A General Strategy towards Superhydrophobic Self-Cleaning and Anti-Corrosion Metallic Surfaces: An Example with Aluminum Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Characterization Methods
3. Results and Discussion
3.1. Optimization of Etching Time and HCl Concentration for SHC
3.2. Surface Morphology and Reaction Mechanism
3.3. Wettability
3.4. Durability and Heat–Humidity Resistance
3.5. Self-Cleaning Performance
3.6. Corrosion Resistance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- López-Ortega, A.; Areitioaurtena, O.; Alves, S.A.; Goitandia, A.M.; Elexpe, I.; Arana, J.L.; Bayón, R. Development of a superhydrophobic and bactericide organic topcoat to be applied on thermally sprayed aluminum coatings in offshore submerged components. Prog. Org. Coat. 2019, 137, 105376. [Google Scholar] [CrossRef]
- The Annual Loss of Marine Corrosion in China is 700 Billion. Available online: https://www.cdstm.cn/frontier/nykj/201804/t20180424_753736.html (accessed on 24 April 2018). (In Chinese).
- Piao, N.; Wang, L.; Anwar, T.; Feng, X.; He, X. Corrosion resistance mechanism of chromate conversion coated aluminium current collector in lithium-ion batteries. Corros. Sci. 2019, 158, 108100. [Google Scholar] [CrossRef]
- Din, R.U.; Jellesen, M.S.; Ambat, R. Performance comparison of steam-based and chromate conversion coatings on AA6060 aluminium alloy. Corrosion 2015, 71, 839–853. [Google Scholar] [CrossRef]
- Xia, L.; Akiyama, E.; Frankel, G.; McCreery, R. Storage and release of soluble hexavalent chromium from chromate conversion coatings equilibrium aspects of CrVI concentration. J. Electrochem. Soc. 2000, 147, 2556–2562. [Google Scholar] [CrossRef]
- Xiang, T.; Han, Y.; Guo, Z.; Wang, R.; Zheng, S.; Li, S.; Li, C.; Dai, X. Fabrication of inherent anticorrosion superhydrophobic surfaces on metals. ACS Sustain. Chem. Eng. 2018, 6, 5598–5606. [Google Scholar] [CrossRef]
- Barthwal, S.; Lim, S.-H. Robust and chemically stable superhydrophobic aluminum-alloy surface with enhanced corrosion-resistance properties. Int. J. Precis. Eng. Manuf. Green Technol. 2020, 7, 481–492. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Iatridi, Z.; Moschakou, M.; Damigos, P.; Bokias, G.; Kallitsis, J.K. Development of Cu2+- and/or phosphonium-based polymeric biocidal materials and their potential application in antifouling paints. Prog. Org. Coat. 2012, 75, 190–199. [Google Scholar] [CrossRef]
- Aroguz, A.Z.; Succedinov, Y.; Gerengi, H.; Bereket, G. Corrosion protection of aluminum alloy AA7020 in NaCl solution by hybrid sol–gel coatings. Prot. Met. Phys. Chem. Surf. 2020, 56, 405–413. [Google Scholar] [CrossRef]
- Njoku, C.N.; Bai, W.; Arukalam, I.O.; Yang, L.; Hou, B.; Njoku, D.I.; Li, Y. Epoxy-based smart coating with self-repairing polyurea-formaldehyde microcapsules for anticorrosion protection of aluminum alloy AA2024. J. Coat. Technol. Res. 2020, 17, 797–813. [Google Scholar] [CrossRef]
- Liu, S.; Liu, L.; Meng, F.; Li, Y.; Wang, F. Protective performance of polyaniline-sulfosalicylic acid/epoxy coating for 5083 aluminum. Materials 2018, 11, 292. [Google Scholar] [CrossRef] [Green Version]
- Njoku, C.N.; Arukalam, I.O.; Bai, W.; Li, Y. Optimizing maleic anhydride microcapsules size for use in self-healing epoxy-based coatings for corrosion protection of aluminum alloy. Mater. Corros. 2018, 69, 1257–1267. [Google Scholar] [CrossRef]
- Dong, C.F.; Xiao, K.; Sheng, H.; An, Y.H.; Li, X.G. Characterizations of UV aging and atmospheric corrosion on epoxy coated 7A04 aluminum alloy. Mater. Sci. Forum 2011, 686, 784–791. [Google Scholar] [CrossRef]
- Chapin, R.E.; Adams, J.; Boekelheide, K.; Gray, L.E., Jr.; Hayward, S.W.; Lees, P.S.J.; McIntyre, B.S.; Portier, K.M.; Schnorr, T.M.; Selevan, S.G.; et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res. Part B 2008, 83, 157–395. [Google Scholar] [CrossRef]
- Wang, S.; Yu, X.; Zhang, Y. Large-scale fabrication of translucent, stretchable and durable superhydrophobic composite films. J. Mater. Chem. A 2017, 5, 23489–23496. [Google Scholar] [CrossRef]
- Zheng, S.; Li, C.; Fu, Q.; Hu, W.; Xiang, T.; Wang, Q.; Du, M.; Liu, X.; Chen, Z. Development of stable superhydrophobic coatings on aluminum surface for corrosion-resistant, self-cleaning, and anti-icing applications. Mater. Des. 2016, 93, 261–270. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, D.; Chen, J.; Liu, G.; Yu, M. Superhydrophobic paper fabricated via nanostructured titanium dioxide-functionalized wood cellulose fibers. J. Mater. Sci. 2020, 55, 7084–7094. [Google Scholar] [CrossRef]
- Xiang, T.; Zhang, M.; Sadiga, H.; Li, Z.; Zhang, M.; Dong, C.; Yang, L.; Chan, W.; Li, C. Slippery liquid-infused porous surface for corrosion protection with self-healing property. Chem. Eng. J. 2018, 345, 147–155. [Google Scholar] [CrossRef]
- Wu, X.H.; Chen, Z. A mechanically robust transparent coating for anti-icing and self-cleaning applications. J. Mater. Chem. A 2018, 6, 16043–16052. [Google Scholar] [CrossRef]
- Qing, Y.; Hu, C.; Yang, C.; An, K.; Tang, F.; Tan, J.; Liu, C. Rough structure of electrodeposition as a template for an ultrarobust self-cleaning surface. ACS Appl. Mater. Interfaces 2017, 9, 16571–16580. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Q.; Liu, J.; Chen, R.; Takahashi, K.; Liu, L.; Li, R.; Liu, P.; Wang, J. Hierarchical flower like double-layer superhydrophobic films fabricated on AZ31 for corrosion protection and self-cleaning. New J. Chem. 2017, 41, 12767–12776. [Google Scholar] [CrossRef]
- Zheng, S.; Bellido-Aguilar, D.A.; Hu, J.; Huang, Y.; Zhao, X.; Wang, Z.; Zeng, X.; Zhang, Q.; Chen, Z. Waterborne bio-based epoxy coatings for the corrosion protection of metallic substrates. Prog. Org. Coat. 2019, 136, 105265. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Q.; Wang, N.; Zheng, X. Fabrication of superhydrophobic green surfaces with good self-cleaning, chemical stability and anti-corrosion properties. J. Mater. Sci. 2019, 54, 13006–13016. [Google Scholar] [CrossRef]
- Hu, C.; Xie, X.; Zheng, H.; Qing, Y.; Ren, K. Facile fabrication of superhydrophobic zinc coatings with corrosion resistance via an electrodeposition process. New J. Chem. 2020, 44, 8890–8901. [Google Scholar] [CrossRef]
- Hu, J.; He, S.; Wang, Z.; Zhu, J.; Wei, L.; Chen, Z. Stearic acid-coated superhydrophobic Fe2O3/Fe3O4 composite film on N80 steel for corrosion protection. Surf. Coat. Technol. 2019, 359, 47–54. [Google Scholar] [CrossRef]
- He, S.; Wang, Z.; Hu, J.; Zhu, J.; Wei, L.; Chen, Z. Formation of superhydrophobic micro-nanostructured iron oxide for corrosion protection of N80 steel. Mater. Des. 2018, 160, 84–94. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, Z.; Wu, H.; Li, L.; Fu, X. Study on preparation of superhydrophobic Ni-Co coating and corrosion resistance by sandblasting-electrodeposition. Coatings 2020, 10, 1164. [Google Scholar] [CrossRef]
- Xiang, T.; Liu, J.; Liu, Q.; Wei, F.; Lv, Z.; Yang, Y.; Shi, L.p.; Li, C.; Chen, D.; Xu, G. Self-healing solid slippery surface with porous structure and enhanced corrosion resistance. Chem. Eng. J. 2021, 417, 128083. [Google Scholar] [CrossRef]
- Zheng, S.; Bellido-Aguilar, D.A.; Wu, X.; Zhan, X.; Huang, Y.; Zeng, X.; Zhang, Q.; Chen, Z. Durable waterborne hydrophobic bio-epoxy coating with improved anti-icing and self-cleaning performance. ACS Sustain. Chem. Eng. 2019, 7, 641–649. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, X.; Tao, J.; Zhu, C.; Lai, Y.; Chen, Z. Icephobic materials: Fundamentals, performance evaluation, and applications. Prog. Mater. Sci. 2019, 103, 509–557. [Google Scholar] [CrossRef]
- Gao, S.; Dong, X.; Huang, J.; Li, S.; Li, Y.; Chen, Z.; Lai, Y. Rational construction of highly transparent superhydrophobic coatings based on a non-particle, fluorine-free and water-rich system for versatile oil-water separation. Chem. Eng. J. 2018, 333, 621–629. [Google Scholar] [CrossRef]
- Ge, B.; Men, X.; Zhu, X.; Zhang, Z. A superhydrophobic monolithic material with tunable wettability for oil and water separation. J. Mater. Sci. 2015, 50, 2365–2369. [Google Scholar] [CrossRef]
- Wang, N.; Tang, L.; Cai, Y.; Tong, W.; Xiong, D. Scalable superhydrophobic coating with controllable wettability and investigations of its drag reduction. Colloids Surf. A 2018, 555, 290–295. [Google Scholar] [CrossRef]
- Espanhol-Soares, M.; Costa, L.; Silva, M.R.A.; Silva, F.S.; Ribeiro, L.M.S.; Gimenes, R. Super-hydrophobic coatings on cotton fabrics using sol-gel technique by spray. J. Sol Gel Sci. Technol. 2020, 95, 22–33. [Google Scholar] [CrossRef]
- El Fouhaili, B.; Ibrahim, A.; Dietlin, C.; Chemtob, A.; Allonas, X.; Croutxé-Barghorn, C. Single-step formation of superhydrophobic surfaces using photobase-catalyzed sol-gel process. Prog. Org. Coat. 2019, 137, 105293. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y.; Liu, G.; Chu, F.; Chen, C.; Zhang, Y.; Tian, H.; Song, Y. Patterning a superhydrophobic area on a facile fabricated superhydrophilic layer based on an inkjet-printed water-soluble polymer template. Langmuir 2020, 36, 9952–9959. [Google Scholar] [CrossRef]
- Liu, Z.W.; Tang, Y.F.; Zhao, K.; Zhang, Q. Superhydrophobic SiO2 micro/nanofibrous membranes with porous surface prepared by freeze electrospinning for oil adsorption. Colloids Surf. A 2019, 568, 356–361. [Google Scholar] [CrossRef]
- Wang, F.; Guo, Z. Insitu growth of durable superhydrophobic Mg-Al layered double hydroxides nanoplatelets on aluminum alloys for corrosion resistance. J. Alloys Compd. 2018, 767, 382–391. [Google Scholar] [CrossRef]
- Wu, W.B.; Liang, R.X.; Lu, L.S.; Wang, W.T.; Ran, X.; Yue, D.D. Preparation of superhydrophobic laser-induced graphene using taro leaf structure as templates. Surf. Coat. Technol. 2020, 393, 125744. [Google Scholar] [CrossRef]
- Fenero, M.; Knez, M.; Saric, I.; Petravic, M.; Grande, H.; Palenzuela, J. Omniphobic etched aluminum surfaces with anti-icing ability. Langmuir 2020, 36, 10916–10922. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Guo, Z.; Shi, T.; Yu, J.; Tang, M.; Huang, X. Fabrication of superhydrophobic surface with improved corrosion inhibition on 6061 aluminum alloy substrate. Appl. Surf. Sci. 2015, 342, 76–83. [Google Scholar] [CrossRef]
- Zheng, S.L.; Li, C.; Fu, Q.T.; Li, M.; Hu, W.; Wang, Q.; Du, M.P.; Liu, X.C.; Chen, Z. Fabrication of self-cleaning superhydrophobic surface on aluminum alloys with excellent corrosion resistance. Surf. Coat. Technol. 2015, 276, 341–348. [Google Scholar] [CrossRef]
- He, S.; Shi, J.; Huang, J.; Hu, J.; Lai, Y.; Chen, Z. Rational designed structured superhydrophobic iron oxide surface towards sustainable anti-corrosion and self-cleaning. Chem. Eng. J. 2021, 416, 127768. [Google Scholar] [CrossRef]
- Feng, L.; Zhu, Y.; Wang, J.; Shi, X. One-step hydrothermal process to fabricate superhydrophobic surface on magnesium alloy with enhanced corrosion resistance and self-cleaning performance. Appl. Surf. Sci. 2017, 422, 566–573. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.; Yu, X.; Wu, H. Low-cost one-step fabrication of superhydrophobic surface on Al alloy. Appl. Surf. Sci. 2011, 257, 7928–7931. [Google Scholar] [CrossRef]
- Shen, X.; Yang, L.; Fan, S.; Yang, Q.; Wu, W.; Zhang, B. Colorful and superhydrophobic titanium surfaces textured by obliquely incident femtosecond laser induced micro/nano structures. Opt. Commun. 2020, 466, 125687. [Google Scholar] [CrossRef]
- Dong, H.; Cheng, M.; Zhang, Y.; Wei, H.; Shi, F. Extraordinary drag-reducing effect of a superhydrophobic coating on a macroscopic model ship at high speed. J. Mater. Chem. A 2013, 1, 5886–5891. [Google Scholar] [CrossRef]
- Qing, Y.; Yang, C.; Hu, C.; Zheng, Y.; Liu, C. A facile method to prepare superhydrophobic fluorinated polysiloxane/ZnO nanocomposite coatings with corrosion resistance. Appl. Surf. Sci. 2015, 326, 48–54. [Google Scholar] [CrossRef]
- Li, D.-W.; Wang, H.-Y.; Liu, Y.; Wei, D.-S.; Zhao, Z.-X. Large-scale fabrication of durable and robust super-hydrophobic spray coatings with excellent repairable and anti-corrosion performance. Chem. Eng. J. 2019, 367, 169–179. [Google Scholar] [CrossRef]
- Feng, L.; Che, Y.; Liu, Y.; Qiang, X.; Wang, Y. Fabrication of superhydrophobic aluminium alloy surface with excellent corrosion resistance by a facile and environment-friendly method. Appl. Surf. Sci. 2013, 283, 367–374. [Google Scholar] [CrossRef]
- Zheng, S.; Bellido-Aguilar, D.A.; Huang, Y.; Zeng, X.; Zhang, Q.; Chen, Z. Mechanically robust hydrophobic bio-based epoxy coatings for anti-corrosion application. Surf. Coat. Technol. 2019, 363, 43–50. [Google Scholar] [CrossRef]
- Badawy, W.A.; Al-Kharafi, F.M.; El-Azab, A.S. Electrochemical behaviour and corrosion inhibition of Al, Al-6061 and Al–Cu in neutral aqueous solutions. Corros. Sci. 1999, 41, 709–727. [Google Scholar] [CrossRef]
- Ghahremaninezhad, A.; Dolati, A. A study on electrochemical growth behavior of the Co–Ni alloy nanowires in anodic aluminum oxide template. J. Alloys Compd. 2009, 480, 275–278. [Google Scholar] [CrossRef]
- Ellinas, K.; Dimitrakellis, P.; Sarkiris, P.; Gogolides, E. A review of fabrication methods, properties and applications of superhydrophobic metals. Processes 2021, 9, 666. [Google Scholar] [CrossRef]
- Liu, X.; Wang, P.; Zhang, D.; Chen, X. Atmospheric corrosion protection performance and mechanism of superhydrophobic surface based on coalescence-induced droplet self-jumping behavior. ACS Appl. Mater. Interfaces 2021, 13, 25438–25450. [Google Scholar] [CrossRef]
- Dong, X.J.; Meng, J.B.; Hu, Y.Z.; Wei, X.T.; Luan, X.S.; Zhou, H.A. Fabrication of self-cleaning superhydrophobic surfaces with improved corrosion resistance on 6061 aluminum alloys. Micromachines 2020, 11, 159. [Google Scholar] [CrossRef] [Green Version]
- Lei, Z.L.; Tian, Z.; Chen, X.; Chen, Y.B.; Bi, J.; Wu, S.B.; Sun, H.R. Large spot diameter nanosecond laser treatment of aluminum alloy sheets for high-speed superhydrophobic hierarchical micro- and nanostructured surface preparation. Surf. Coat. Technol. 2019, 361, 249–254. [Google Scholar] [CrossRef]
- Grignard, B.; Vaillant, A.; de Coninck, J.; Piens, M.; Jonas, A.M.; Detrembleur, C.; Jerome, C. Electrospinning of a functional perfluorinated block copolymer as a powerful route for imparting superhydrophobicity and corrosion resistance to aluminum substrates. Langmuir 2011, 27, 335–342. [Google Scholar] [CrossRef]
- Tao, Y.T. Structural comparison of self-assembled monolayers of n-alkanoic acids on the surfaces of silver, copper, and aluminum. J. Am. Chem. Soc. 1993, 115, 4350–4358. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, T.; Chen, S.; Liu, T.; Cheng, S. Structure stability and corrosion inhibition of super-hydrophobic film on aluminum in seawater. Appl. Surf. Sci. 2008, 255, 2978–2984. [Google Scholar] [CrossRef]
- Ji, H.; Chen, G.; Yang, J.; Hu, J.; Song, H.; Zhao, Y. A simple approach to fabricate stable superhydrophobic glass surfaces. Appl. Surf. Sci. 2013, 266, 105–109. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Wolansky, G.; Marmur, A. Apparent contact angles on rough surfaces: The Wenzel equation revisited. Colloids Surf. A 1999, 156, 381–388. [Google Scholar] [CrossRef]
- Johnson, R.E.; Dettre, R.H. Contact angle hysteresis. III. Study of an idealized heterogeneous surface. J. Phys. Chem. 1964, 68, 1744–1750. [Google Scholar] [CrossRef]
- Liu, W.; Luo, Y.; Sun, L.; Wu, R.; Jiang, H.; Liu, Y. Fabrication of the superhydrophobic surface on aluminum alloy by anodizing and polymeric coating. Appl. Surf. Sci. 2013, 264, 872–878. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Xu, W.; Song, J.; Sun, J.; Lu, Y.; Yu, Z. Rapid fabrication of large-area, corrosion-resistant superhydrophobic Mg alloy surfaces. ACS Appl. Mater. Interfaces 2011, 3, 4404–4414. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, H.; Wang, Z.; Liu, Y. Superhydrophobic aluminum alloy surface: Fabrication, structure, and corrosion resistance. Colloids Surf. A 2014, 441, 319–325. [Google Scholar] [CrossRef]
- Saleema, N.; Sarkar, D.K.; Gallant, D.; Paynter, R.W.; Chen, X.G. Chemical nature of superhydrophobic aluminum alloy surfaces produced via a one-step process using fluoroalkyl-silane in a base medium. ACS Appl. Mater. Interfaces 2011, 3, 4775–4781. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Chen, S.; Cheng, S.; Tian, J.; Chang, X.; Yin, Y. Corrosion behavior of super-hydrophobic surface on copper in seawater. Electrochim. Acta 2007, 52, 8003–8007. [Google Scholar] [CrossRef]








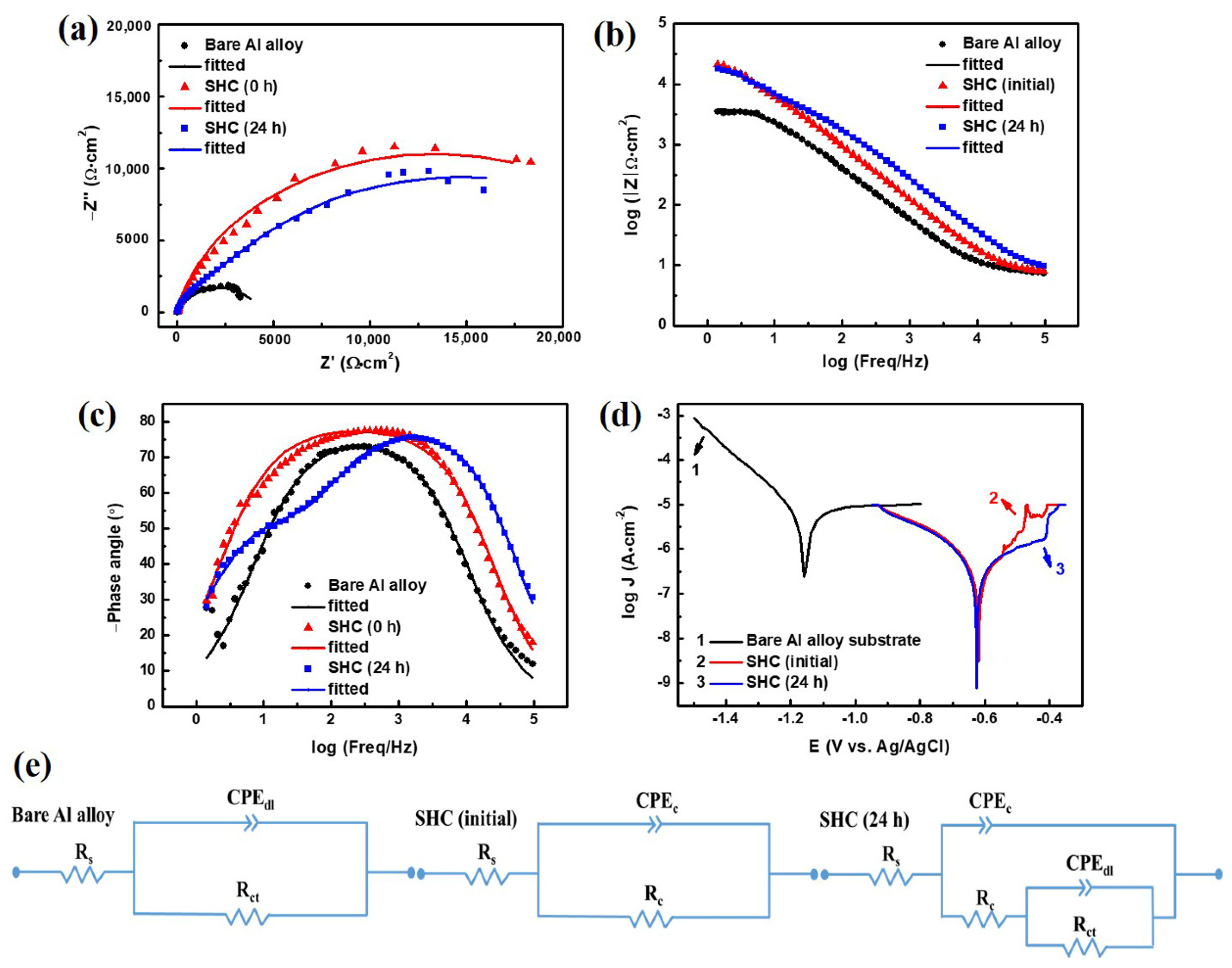

| Samples | Rs (Ω·cm2) | CPEdl | Rct (Ω·cm2) | CPEc | Rc (Ω·cm2) | ||
|---|---|---|---|---|---|---|---|
| Yo-dl | ndl | Yo-c (S·sn·cm−2) | nc | ||||
| (S·sn·cm−2) | |||||||
| Bare Al alloy | 7.270 | 9.179 × 10−6 | 0.8647 | 4.238 × 103 | - | - | - |
| SHC (initial) | 7.131 | - | - | - | 3.725 × 10−6 | 0.8795 | 2.666 × 104 |
| SHC (24 h) | 7.531 | 5.521 × 10−6 | 0.7374 | 2.376 × 104 | 1.479 × 10−6 | 0.8920 | 4.321 × 103 |
| Samples | Ecorr (V vs. Ag/AgCl) | Jcorr (A·cm−2) | Inhibition Efficiency (η %) |
|---|---|---|---|
| Bare Al alloy | −1.160 | 1.070 × 10−5 | - |
| SHC (initial) | −0.620 | 3.171 × 10−7 | 97 |
| SHC (24 h) | −0.626 | 5.123 × 10−7 | 95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, S.; Li, C.; Zhang, Y.; Xiang, T.; Cao, Y.; Li, Q.; Chen, Z. A General Strategy towards Superhydrophobic Self-Cleaning and Anti-Corrosion Metallic Surfaces: An Example with Aluminum Alloy. Coatings 2021, 11, 788. https://doi.org/10.3390/coatings11070788
Zheng S, Li C, Zhang Y, Xiang T, Cao Y, Li Q, Chen Z. A General Strategy towards Superhydrophobic Self-Cleaning and Anti-Corrosion Metallic Surfaces: An Example with Aluminum Alloy. Coatings. 2021; 11(7):788. https://doi.org/10.3390/coatings11070788
Chicago/Turabian StyleZheng, Shunli, Cheng Li, Yupeng Zhang, Tengfei Xiang, Ying Cao, Quanli Li, and Zhong Chen. 2021. "A General Strategy towards Superhydrophobic Self-Cleaning and Anti-Corrosion Metallic Surfaces: An Example with Aluminum Alloy" Coatings 11, no. 7: 788. https://doi.org/10.3390/coatings11070788
APA StyleZheng, S., Li, C., Zhang, Y., Xiang, T., Cao, Y., Li, Q., & Chen, Z. (2021). A General Strategy towards Superhydrophobic Self-Cleaning and Anti-Corrosion Metallic Surfaces: An Example with Aluminum Alloy. Coatings, 11(7), 788. https://doi.org/10.3390/coatings11070788









