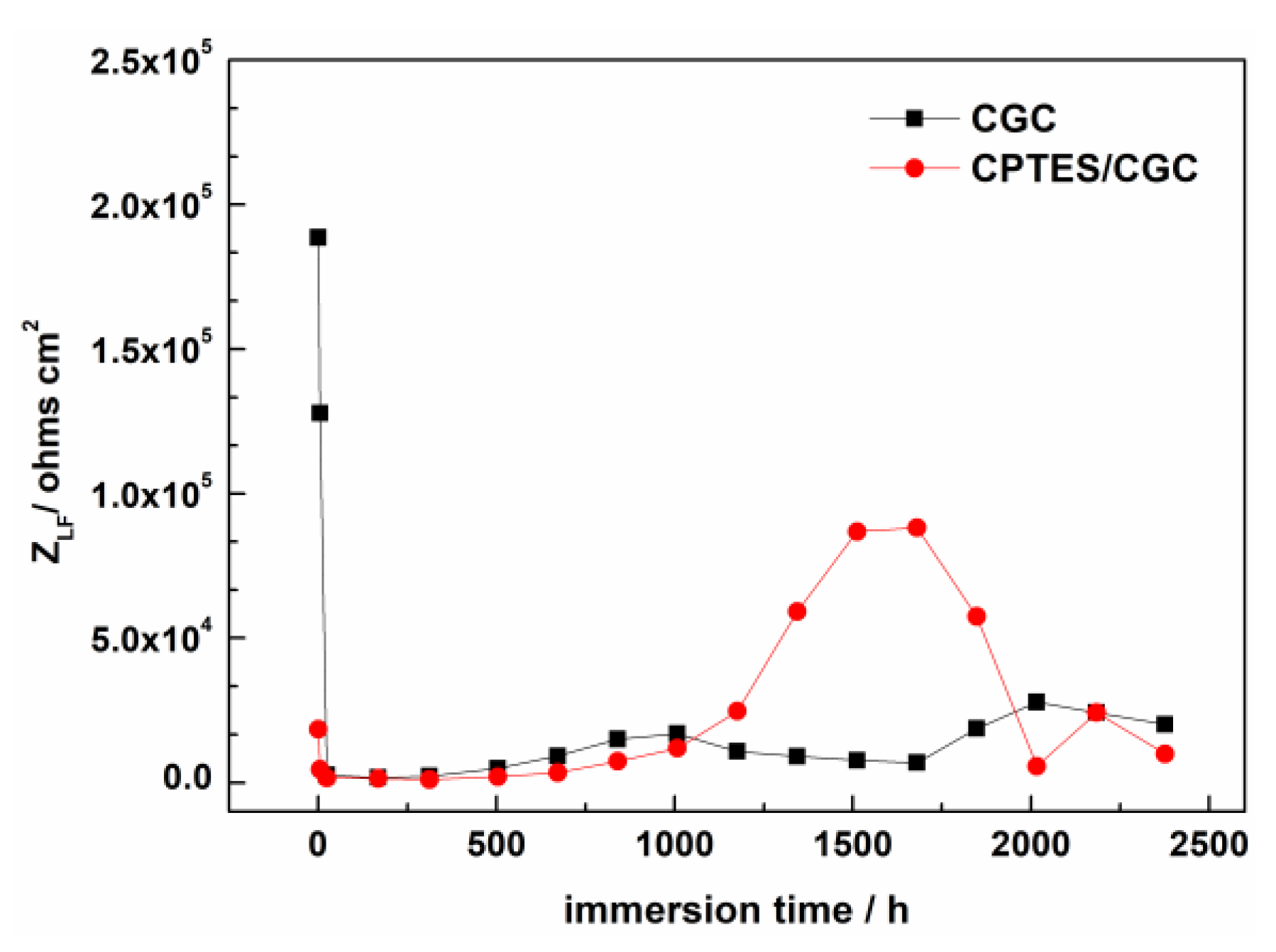
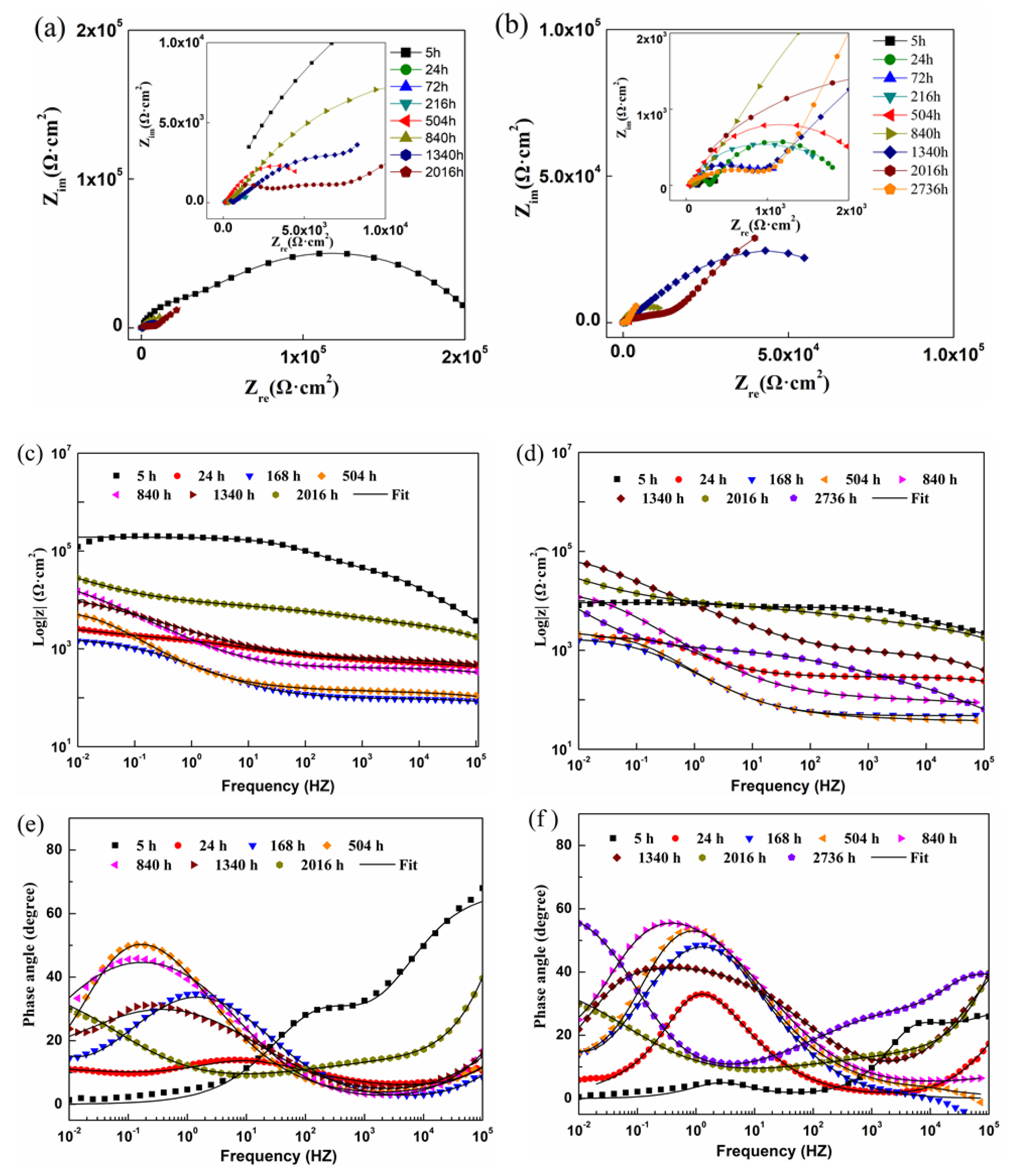
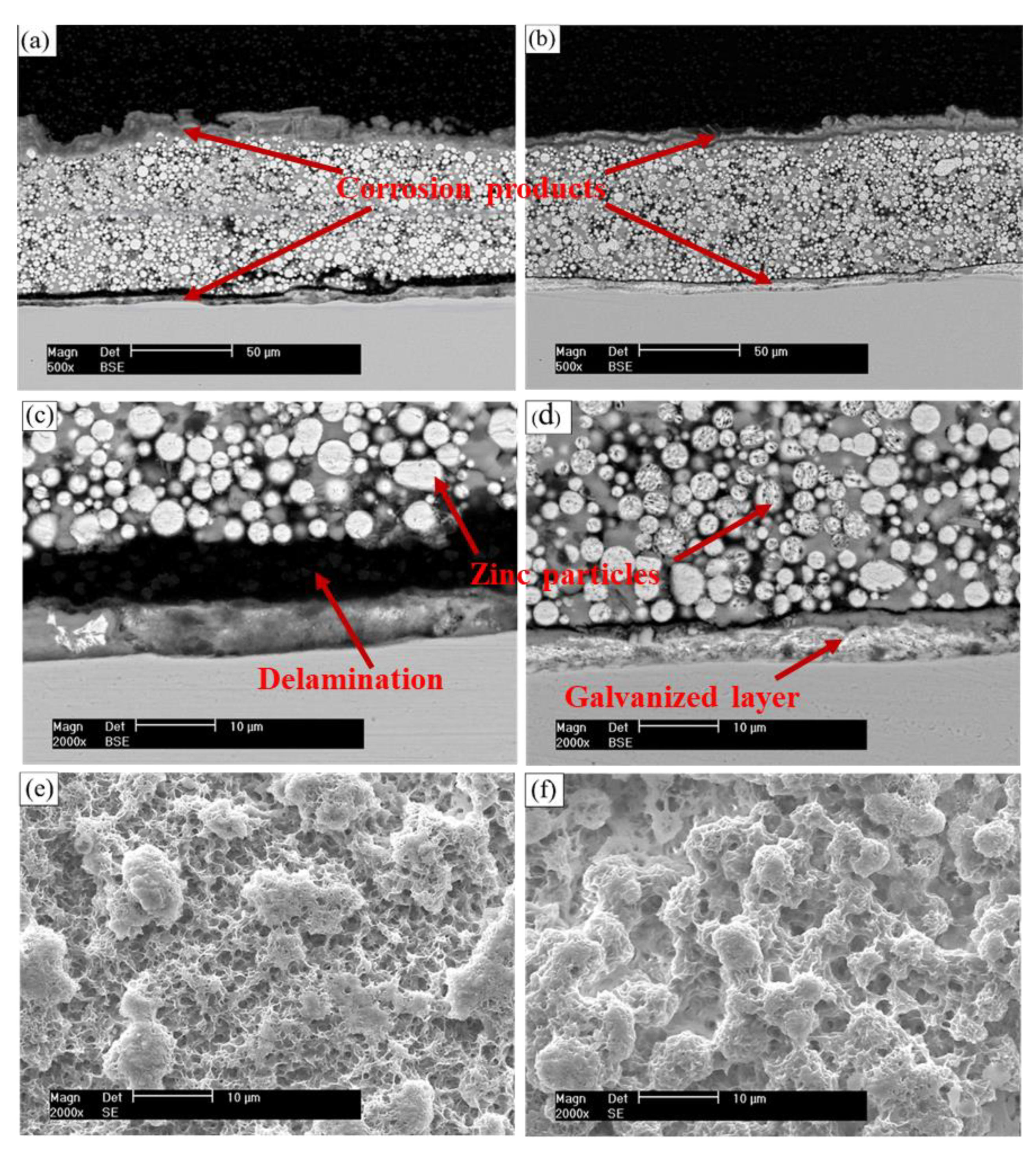
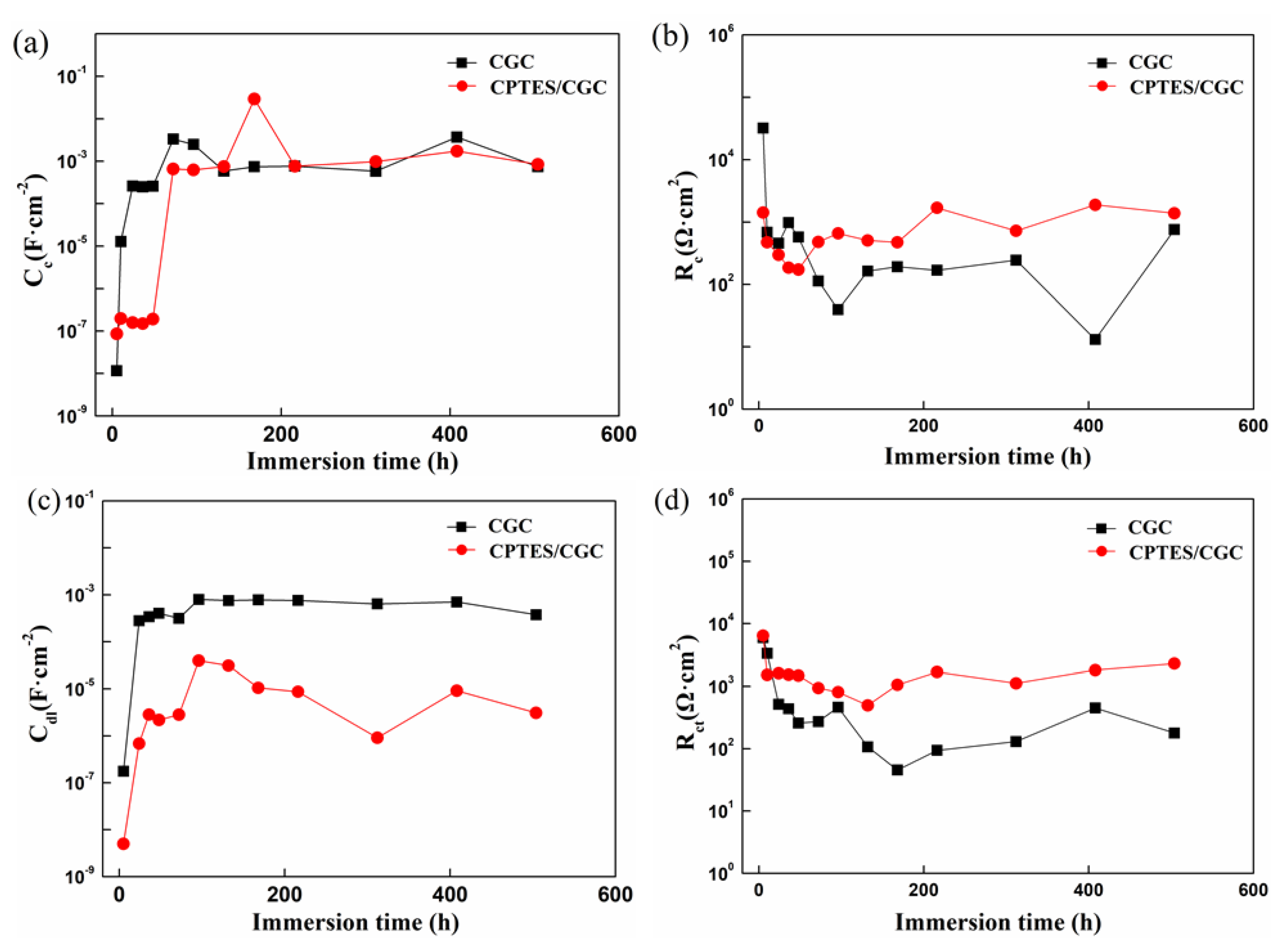
3.4.2. Electrochemical Impedance Spectroscopy Test
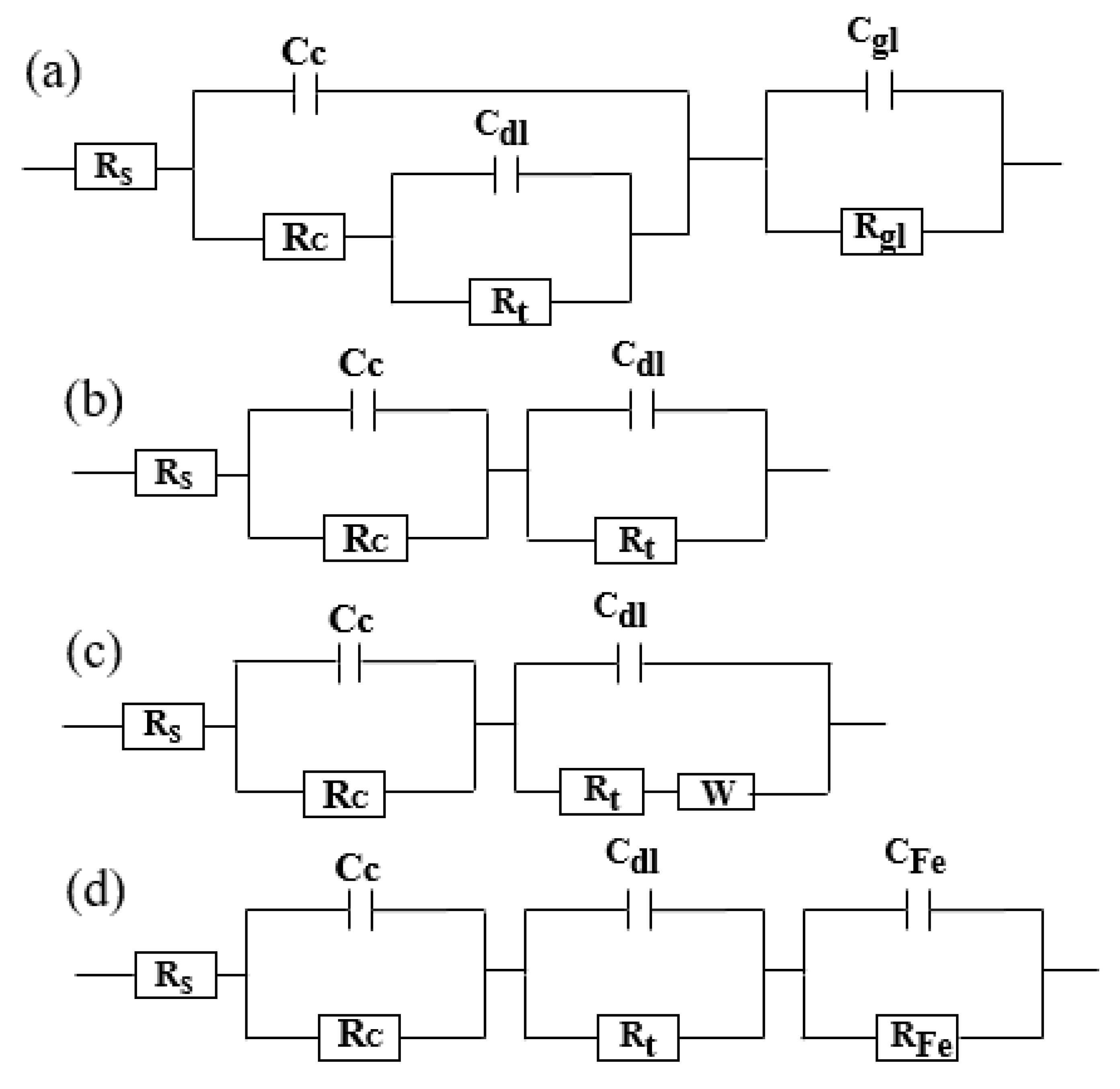
3.4.3. Equivalent Circuits Fitting and Fitting Parameters
4. Conclusions
- (1)
- Water contact angle experimental results showed that wettability of the CPTES/CGC increase, compared with that of the CGC, which benefited to coating/substrate interface adhesion.
- (2)
- The FTIR and XPS results proved crosslinking networks occurred in the CPTES/CGC and its interface due to Si–O–Si, Si–O–C and Si–O–Zn bond formation.
- (3)
- The pull-off adhesion experimental results revealed that dry adhesion and wet adhesion of CPTES/CGC increase by 50% and 200%, respectively compared with CGC, attributed to wettability improvement and crosslinking network formation.
- (4)
- Electrochemical results indicated that the cathodic protection and barrier effects of CGC were improved by CPTES, especially the cathodic protection effect time increased threefold due to adhesion enhancement and three-dimensional network construction.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marder, A.R. The metallurgy of zinc-coated steel. Prog. Mater. Sci. 2000, 45, 191–271. [Google Scholar] [CrossRef]
- Li, C.; Liang, T.; Ma, R.; Du, A.; Fan, Y.; Zhao, X.; Cao, X. Superhydrophobic surface containing cerium salt and organosilane for corrosion protection of galvanized steel. J. Alloys Compd. 2020, 825, 153921. [Google Scholar] [CrossRef]
- Liu, Y.W.; Wang, Z.Y.; Cao, G.W.; Cao, Y.; Huo, Y. Study on corrosion behavior of zinc exposed in coastal-industrial atmospheric environment. Mater. Chem. Phys. 2017, 198, 243–249. [Google Scholar] [CrossRef]
- Cabral, A.M.; Trabelsi, W.; Serra, R.; Montemor, M.F.; Zheludkevich, M.L.; Ferreira, M.G.S. The corrosion resistance of hot dip galvanised steel and AA2024-T3 pre-treated with bis- triethoxysilylpropyl tetrasulfide solutions doped with Ce(NO3)3. Corros. Sci. 2006, 48, 3740–3758. [Google Scholar] [CrossRef]
- Zhu, D.Q.; van Ooij, W.J. Enhanced corrosion resistance of AA 2024-T3 and hot-dip galvanized steel using a mixture of bis- triethoxysilylpropyl tetrasulfide and bis- trimethoxysilylpropyl amine. Electrochim. Acta 2004, 49, 1113–1125. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Attar, M.M. An evaluation of the corrosion resistance and adhesion properties of an epoxy-nanocomposite on a hot-dip galvanized steel (HDG) treated by different kinds of conversion coatings. Surf. Coat. Technol. 2011, 205, 4649–4657. [Google Scholar] [CrossRef]
- Lin, B.-L.; Lu, J.-T.; Kong, G. Effect of molybdate post-sealing on the corrosion resistance of zinc phosphate coatings on hot-dip galvanized steel. Corros. Sci. 2008, 50, 962–967. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Attar, M.M. Studying the effects of micro and nano sized ZnO particles on the corrosion resistance and deterioration behavior of an epoxy-polyamide coating on hot-dip galvanized steel. Prog. Org. Coat. 2011, 71, 314–328. [Google Scholar] [CrossRef]
- Schaefer, K.; Miszczyk, A. Improvement of electrochemical action of zinc-rich paints by addition of nanoparticulate zinc. Corros. Sci. 2013, 66, 380–391. [Google Scholar] [CrossRef]
- Shreepathi, S.; Bajaj, P.; Mallik, B.P. Electrochemical impedance spectroscopy investigations of epoxy zinc rich coatings: Role of Zn content on corrosion protection mechanism. Electrochim. Acta 2010, 55, 5129–5134. [Google Scholar] [CrossRef]
- Xu, L.; Liu, F.; Han, E.-H.; Ke, W. Corrosion resistance and mechanism of one-component organic Zn15Al-rich coating. Prog. Org. Coat. 2019, 132, 305–315. [Google Scholar] [CrossRef]
- Yun, T.H.; Park, J.H.; Kim, J.-S.; Park, J.M. Effect of the surface modification of zinc powders with organosilanes on the corrosion resistance of a zinc pigmented organic coating. Prog. Org. Coat. 2014, 77, 1780–1788. [Google Scholar] [CrossRef]
- Bastos, A.C.; Zheludkevich, M.L.; Klüppel, I.; Grundmeier, G.; Ferreira, M.G.S. Modification of zinc powder to improve the corrosion resistance of weldable primers. Prog. Org. Coat. 2010, 69, 184–192. [Google Scholar] [CrossRef]
- Xu, L.; Liu, F.; Wang, Z.; Ke, W.; Han, E.-H.; Jie, G.; Wang, J.; Huang, H. The effect of surface modification of zinc particles with phosphoric acid on the corrosion resistance of cold galvanizing coatings. Prog. Org. Coat. 2018, 114, 90–101. [Google Scholar] [CrossRef]
- Xu, L.; Liu, F.; Liu, M.; Wang, Z.; Qian, Z.; Ke, W.; Han, E.-H.; Jie, G.; Wang, J.; Zhu, L. Fabrication of repairable superhydrophobic surface and improved anticorrosion performance based on zinc-rich coating. Prog. Org. Coat. 2019, 137, 105335. [Google Scholar] [CrossRef]
- Kalendová, A. Effects of particle sizes and shapes of zinc metal on the properties of anticorrosive coatings. Prog. Org. Coat. 2003, 46, 324–332. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Ma, A.B.; Jiang, J.H.; Song, D.; Chen, J.Q.; Yang, D.H. Anti-corrosion performance of waterborne Zn-rich coating with modified silicon-based vehicle and lamellar Zn(Al) pigments. Prog. Nat. Sci. 2012, 22, 326–333. [Google Scholar] [CrossRef] [Green Version]
- Hare, C.H.; Kurnas, J.S. Reduced PVC and the design of metal primers. J. Coat. Technol. 2000, 72, 21–27. [Google Scholar] [CrossRef]
- Kalendova, A.; Kalenda, P.; Vesely, D. Comparison of the efficiency of inorganic nonmetal pigments with zinc powder in anticorrosion paints. Prog. Org. Coat. 2006, 57, 1–10. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Moghadam, M.H.M.; Shohani, N.; Mandavian, M. Effects of highly crystalline and conductive polyaniline/graphene oxide composites on the corrosion protection performance of a zinc-rich epoxy coating. Chem. Eng. J. 2017, 320, 363–375. [Google Scholar] [CrossRef]
- Arman, S.Y.; Ramezanzadeh, B.; Farghadani, S.; Mehdipour, M.; Rajabi, A. Application of the electrochemical noise to investigate the corrosion resistance of an epoxy zinc-rich coating loaded with lamellar aluminum and micaceous iron oxide particles. Corros. Sci. 2013, 77, 118–127. [Google Scholar] [CrossRef]
- Cho, S.; Chiu, T.M.; Castaneda, H. Electrical and electrochemical behavior of a zinc-rich epoxy coating system with carbon nanotubes as a diode-like material. Electrochim. Acta 2019, 316, 189–201. [Google Scholar] [CrossRef]
- Feliu, S.; Morcillo, M.; Feliu, S. Deterioration of cathodic protection action of zinc-rich paint coatings in atmospheric exposure. Corrosion 2001, 57, 591–597. [Google Scholar] [CrossRef]
- Fedel, M.; Olivier, M.; Poelman, M.; Deflorian, F.; Rossi, S.; Druart, M.E. Corrosion protection properties of silane pre-treated powder coated galvanized steel. Prog. Org. Coat. 2009, 66, 118–128. [Google Scholar] [CrossRef]
- Pourhashem, S.; Vaezi, M.R.; Rashidi, A.; Bagherzadeh, M.R. Distinctive roles of silane coupling agents on the corrosion inhibition performance of graphene oxide in epoxy coatings. Prog. Org. Coat. 2017, 111, 47–56. [Google Scholar] [CrossRef]
- Jiang, M.Y.; Wu, L.K.; Hu, J.M.; Zhang, J.Q. Silane-incorporated epoxy coatings on aluminum alloy (AA2024). Part 1: Improved corrosion performance. Corros. Sci. 2015, 92, 118–126. [Google Scholar] [CrossRef]
- Iglesias, J.G.; Gonzalez-Benito, J.; Aznar, A.J.; Bravo, J.; Baselga, J. Effect of glass fiber surface treatments on mechanical strength of epoxy based composite materials. J. Colloid Interface Sci. 2002, 250, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Davis, S.R.; Brough, A.R.; Atkinson, A. Formation of silica/epoxy hybrid network polymers. J. Non Cryst. Solids 2003, 315, 197–205. [Google Scholar] [CrossRef]
- Liu, B.; Fang, Z.G.; Wang, H.B.; Wang, T. Effect of cross linking degree and adhesion force on the anti-corrosion performance of epoxy coatings under simulated deep sea environment. Prog. Org. Coat. 2013, 76, 1814–1818. [Google Scholar] [CrossRef]
- Chico, B.; Galván, J.C.; de la Fuente, D.; Morcillo, M. Electrochemical impedance spectroscopy study of the effect of curing time on the early barrier properties of silane systems applied on steel substrates. Prog. Org. Coat. 2007, 60, 45–53. [Google Scholar] [CrossRef]
- Ji, W.-G.; Hu, J.-M.; Zhang, J.-Q.; Cao, C.-N. Reducing the water absorption in epoxy coatings by silane monomer incorporation. Corros. Sci. 2006, 48, 3731–3739. [Google Scholar] [CrossRef]
- Ji, W.-G.; Hu, J.-M.; Liu, L.; Zhang, J.-Q.; Cao, C.-N. Water uptake of epoxy coatings modified with γ-APS silane monomer. Prog. Org. Coat. 2006, 57, 439–443. [Google Scholar] [CrossRef]
- Wang, P.; Schaefer, D.W. Why does Silane Enhance the Protective Properties of Epoxy Films? Langmuir 2008, 24, 13496–13501. [Google Scholar] [CrossRef] [PubMed]
- Montemor, M.F.; Cabral, A.M.; Zheludkevich, M.L.; Ferreira, M.G.S. The corrosion resistance of hot dip galvanized steel pretreated with Bis-functional silanes modified with microsilica. Surf. Coat. Technol. 2006, 200, 2875–2885. [Google Scholar] [CrossRef]
- ISO 4624: 2016 Paints and Varnishes— Pull-off Test for Adhesion; ISO: Geneve, Switzerland, 2016.
- Zhang, D.; Qian, H.; Wang, L.; Li, X. Comparison of barrier properties for a superhydrophobic epoxy coating under different simulated corrosion environments. Corros. Sci. 2016, 103, 230–241. [Google Scholar] [CrossRef]
- Wang, X.; Hu, J.; Li, Y.; Zhang, J.; Ding, Y. The surface properties and corrosion resistance of fluorinated polyurethane coatings. J. Fluor. Chem. 2015, 176, 14–19. [Google Scholar] [CrossRef]
- Chibowski, E.; Perea-Carpio, R. Problems of contact angle and solid surface free energy determination. Adv. Colloid Interface Sci. 2002, 98, 245–264. [Google Scholar] [CrossRef]
- Li, D.; Neumann, A.W. Contact angles on hydrophobic solid surfaces and their interpretation. J. Colloid Interface Sci. 1992, 148, 190–200. [Google Scholar] [CrossRef]
- Vakili, H.; Ramezanzadeh, B.; Amini, R. The corrosion performance and adhesion properties of the epoxy coating applied on the steel substrates treated by cerium-based conversion coatings. Corros. Sci. 2015, 94, 466–475. [Google Scholar] [CrossRef]
- Rodic, P.; Iskra, J.; Milosev, I. A hybrid organic-inorganic sol-gel coating for protecting aluminium alloy 7075-T6 against corrosion in Harrison’ s solution. J. Sol Gel Sci. Technol. 2014, 70, 90–103. [Google Scholar] [CrossRef]
- Rodic, P.; Korosec, R.C.; Kapun, B.; Mertelj, A.; Milosev, I. Acrylate-Based Hybrid Sol-Gel Coating for Corrosion Protection of AA7075-T6 in Aircraft Applications: The Effect of Copolymerization Time. Polymers 2020, 12, 948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, C.; Shao, Q.; Wu, M.; Zheng, B.; Guo, Z.; Yi, J.; Zhang, J.; Lin, J.; Wu, S.; Dong, M.; et al. 2-(3,4-Epoxy) ethyltriethoxysilane-modified waterborne acrylic resin: Preparation and property analysis. Polymer 2020, 190, 122196. [Google Scholar] [CrossRef]
- Wu, G.; Ma, L.; Jiang, H. The roles of surface wettability and roughness of carbon fibers in interfacial enhancement of silicone resin composites. Polym. Compos. 2019, 40, E255–E264. [Google Scholar] [CrossRef]
- Rodič, P.; Kapun, B.; Panjan, M.; Milošev, I. Easy and Fast Fabrication of Self-Cleaning and Anti-Icing Perfluoroalkyl Silane Film on Aluminium. Coatings 2020, 10, 234. [Google Scholar] [CrossRef] [Green Version]
- NIST X-ray Photoelectron Spectroscopy Database. NIST Standard Reference Database Number 20; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2000; Volume 2000. [CrossRef]
- Uzoma, P.C.; Liu, F.; Xu, L.; Zhang, Z.; Han, E.-H.; Ke, W.; Arukalam, I.O. Superhydrophobicity, conductivity and anticorrosion of robust siloxane-acrylic coatings modified with graphene nanosheets. Prog. Org. Coat. 2019, 127, 239–251. [Google Scholar] [CrossRef]
- Meroufel, A.; Touzain, S. EIS characterisation of new zinc-rich powder coatings. Prog. Org. Coat. 2007, 59, 197–205. [Google Scholar] [CrossRef]
- Abreu, C.M.; Izquierdo, M.; Keddam, M.; Nóvoa, X.R.; Takenouti, H. Electrochemical behaviour of zinc-rich epoxy paints in 3% NaCl solution. Electrochim. Acta 1996, 41, 2405–2415. [Google Scholar] [CrossRef]
- Bonnel, K.; Le Pen, C.; Pébère, N.E.I.S. characterization of protective coatings on aluminum alloys. Electrochim. Acta 1999, 44, 4259–4267. [Google Scholar] [CrossRef]
- Marchebois, H.; Keddam, M.; Savall, C.; Bernard, J.; Touzain, S. Zinc-rich powder coatings characterisation in artificial sea water: EIS analysis of the galvanic action. Electrochim. Acta 2004, 49, 1719–1729. [Google Scholar] [CrossRef]
- Armstrong, R.D.; Wright, J.D. Impedance studies of poly ethylmethacrylate coatings formed upon tin-free steel. Corros. Sci. 1992, 33, 1529–1539. [Google Scholar] [CrossRef]
- Naderi, R.; Attar, M.M.; Moayed, M.H. EIS examination of mill scale on mild steel with polyester-epoxy powder coating. Prog. Org. Coat. 2004, 50, 162–165. [Google Scholar] [CrossRef]


| Samples | Zinc Powders (wt %) | Resin (wt %) | Solvent (wt %) | Silane (wt %) |
|---|---|---|---|---|
| CGC | 69.5 | 17.4 | 13.1 | — |
| CPTES/CGC | 69.5 | 17.4 | 11.1 | 2 |
| Sample | Wa | ||
|---|---|---|---|
| CGC | 98.5 ± 0.87 | 6.20 ± 0.11 | 2.40 ± 0.06 |
| CPTES/CGC | 91.0 ± 0.71 | 7.15 ± 0.09 | 2.86 ± 0.04 |
| Samples | Dry Adhesion (MPa) | Modality of Failure | Wet Adhesion (MPa) | Modality of Failure | Adhesion Loss |
|---|---|---|---|---|---|
| CGC | 2.0 (2.28 ± 0.12) | 50% A/B, 50% B | 1.0 (1.12 ± 0.28) | 90% A/B, 10% B | 50.9% |
| CPTES/CGC | 3.0 (3.36 ± 0.44) | 100% B | 3.0 (2.64 ± 0.24) | 10% A/B, 90% B | 21.4% |
| Samples | Icorr (A/cm2) | Ecorr (V/SCE) | Ba (mV) | Bc (mV) | Rp ( cm2) |
|---|---|---|---|---|---|
| CGC | 8.59 × 10−5 (± 1.04 × 10−5) | −0.98 (± 0.0055) | 274.18 (± 15.65) | −465.49 (± 31.33) | 3.41 × 103 (± 154.0) |
| CPTES/CGC | 1.55 × 10−6 (± 1.625 × 10−7) | −0.85 (± 0.014) | 256.53 (± 32.10) | −331.32 (± 60.90) | 4.74 × 105 (± 3.16 × 105) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, Q.; Gao, N.; Nwokolo, I.K.; Zhang, W.; Ma, L.; Liu, F.; Han, E.-H. Interface Characteristics and Anticorrosion Performances of Cold Galvanizing Coatings Incorporated with ?-chloropropyl Triethoxysilane on Hot-Dip Galvanized Steel. Coatings 2021, 11, 402. https://doi.org/10.3390/coatings11040402
Li J, Wang Q, Gao N, Nwokolo IK, Zhang W, Ma L, Liu F, Han E-H. Interface Characteristics and Anticorrosion Performances of Cold Galvanizing Coatings Incorporated with ?-chloropropyl Triethoxysilane on Hot-Dip Galvanized Steel. Coatings. 2021; 11(4):402. https://doi.org/10.3390/coatings11040402
Chicago/Turabian StyleLi, Jiwen, Qiumeng Wang, Ningjie Gao, Izuchukwu Kenneth Nwokolo, Wanyu Zhang, Lin Ma, Fuchun Liu, and En-Hou Han. 2021. "Interface Characteristics and Anticorrosion Performances of Cold Galvanizing Coatings Incorporated with ?-chloropropyl Triethoxysilane on Hot-Dip Galvanized Steel" Coatings 11, no. 4: 402. https://doi.org/10.3390/coatings11040402
APA StyleLi, J., Wang, Q., Gao, N., Nwokolo, I. K., Zhang, W., Ma, L., Liu, F., & Han, E.-H. (2021). Interface Characteristics and Anticorrosion Performances of Cold Galvanizing Coatings Incorporated with ?-chloropropyl Triethoxysilane on Hot-Dip Galvanized Steel. Coatings, 11(4), 402. https://doi.org/10.3390/coatings11040402







