Superlow Friction of a-C:H Coatings in Vacuum: Passivation Regimes and Structural Characterization of the Sliding Interfaces
Abstract
:1. Introduction
2. Methods
2.1. Computational Details
2.2. Experimental Details
2.2.1. Materials
2.2.2. Friction Tests
2.2.3. Surface Analyses
3. Results
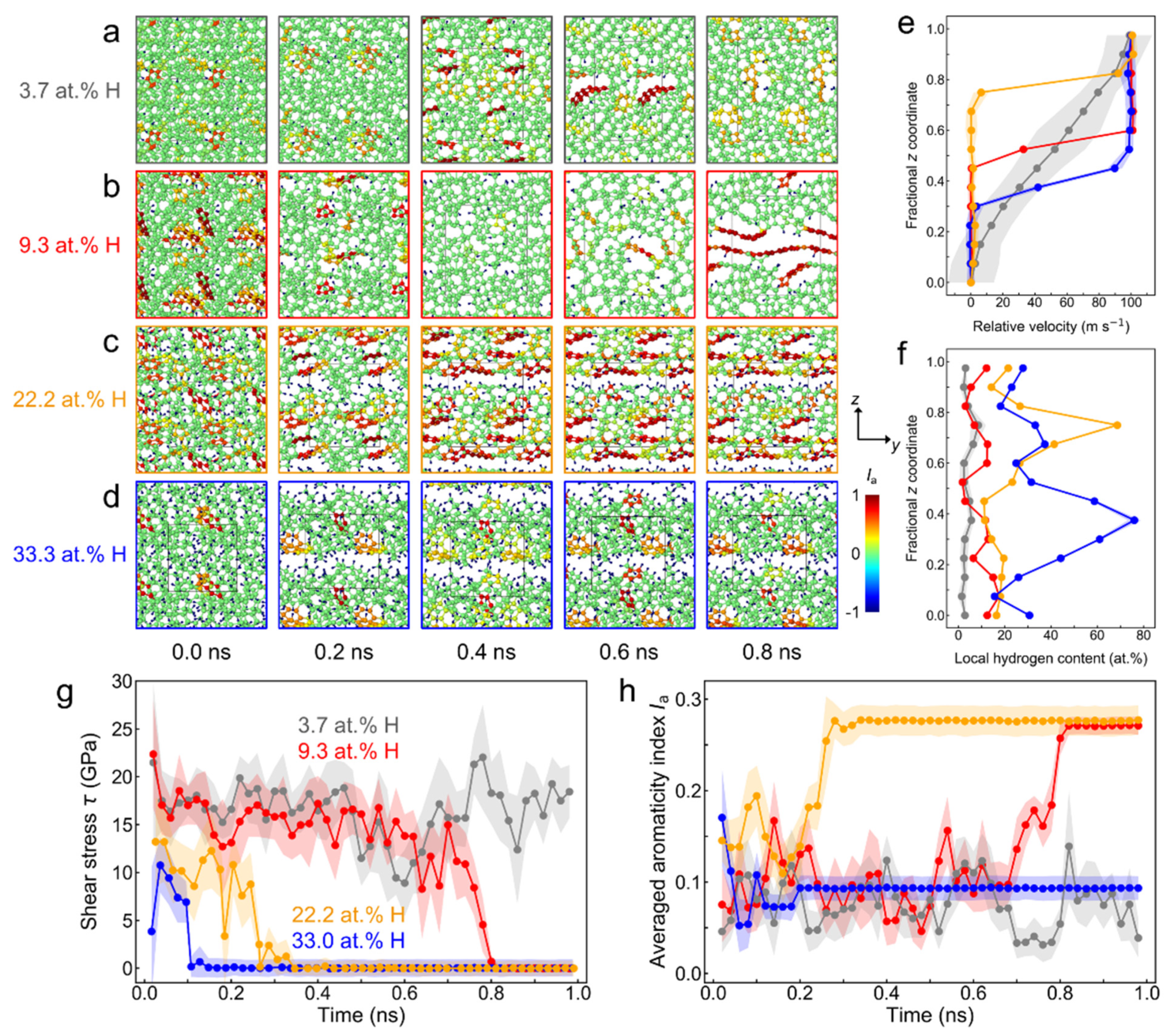
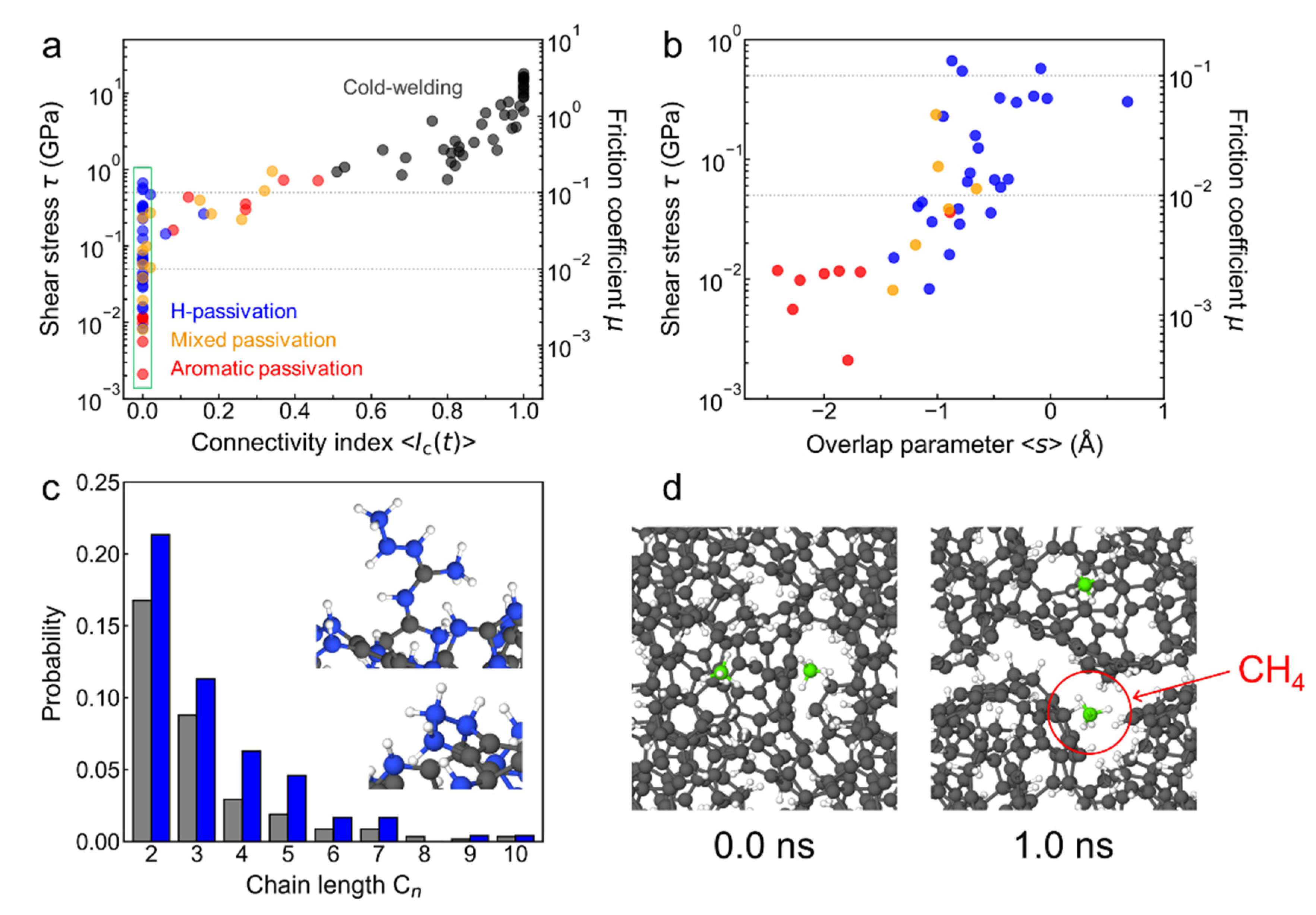
3.1. Quantum Molecular Dynamics Shearing Simulations
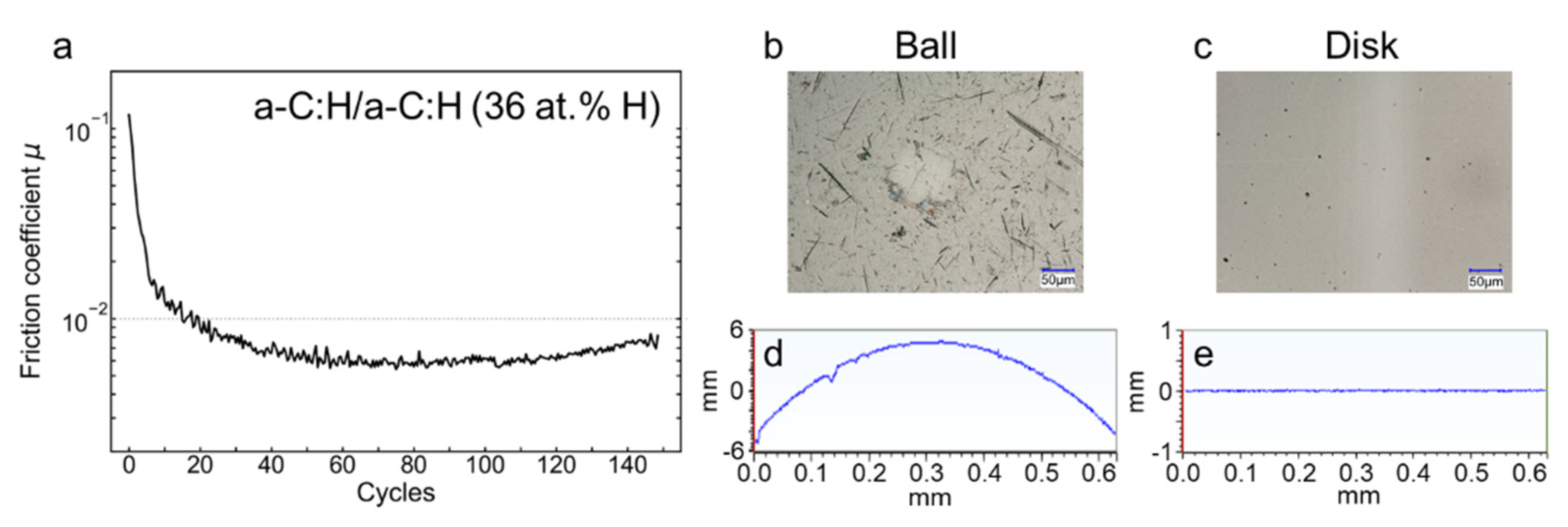
3.2. Reciprocating Friction Experiments
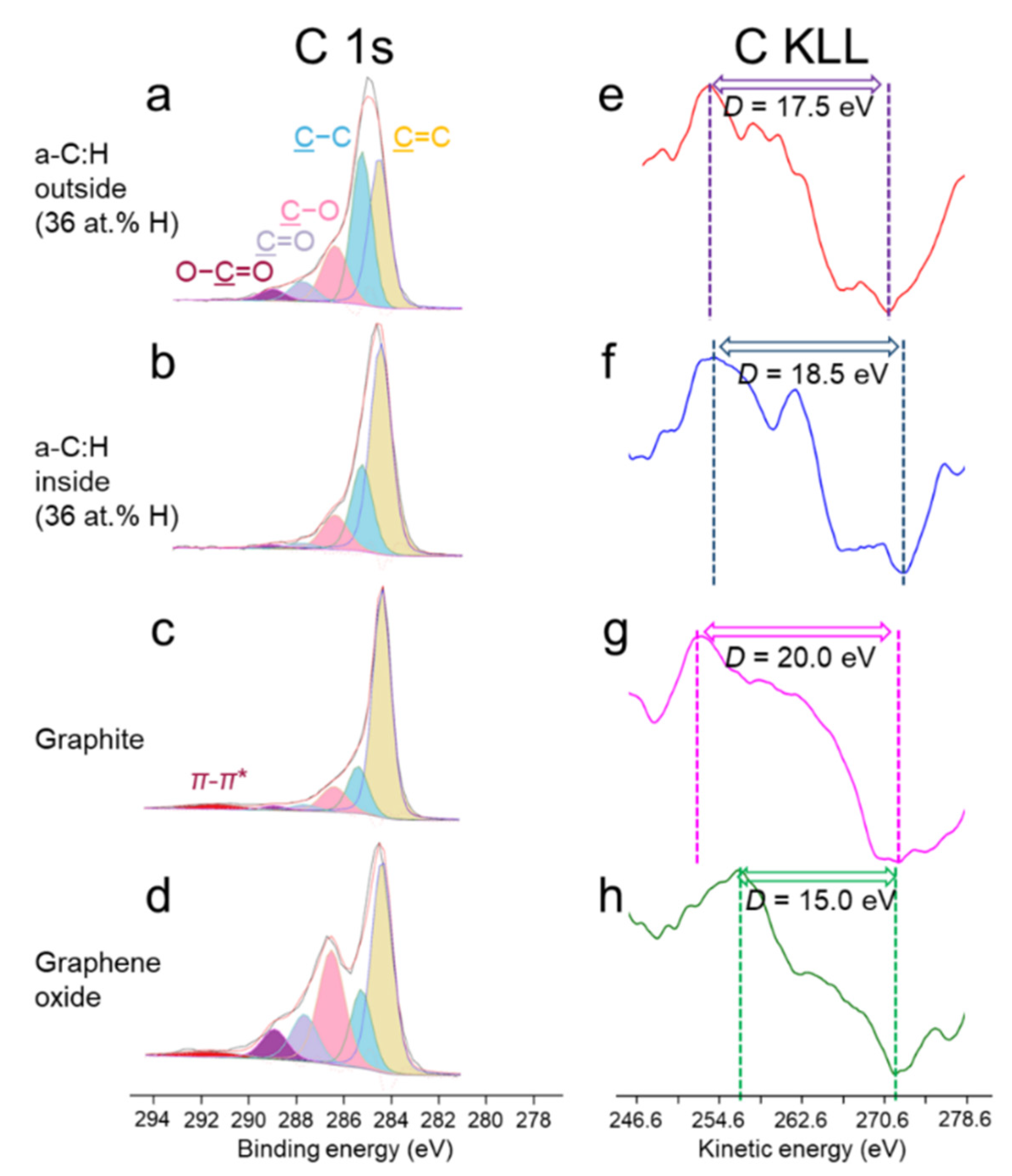
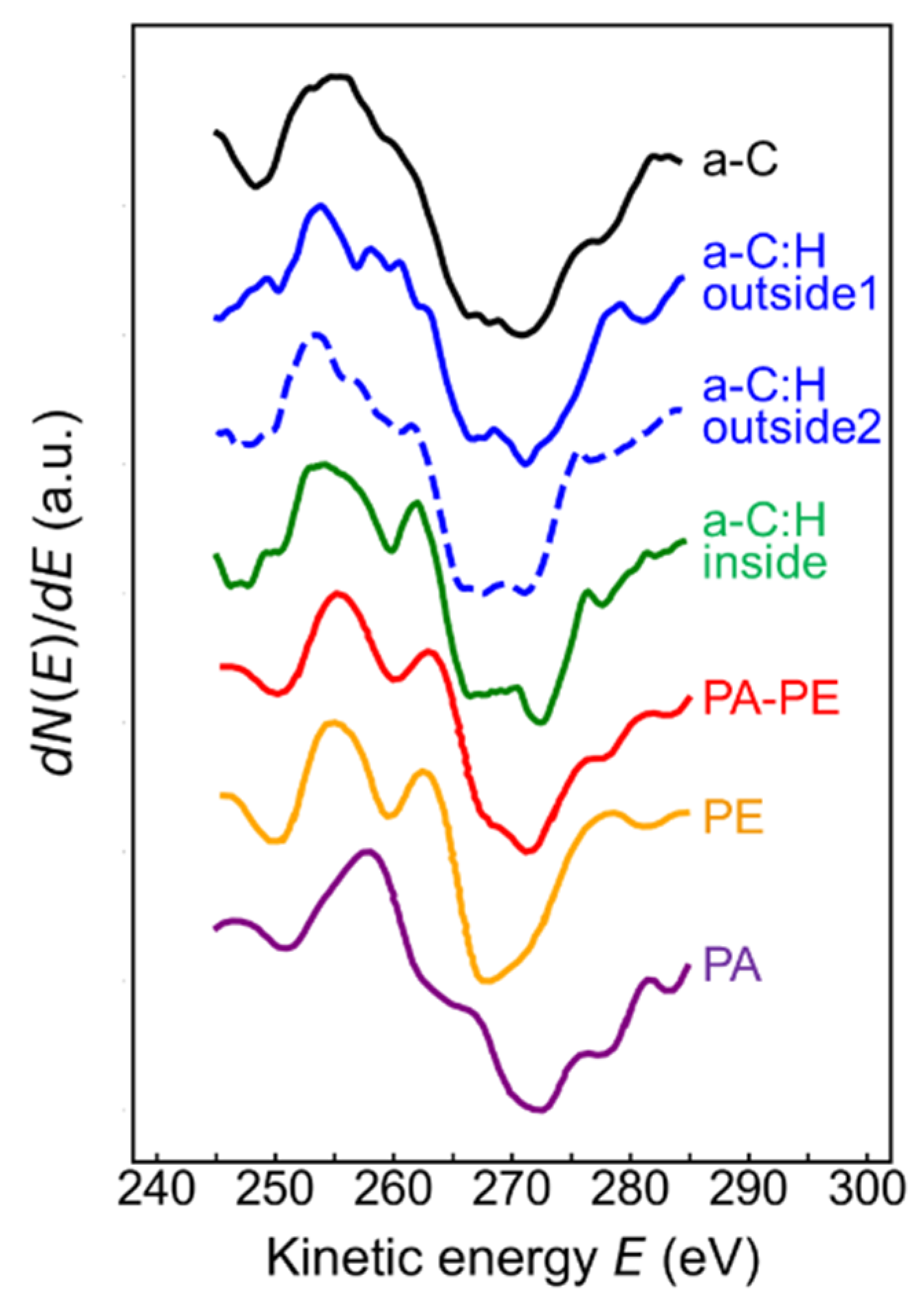
3.3. XPS/XAES Surface Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holmberg, K.; Andersson, P.; Erdemir, A. Global energy consumption due to friction in passenger cars. Tribol. Int. 2012, 47, 221–234. [Google Scholar] [CrossRef]
- Vetter, J. 60 years of DLC coatings: Historical highlights and technical review of cathodic arc processes to synthesize various DLC types, and their evolution for industrial applications. Surf. Coat. Technol. 2014, 257, 213–240. [Google Scholar] [CrossRef]
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R Rep. 2002, 37, 129–281. [Google Scholar] [CrossRef] [Green Version]
- Salinas Ruiz, V.R.; Kuwahara, T.; Galipaud, J.; Masenelli-Varlot, K.; Hassine, M.B.; Héau, C.; Stoll, M.; Mayrhofer, L.; Moras, G.; Martin, J.M.; et al. Interplay of mechanics and chemistry governs wear of diamond-like carbon coatings interacting with ZDDP-additivated lubricants. Nat. Commun. 2021, 12, 4550. [Google Scholar] [CrossRef] [PubMed]
- Erdemir, A.; Eryilmaz, O.L.; Fenske, G. Synthesis of diamondlike carbon films with superlow friction and wear properties. J. Vac. Sci. Technol. A 2000, 18, 1987–1992. [Google Scholar] [CrossRef] [Green Version]
- Donnet, C.; Fontaine, J.; Grill, A.; Le Mogne, T. The role of hydrogen on the friction mechanism of diamond-like carbon films. Tribol. Lett. 2001, 9, 137–142. [Google Scholar] [CrossRef]
- Erdemir, A. The role of hydrogen in tribological properties of diamond-like carbon films. Surf. Coat. Technol. 2001, 146, 292–297. [Google Scholar] [CrossRef]
- Reichenbach, T.; Mayrhofer, L.; Kuwahara, T.; Moseler, M.; Moras, G. Steric effects control dry friction of H- and F-terminated carbon surfaces. ACS Appl. Mater. Interfaces 2020, 12, 8805–8816. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, C.; Kato, T.; Yang, X.; Wu, S.; Wang, R.; Nosaka, M.; Luo, J. Evolution of tribo-induced interfacial nanostructures governing superlubricity in a-C:H and a-C:H:Si films. Nat. Commun. 2017, 8, 1675. [Google Scholar] [CrossRef]
- Kuwahara, T.; Moras, G.; Moseler, M. Friction Regimes of Water-Lubricated Diamond (111): Role of Interfacial Ether Groups and Tribo-Induced Aromatic Surface Reconstructions. Phys. Rev. Lett. 2017, 119, 096101. [Google Scholar] [CrossRef]
- Kuwahara, T.; Moras, G.; Moseler, M. Role of oxygen functional groups in the friction of water-lubricated low-index diamond surfaces. Phys. Rev. Mater. 2018, 2, 073606. [Google Scholar] [CrossRef]
- Kuwahara, T.; Romero, P.A.; Makowski, S.; Weihnacht, V.; Moras, G.; Moseler, M. Mechano-chemical decomposition of organic friction modifiers with multiple reactive centres induces superlubricity of ta-C. Nat. Commun. 2019, 10, 151. [Google Scholar] [CrossRef] [Green Version]
- Long, Y.; Kuwahara, T.; De Barros Bouchet, M.I.; Ristić, A.; Dörr, N.; Lubrecht, T.; Dupuy, L.; Moras, G.; Martin, J.M.; Moseler, M. In Situ Synthesis of Graphene Nitride Nanolayers on Glycerol-Lubricated Si3N4 for Superlubricity Applications. ACS Appl. Nano Mater. 2021, 4, 2721–2732. [Google Scholar] [CrossRef]
- Elstner, M.; Porezag, D.; Jungnickel, G.; Elsner, J.; Haugk, M.; Frauenheim, T.; Suhai, S.; Seifert, G. Self-consistent-charge density-functional tight-binding method for simulations of complex materials properties. Phys. Rev. B 1998, 58, 7260–7268. [Google Scholar] [CrossRef]
- Interatomic Potentials Library Atomistica. Available online: http://www.atomistica.org/ (accessed on 1 September 2021).
- Lees, A.W.; Edwards, S.F. The computer study of transport processes under extreme conditions. J. Phys. C Solid State Phys. 1972, 5, 1921–1929. [Google Scholar] [CrossRef]
- Peters, E.A.J.F. Elimination of time step effects in DPD. Europhys. Lett. 2004, 66, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Frenkel, D.; Smit, B. Understanding Molecular Simulations: From Algorithms to Applications, 2nd ed.; Academic Press: New York, NY, USA, 2001; ISBN 978-0-12-267351-1. [Google Scholar]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef] [Green Version]
- Wong-ekkabut, J.; Karttunen, M. The good, the bad and the user in soft matter simulations. Biochim. Biophys. Acta Biomembr. 2016, 1858, 2529–2538. [Google Scholar] [CrossRef]
- Frizzo, C.P.; Martins, M.A.P. Aromaticity in heterocycles: New HOMA index parametrization. Struct. Chem. 2012, 23, 375–380. [Google Scholar] [CrossRef]
- Franzblau, D.S. Computation of ring statistics for network models of solids. Phys. Rev. B 1991, 44, 4925–4930. [Google Scholar] [CrossRef]
- Choi, E.-Y.; Choi, W.S.; Lee, Y.B.; Noh, Y.-Y. Production of graphene by exfoliation of graphite in a volatile organic solvent. Nanotechnology 2011, 22, 365601. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Jung, I.; Piner, R.D.; An, S.J.; Li, X.; Velamakanni, A.; Ruoff, R.S. Colloidal Suspensions of Highly Reduced Graphene Oxide in a Wide Variety of Organic Solvents. Nano Lett. 2009, 9, 1593–1597. [Google Scholar] [CrossRef] [PubMed]
- Le Mogne, T.; Martin, J.M.; Grossiord, C. Imaging the chemistry of transfer films in the AES/XPS analytical UHV tribotester. Lubr. Front. 1999, 36, 413–421. [Google Scholar] [CrossRef]
- Wagner, C.D.; Davis, L.E.; Zeller, M.V.; Taylor, J.A.; Raymond, R.H.; Gale, L.H. Empirical Atomic Sensitivity Factors for Quantitative Analysis by Electron Spectroscopy for Chemical Analysis. Surf. Interface Anal. 1981, 3, 211–225. [Google Scholar] [CrossRef]
- Sohbatzadeh, F.; Shakerinasab, E.; Eshghabadi, M.; Ghasemi, M. Characterization and performance of coupled atmospheric pressure argon plasma jet with n-hexane electrospray for hydrophobic layer coatings on cotton textile. Diam. Relat. Mater. 2019, 91, 34–45. [Google Scholar] [CrossRef]
- Lascovich, J.C.; Giorgi, R.; Scaglione, S. Evaluation of the sp2/sp3 ratio in amorphous carbon structure by XPS and XAES. Appl. Surf. Sci. 1991, 47, 17–21. [Google Scholar] [CrossRef]
- Turgeon, S.; Paynter, R.W. On the determination of carbon sp2/sp3 ratios in polystyrene-polyethylene copolymers by photoelectron spectroscopy. Thin Solid Films 2001, 394, 44–48. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lyu, J.; Kang, S.; Lu, S.J.; Bielawski, C.W. Ascertaining the Carbon Hybridization States of Synthetic Polymers with X-ray Induced Auger Electron Spectroscopy. J. Phys. Chem. C 2018, 122, 11855–11861. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuwahara, T.; Long, Y.; De Barros Bouchet, M.-I.; Martin, J.M.; Moras, G.; Moseler, M. Superlow Friction of a-C:H Coatings in Vacuum: Passivation Regimes and Structural Characterization of the Sliding Interfaces. Coatings 2021, 11, 1069. https://doi.org/10.3390/coatings11091069
Kuwahara T, Long Y, De Barros Bouchet M-I, Martin JM, Moras G, Moseler M. Superlow Friction of a-C:H Coatings in Vacuum: Passivation Regimes and Structural Characterization of the Sliding Interfaces. Coatings. 2021; 11(9):1069. https://doi.org/10.3390/coatings11091069
Chicago/Turabian StyleKuwahara, Takuya, Yun Long, Maria-Isabel De Barros Bouchet, Jean Michel Martin, Gianpietro Moras, and Michael Moseler. 2021. "Superlow Friction of a-C:H Coatings in Vacuum: Passivation Regimes and Structural Characterization of the Sliding Interfaces" Coatings 11, no. 9: 1069. https://doi.org/10.3390/coatings11091069





