Chemical Composition and Corrosion Behavior of a-C:H/DLC Film-Coated Titanium Substrate in Simulated PEMFC Environment
Abstract
:1. Introduction
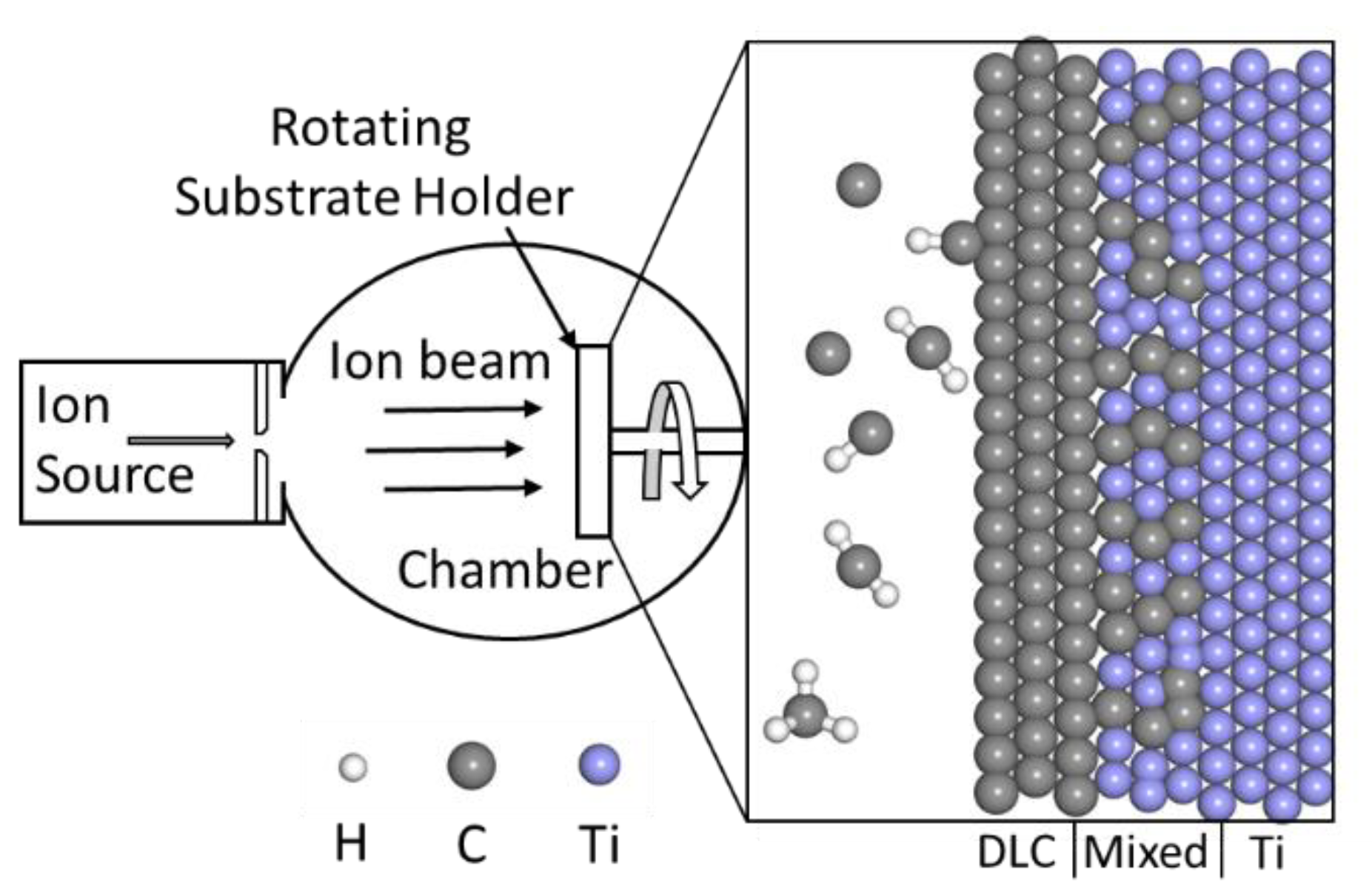
2. Materials and Methods
3. Results and Discussions
3.1. Surface Topography
3.2. Raman Analysis
3.3. TEM Analysis
3.4. XPS Characterization
3.5. ICR Test
3.6. Corrosion Behavior of DLC Film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dodds, P.E.; Staffell, I.; Hawkes, A.; Li, F.G.N.; Grunewald, P.; Mcdowall, W.; Ekins, P. Hydrogen and fuel cell technologies for heating: A review. Int. J. Hydrogen Energy 2015, 40, 2065–2083. [Google Scholar] [CrossRef] [Green Version]
- Kumagai, M.; Myung, S.; Kuwata, S.; Asaishi, R.; Katada, Y.; Yashiro, H. Application of ni-free high nitrogen stainless steel for bipolar plates of proton exchange membrane fuel cells. Electrochim. Acta 2009, 54, 1127–1133. [Google Scholar] [CrossRef]
- Dhakate, S.R.; Sharma, S.P.; Borah, M.; Mathur, R.B.; Dhami, T.L. Expanded graphite-based electrically conductive composites as bipolar plate for pem fuel cell. Int. J. Hydrogen Energy 2008, 33, 7146–7152. [Google Scholar] [CrossRef]
- Asri, N.F.; Husaini, T.; Sulong, A.B.; Majlan, E.H.; Daud, W.R.W. Coating of stainless steel and titanium bipolar plates for anticorrosion in pemfc: A review. Int. J. Hydrogen Energy 2017, 42, 9135–9148. [Google Scholar] [CrossRef]
- Mawdsley, J.; Carter, J.D.; Wang, X.; Niyogi, S.; Fan, C.Q.; Koc, R.; Osterhout, G. Composite-coated aluminum bipolar plates for pem fuel cells. J. Power Sources 2013, 231, 106–112. [Google Scholar] [CrossRef]
- Hatada, R.; Flege, S.; Bobrich, A.; Ensinger, W.; Baba, K. Surface modification and corrosion properties of implanted and dlc coated stainless steel by plasma based ion implantation and deposition. Surf. Coat. Technol. 2014, 256, 23–29. [Google Scholar] [CrossRef]
- Mingge, W.; Congda, L.; Tao, H.; Guohai, C.; Donghui, W.; Haifeng, Z.; Dong, Z.; Aiying, W. Chromium interlayer amorphous carbon film for 304 stainless steel bipolar plate of proton exchange membrane fuel cell. Surf. Coat. Technol. 2016, 307, 374–381. [Google Scholar] [CrossRef]
- Lee, S.; Kakati, N.; Maiti, J.; Jee, S.H.; Kalita, D.J.; Yoon, Y.S. Corrosion and electrical properties of crn- and tin-coated 316l stainless steel used as bipolar plates for polymer electrolyte membrane fuel cells. Thin Solid Film. 2013, 529, 374–379. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, H.; Liu, C.; Huang, M.; Chen, J.; Shih, H.C. Structure and characterization of the multilayered ti-dlc films by fcva. Diam. Relat. Mater. 2010, 19, 1034–1039. [Google Scholar] [CrossRef]
- Yatsuzuka, M.; Tateiwa, J.; Uchida, H. Evaluation of pinhole defect in dlc film prepared by hybrid process of plasma-based ion implantation and deposition. Vacuum 2006, 80, 1351–1355. [Google Scholar] [CrossRef]
- Toro, R.G.; Calandra, P.; Cortese, B.; De Caro, T.; Brucale, M.; Mezzi, A.; Federici, F.; Caschera, D. Argon and hydrogen plasma influence on the protective properties of diamond-like carbon films as barrier coating. Surf. Interfaces 2017, 6, 60–71. [Google Scholar] [CrossRef]
- Lin, K.; Li, X.; Tian, L.; Dong, H. Active screen plasma surface co-alloying of 316 austenitic stainless steel with both nitrogen and niobium for the application of bipolar plates in proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2015, 40, 10281–10292. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Hou, M.; Lin, G.; Han, Z.; Fu, Y.; Sun, S.; Shao, Z.; Yi, B. Performance of ti-ag-deposited titanium bipolar plates in simulated unitized regenerative fuel cell (urfc) environment. Int. J. Hydrogen Energy 2011, 36, 5695–5701. [Google Scholar] [CrossRef]
- Jung, H.; Huang, S.; Ganesan, P.; Popov, B.N. Performance of gold-coated titanium bipolar plates in unitized regenerative fuel cell operation. J. Power Sources 2009, 194, 972–975. [Google Scholar] [CrossRef]
- Han, B.B.; Ju, D.Y.; Chai, M.R.; Zhao, H.J.; Sato, S. Corrosion resistance of dlc film-coated sus316l steel prepared by ion beam enhanced deposition. Adv. Mater. Sci. Eng. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Kavanagh, J.; Matthews, A.; Leyland, A. The combined effects of cu and ag on the nanostructure and mechanical properties of crcuagn pvd coatings. Surf. Coat. Technol. 2015, 284, 101–111. [Google Scholar] [CrossRef]
- Sioshansi, P.; Tobin, E.J. Surface treatment of biomaterials by ion beam processes. Surf. Coat. Technol. 1996, 83, 175–182. [Google Scholar] [CrossRef]
- Martigonzalez, J.; Bertran, E. Mechanical and surface characterization of diamond-like carbon coatings onto polymeric substrate. arXiv 2015, arXiv:1509.08512. [Google Scholar]
- Voevodin, A.A.; Donley, M.S.; Zabinski, J.S. Pulsed laser deposition of diamond-like carbon wear protective coatings: A review. Surf. Coat. Technol. 1997, 92, 42–49. [Google Scholar] [CrossRef]
- Kesavan, T.; Partheeban, T.; Vivekanantha, M.; Kundu, M.; Maduraiveeran, G.; Sasidharan, M. Hierarchical nanoporous activated carbon as potential electrode materials for high performance electrochemical supercapacitor. Microporous Mesoporous Mater. 2019, 274, 236–244. [Google Scholar] [CrossRef]
- Feng, K.; Cai, X.; Sun, H.; Li, Z.; Chu, P.K. Carbon coated stainless steel bipolar plates in polymer electrolyte membrane fuel cells. Diam. Relat. Mater. 2010, 19, 1354–1361. [Google Scholar] [CrossRef]
- Ortizmedina, J.; Kitano, H.; Morelosgomez, A.; Wang, Z.; Araki, T.; Kang, C.; Hayashi, T.; Takeuchi, K.; Kawaguchi, T.; Tanioka, A. Nanostructured carbon-based membranes: Nitrogen doping effects on reverse osmosis performance. NPG Asia Mater. 2016, 8. [Google Scholar]
- Ding, W.; Guo, Y.; Ju, D.Y.; Sato, S.; Tsunoda, T. The effect of CH4/H2 ratio on the surface properties of hdpe treated by chx ion beam bombardment. Mod. Phys. Lett. B 2016, 30, 1650214. [Google Scholar] [CrossRef]
- Liang, J.H.; Chen, M.H.; Tsai, W.F.; Lee, S.C.; Ai, C.F. Characteristics of diamond-like carbon film synthesized on aisi 304 austenite stainless steel using plasma immersion ion implantation and deposition. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2007, 257, 696–701. [Google Scholar] [CrossRef]
- Han, B.; Ju, D.; Sato, S.; Zhao, H. Plasma preparation method and tribological properties of diamond-like carbon coating on magnesium alloy az31 substrate. Sci. China-Technol. Sci. 2019, 62, 1939–1947. [Google Scholar] [CrossRef]
- Kato, H.; Itagaki, N.; Im, H.J. Growth and raman spectroscopy of thickness-controlled rotationally faulted multilayer graphene. Carbon 2019, 141, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, N.; Rismaniyazdi, E.; Yeo, R.J.; Goohpattader, P.S.; Satyanarayana, N.; Srinivasan, N.; Druz, B.; Tripathy, S.; Bhatia, C.S. Probing the role of an atomically thin sinx interlayer on the structure of ultrathin carbon films. Sci. Rep. 2015, 4, 5021. [Google Scholar] [CrossRef] [Green Version]
- Gao, K.; Wang, Y.; Wei, X.; Qiang, L.; Zhang, B.; Zhang, J. Hydrogenated amorphous carbon films with different nanostructure: A comparative study. Chem. Phys. Lett. 2019, 715, 330–334. [Google Scholar] [CrossRef]
- Ghosh, B.; Guzmanolivos, F.; Espinozagonzalez, R. Plasmon-enhanced optical absorption with graded bandgap in diamond-like carbon (dlc) films. J. Mater. Sci. 2017, 52, 218–228. [Google Scholar] [CrossRef]
- Jo, Y.J.; Zhang, T.F.; Son, M.J.; Kim, K.H. Synthesis and electrochemical properties of ti-doped dlc films by a hybrid pvd/pecvd process. Appl. Surf. Sci. 2018, 433, 1184–1191. [Google Scholar] [CrossRef]
- Meng, W.J.; Gillispie, B.A. Mechanical properties of ti-containing and w-containing diamond-like carbon coatings. J. Appl. Phys. 1998, 84, 4314–4321. [Google Scholar] [CrossRef]
- Guo, T.; Kong, C.; Li, X.; Guo, P.; Wang, Z.; Wang, A. Microstructure and mechanical properties of ti/al co-doped dlc films: Dependence on sputtering current, source gas, and substrate bias. Appl. Surf. Sci. 2017, 410, 51–59. [Google Scholar] [CrossRef]
- Magnuson, M.; Lewin, E.; Hultman, L.; Jansson, U. Electronic structure and chemical bonding of nanocrystalline-tic/amorphous-c nanocomposites. Phys. Rev. B 2009, 80, 235108. [Google Scholar] [CrossRef] [Green Version]
- Mangolini, F.; Krick, B.A.; Jacobs, T.D.B.; Khanal, S.; Streller, F.; Mcclimon, J.B.; Hilbert, J.; Prasad, S.V.; Scharf, T.W.; Ohlhausen, J.A. Effect of silicon and oxygen dopants on the stability of hydrogenated amorphous carbon under harsh environmental conditions. Carbon 2018, 130, 127–136. [Google Scholar] [CrossRef]
- Huang, N.B.; Yu, H.; Xu, L.; Zhan, S.; Sun, M.; Kirk, D.W. Corrosion kinetics of 316l stainless steel bipolar plate with chromiumcarbide coating in simulated pemfc cathodic environment. Results Phys. 2016, 6, 730–736. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, M.; Tanaka, T.; Watanabe, S.; Shinohara, M.; Lee, J.; Takagi, T. Improvement of oxygen barrier of pet film with diamond-like carbon film by plasma-source ion implantation. Surf. Coat. Technol. 2003, 174, 1033–1037. [Google Scholar] [CrossRef]
- Lyons, K.S.; Gould, B.D. Lightweight titanium metal bipolar plates for pem fuel cells. Mater. Sci. Forum 2016, 879, 613–618. [Google Scholar] [CrossRef]














| Substrate | Titanium | |||
|---|---|---|---|---|
| CH4/H2 | 1:1 | 1:0 | ||
| Deposition time | 6 h | 12 h | 6 h | 12 h |
| CH4/H2 = 1:1 | CH4/H2 = 1:0 | |||
|---|---|---|---|---|
| Deposition time | 6 h | 12 h | 6 h | 12 h |
| ID/IG | 0.877 | 0.853 | 0.835 | 0.827 |
| G peak (cm−1) | 1556.59 | 1557.96 | 1560.55 | 1565.77 |
| FWHM (G) (cm−1) | 133.81 | 134.23 | 135.31 | 138.68 |
| CH4/H2 = 1:1 | CH4/H2 = 1:0 | |||
|---|---|---|---|---|
| Deposition time | 6 h | 12 h | 6 h | 12 h |
| ICR (mΩ·cm2) | 7 | 17.3 | 16.5 | 22.4 |
| Substrate | CH4/H2 = 1:1 | CH4/H2 = 1:0 | |||
|---|---|---|---|---|---|
| Deposition time | 6 h | 12 h | 6 h | 12 h | |
| ICP (ppm) | 0.58 | 0.49 | 0.43 | 0.37 | 0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, B.; Yan, M.; Ju, D.; Chai, M.; Sato, S. Chemical Composition and Corrosion Behavior of a-C:H/DLC Film-Coated Titanium Substrate in Simulated PEMFC Environment. Coatings 2021, 11, 820. https://doi.org/10.3390/coatings11070820
Han B, Yan M, Ju D, Chai M, Sato S. Chemical Composition and Corrosion Behavior of a-C:H/DLC Film-Coated Titanium Substrate in Simulated PEMFC Environment. Coatings. 2021; 11(7):820. https://doi.org/10.3390/coatings11070820
Chicago/Turabian StyleHan, Beibei, Mengyuan Yan, Dongying Ju, Maorong Chai, and Susumu Sato. 2021. "Chemical Composition and Corrosion Behavior of a-C:H/DLC Film-Coated Titanium Substrate in Simulated PEMFC Environment" Coatings 11, no. 7: 820. https://doi.org/10.3390/coatings11070820
APA StyleHan, B., Yan, M., Ju, D., Chai, M., & Sato, S. (2021). Chemical Composition and Corrosion Behavior of a-C:H/DLC Film-Coated Titanium Substrate in Simulated PEMFC Environment. Coatings, 11(7), 820. https://doi.org/10.3390/coatings11070820






