Screening of Essential Oils and Effect of a Chitosan-Based Edible Coating Containing Cinnamon Oil on the Quality and Microbial Safety of Fresh-Cut Potatoes
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Preparation of Bacterial Inoculum
2.2. Vegetables
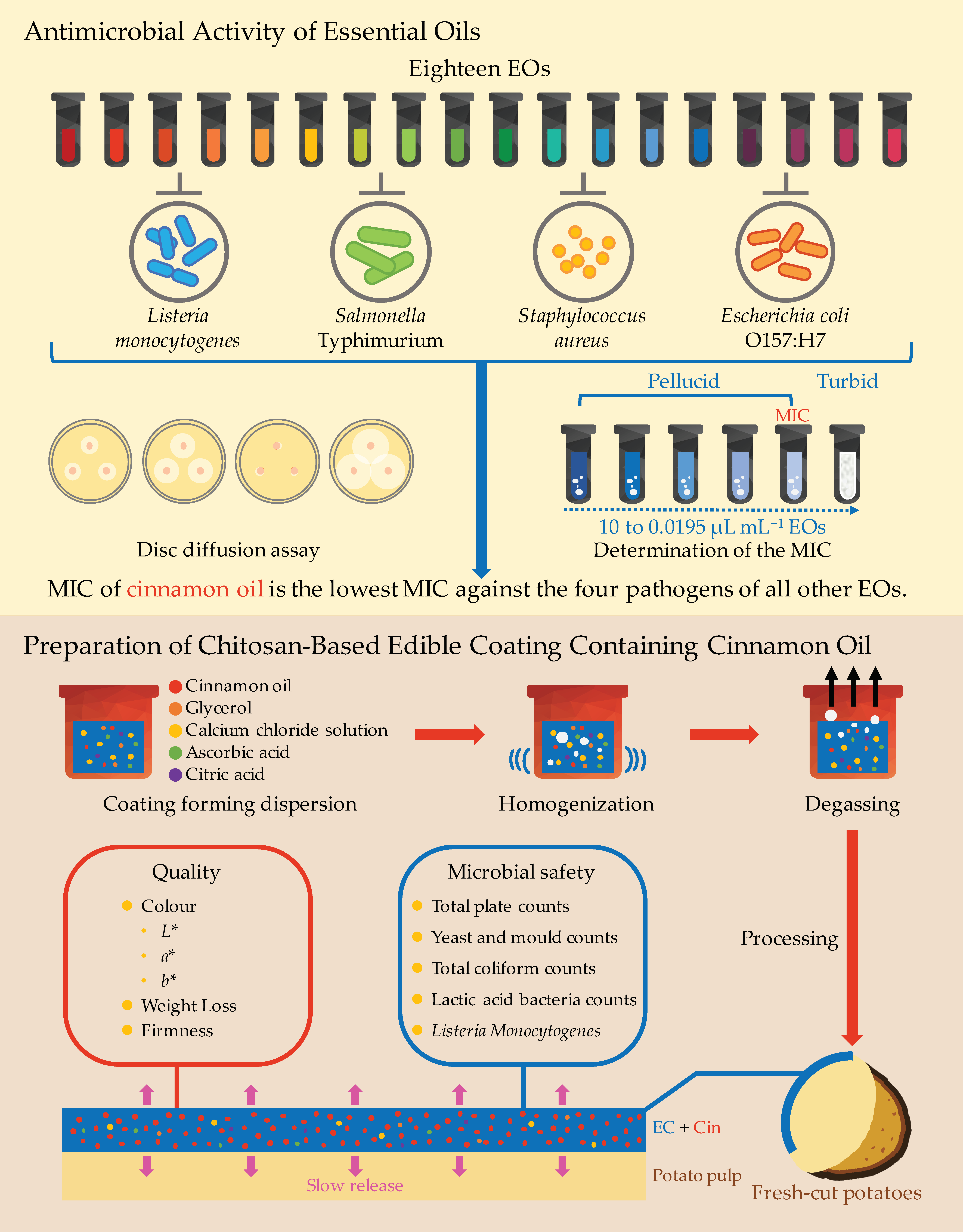
2.3. Antimicrobial Activity of Essential Oils
2.3.1. Essential Oils
2.3.2. Disc Diffusion Assay
2.3.3. Determination of the Minimal Inhibitory Concentration (MIC)
2.4. Preparation of Chitosan Edible Coating
2.5. Processing and Packaging of Fresh-Cut Potatoes
2.6. Determination of Potato Colour, Weight Loss, and Firmness
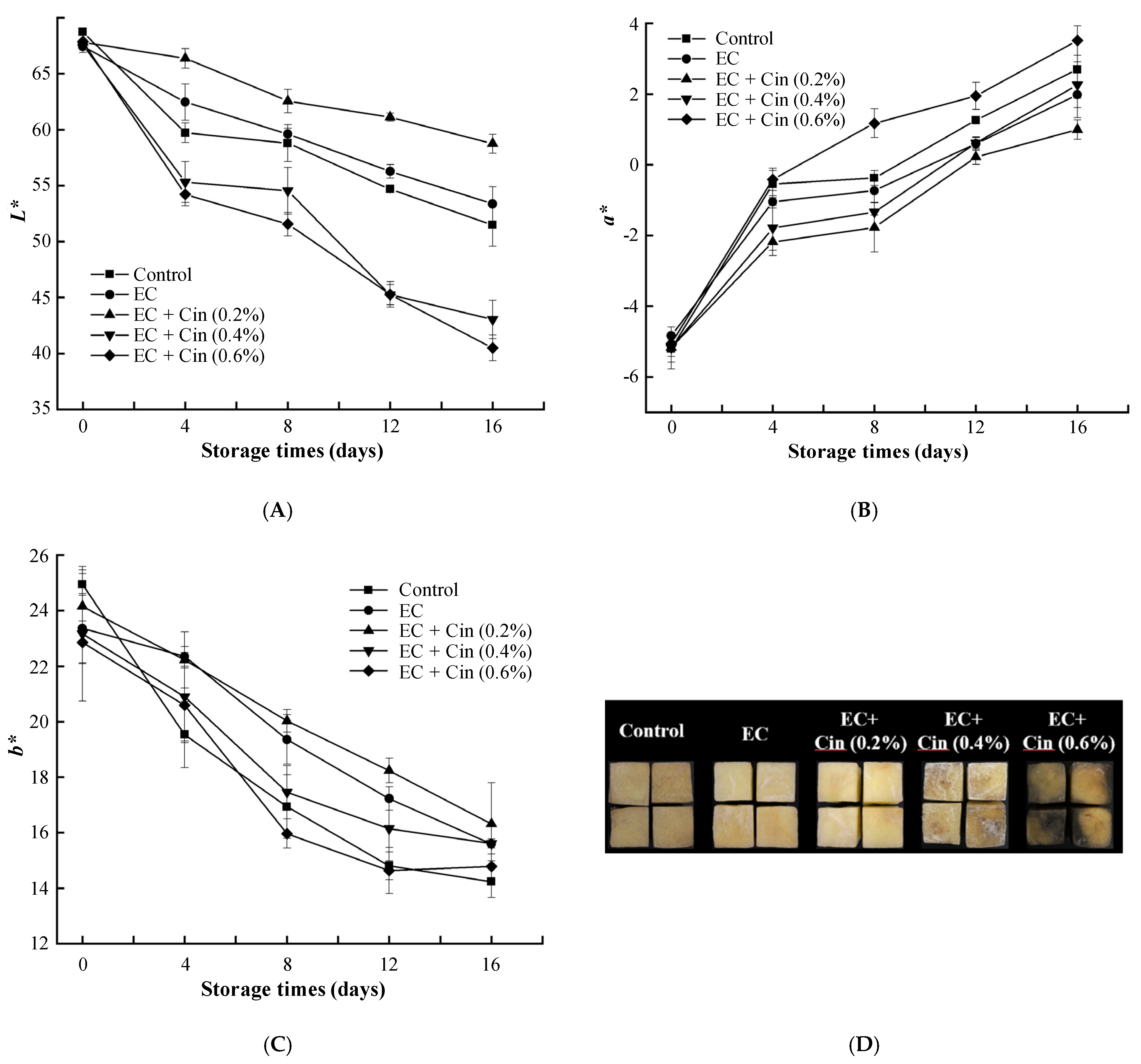
2.6.1. Colour
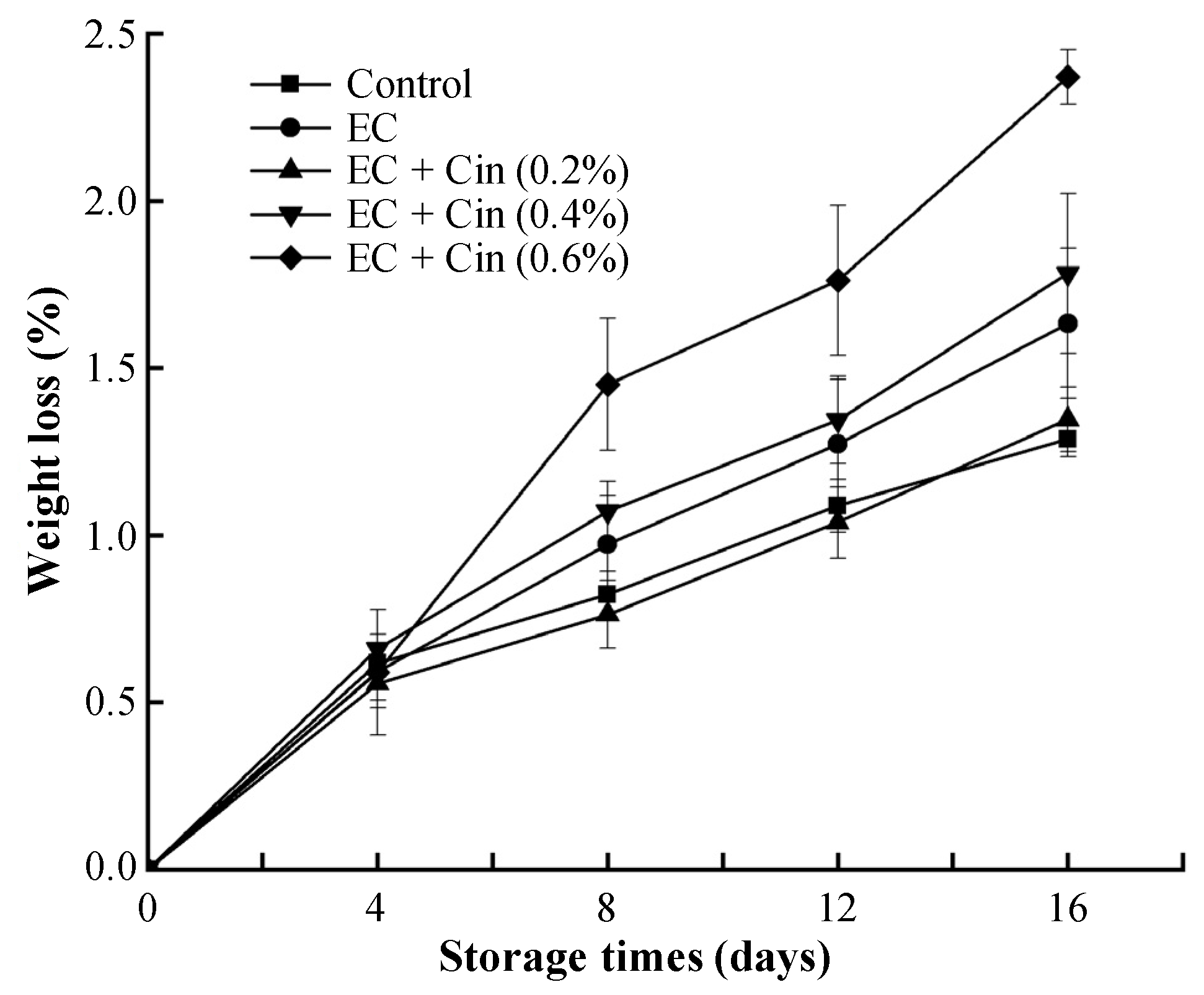
2.6.2. Weight Loss
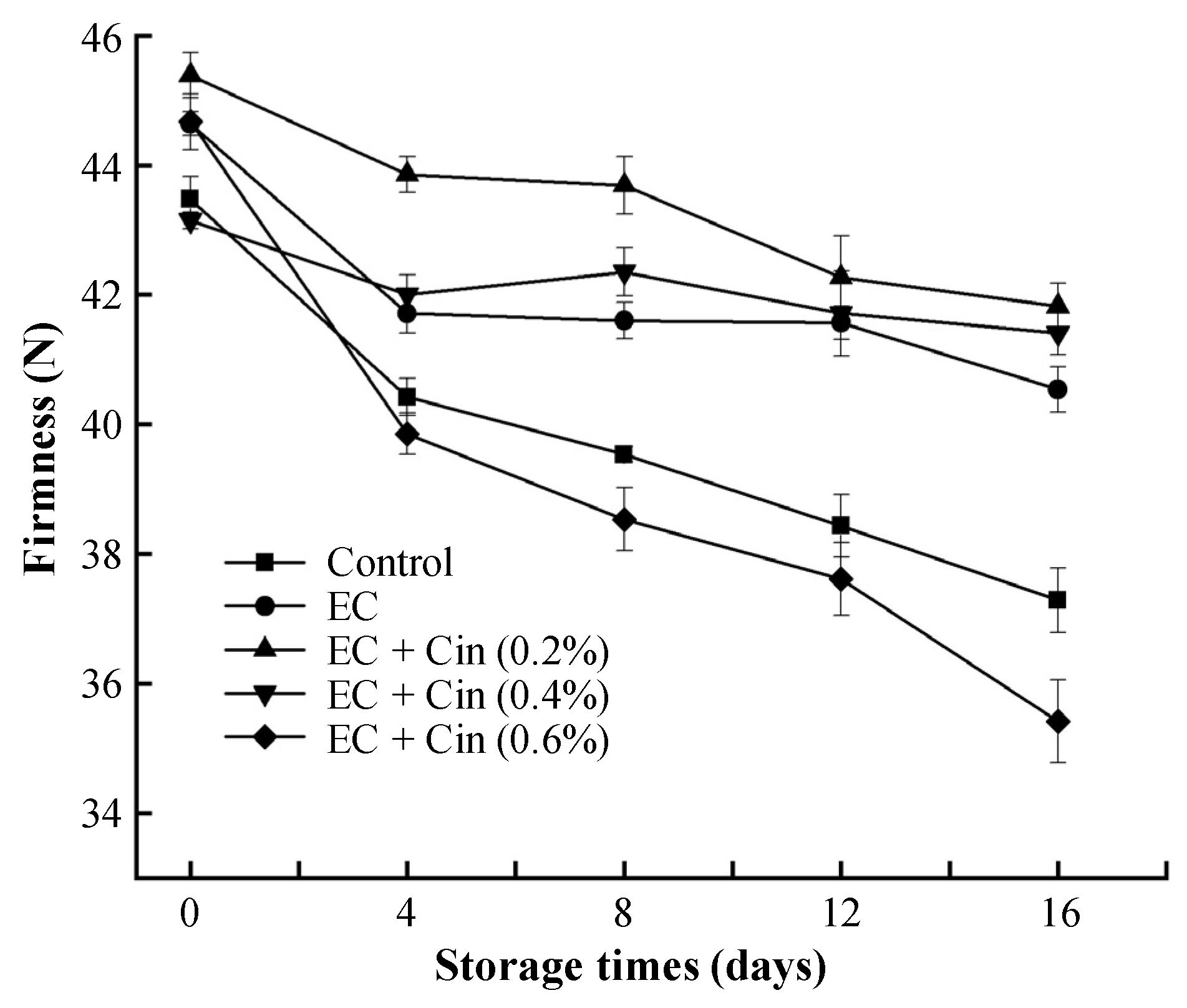
2.6.3. Firmness
2.7. Naturally Occurring Microorganisms
2.8. Inoculation and Analysis of Listeria monocytogenes
2.9. Statistical Analysis
3. Results and Discussion
3.1. Antimicrobial Assay of Essential Oils
3.2. Determination of Minimal Inhibitory Concentration
3.3. Effects of Cinnamon Oil on the Quality of Fresh-Cut Potatoes
3.3.1. Colour
3.3.2. Weight Loss
3.3.3. Firmness
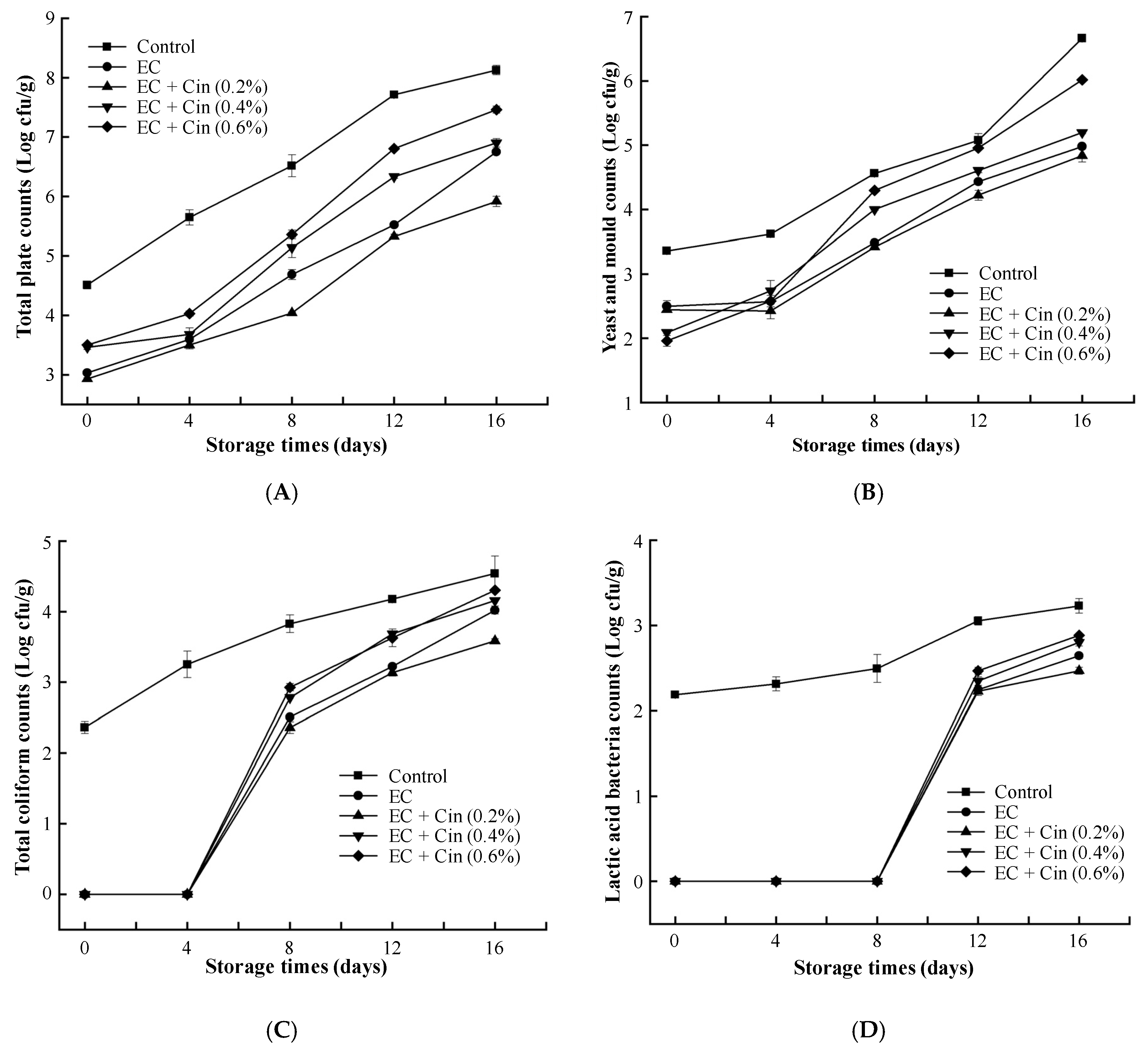
3.4. Microbiological Analysis
3.5. Listeria monocytogenes Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Zhao, D. Transcriptome analysis of scions grafted to potato rootstock for improving late blight resistance. BMC Plant Biol. 2021, 21, 272. [Google Scholar] [CrossRef]
- Dogbe, W.; Revoredo-Giha, C. Nutritional implications of trade-offs between fresh and processed potato products in the United Kingdom (UK). Front. Nutr. 2021, 7, 614176. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.Q.; Zhao, H.B.; Liu, Z.D.; He, J.; Liu, W.Z. Research progress and development of mechanized potato planters: A review. Agriculture 2021, 11, 521. [Google Scholar] [CrossRef]
- Vithu, P.; Sanjaya, K.D.; Kalpana, R. Post-harvest processing and utilization of sweet potato: A review. Food Rev. Int. 2019, 35, 726–762. [Google Scholar]
- Van Haute, S.; Sampers, I.; Holvoet, K.; Uyttendaele, M. Physicochemical quality and chemical safety of chlorine as a reconditioning agent and wash water disinfectant for fresh-cut lettuce washing. Appl. Environ. Microbiol. 2013, 79, 2850–2861. [Google Scholar] [CrossRef] [PubMed]
- Batool, T.; Ali, S.; Seleiman, M.F.; Naveed, N.H.; Ali, A.; Ahmed, K.; Abid, M.; Rizwan, M.; Shahid, M.R.; Alotaibi, M.; et al. Plant growth promoting rhizobacteria alleviates drought stress in potato in response to suppressive oxidative stress and antioxidant enzymes activities. Sci. Rep. 2020, 10, 16975. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, M.; Chambers, D.; Iv, E.C.; Talavera, M. Determining which cooking method provides the best sensory differentiation of potatoes. Foods 2020, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Robitaille, M.C.; Merrill, M.; Teferra, K.; Kim, C.; Raphael, M.P. Mechanisms of cell damage due to mechanical impact: An in vitro investigation. Sci. Rep. 2020, 10, 12009. [Google Scholar] [CrossRef] [PubMed]
- Agriopoulou, S.; Stamatelopoulou, E.; Sachadyn-Król, M.; Varzakas, T. Lactic acid bacteria as antibacterial agents to extend the shelf life of fresh and minimally processed fruits and vegetables: Quality and safety aspects. Microorganisms 2020, 8, 952. [Google Scholar] [CrossRef] [PubMed]
- Blancas-Benitez, F.J.; Montaño-Leyva, B.; Aguirre-Güitrón, L.; Moreno-Hernández, C.L.; Fonseca-Cantabrana, Á.; Romero-Islas, L.; González-Estrada, R. Impact of edible coatings on quality of fruits: A review. Food Control 2022, 139, 109063. [Google Scholar] [CrossRef]
- Valencia-Chamorro, S.A.; Palou, L.; Del Río, M.A.; Pérez-Gago, M.B. Antimicrobial edible films and coatings for fresh and minimally processed fruits and vegetables: A review. Crit. Rev. Food Sci. 2011, 51, 872–900. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, B.; Zhao, J. Dispersion process and effect of oleic acid on properties of cellulose sulfate-oleic acid composite film. Materials 2015, 8, 2346–2360. [Google Scholar] [CrossRef]
- Petriccione, M.; Mastrobuoni, F.; Pasquariello, M.S.; Zampella, L.; Nobis, E.; Capriolo, G.; Scortichini, M. Effect of chitosan coating on the postharvest quality and antioxidant enzyme system response of strawberry fruit during cold storage. Foods 2015, 4, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Meng, X.; Meng, J.; Khan, M.I.H.; Dai, L.; Khan, A.; An, X.; Zhang, J.; Huq, T.; Ni, Y. Chitosan as a preservative for fruits and vegetables: A review on chemistry and antimicrobial properties. J. Bioresour. Bioprod. 2019, 4, 11–21. [Google Scholar] [CrossRef]
- Oyom, W.; Zhang, Z.; Bi, Y.; Tahergorabi, R. Application of starch-based coatings incorporated with antimicrobial agents for preservation of fruits and vegetables: A review. Prog. Org. Coat. 2022, 166, 106800. [Google Scholar] [CrossRef]
- Kumar, P.; Sethi, S.; Sharma, R.R.; Singh, S.; Varghese, E. Improving the shelf life of fresh-cut ‘Royal Delicious’ apple with edible coatings and anti-browning agents. J. Food Sci. Technol. 2018, 55, 3767–3778. [Google Scholar] [CrossRef] [PubMed]
- Tharanathan, R.N.; Kittur, F.S. Chitin—The undisputed biomolecule of great potential. Crit. Rev. Food Sci. Nutr. 2003, 43, 61–87. [Google Scholar] [CrossRef]
- Tokatlı, K.; Demirdöven, A. Effects of chitosan edible film coatings on the physicochemical and microbiological qualities of sweet cherry (Prunus avium L.). Sci. Hortic. 2020, 259, 108656. [Google Scholar] [CrossRef]
- Jeon, Y.I.; Kamil, J.Y.V.A.; Shahidi, F. Chitosan as an edible invisible film for quality preservation of herring and Atlantic cod. J. Agric. Food Chem. 2002, 20, 5167–5178. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, Q.; Critzer, F.; Davidson, P.M.; Zhong, Q. Organic thyme oil emulsion as an alternative washing solution to enhance the microbial safety of organic cantaloupes. Food Control 2016, 67, 31–38. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Zhang, R.; Lan, W.; Qin, W. Fabrication of electrospun polylactic acid/cinnamaldehyde/β-cyclodextrin fibers as an antimicrobial wound dressing. Polymers 2017, 9, 464. [Google Scholar] [CrossRef]
- Kawatra, P.; Rajagopalan, R. Cinnamon: Mystic powers of a minute ingredient. Pharmacogn. Res. 2015, 7, S1–S6. [Google Scholar] [CrossRef] [PubMed]
- Kosari, F.; Taheri, M.; Moradi, A.; Alni, R.H.; Alikhani, M.Y. Evaluation of cinnamon extract effects on clbB gene expression and biofilm formation in Escherichia coli strains isolated from colon cancer patients. BMC Cancer 2020, 20, 267. [Google Scholar] [CrossRef]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Graü, M.A.; Raybaudi-Massilia, R.M.; Soliva-Fortuny, R.C.; AvenaBustillos, R.J.; McHugh, T.H.; Martní-Belloso, O. Apple puree-alginate edible coating as carrier of antimicrobial agents to prolong shelf-life of fresh-cut apples. Postharvest Biol. Technol. 2007, 45, 254–264. [Google Scholar] [CrossRef]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Martín-Belloso, O. Edible alginate-based coating as carrier of antimicrobials to improve shelf-life and safety of fresh-cut melon. Int. J. Food Microbiol. 2008, 121, 313–327. [Google Scholar] [CrossRef]
- Azarakhsha, N.; Osmana, A.; Ghazalia, H.M.; Tan, C.P.; Adzahan, N.M. Lemongrass essential oil incorporated into alginate-based edible coating for shelf-life extension and quality retention of fresh-cut pineapple. Postharvest Biol. Technol. 2014, 88, 1–7. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Use of antimicrobial nanoemulsions as edible coatings: Impact on safety and quality attributes of fresh-cut Fuji apples. Postharvest Biol. Technol. 2015, 105, 8–16. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, M.; Feng, X.; Yao, Z.; Wang, T.; Xu, C. Effect of lemon essential oil-enriched coating on the postharvest storage quality of citrus fruits. Food Sci. Technol. 2022, 42, e125421. [Google Scholar] [CrossRef]
- Joshua, P.V.; Di, L.; Linda, J.H.; Donald, W.S.; Michelle, D.D. Fate of Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella on fresh-cut celery. Food Microbiol. 2013, 34, 151–157. [Google Scholar]
- Shen, X.; Zhang, M.; Devahastin, S.; Guo, Z. Effects of pressurized argon and nitrogen treatments in combination with modified atmosphere on quality characteristics of fresh-cut potatoes. Postharvest Biol. Technol. 2019, 149, 159–165. [Google Scholar] [CrossRef]
- Ferreira, L.R.; Rosário, D.K.A.; Silva, P.I.; Carneiro, J.C.S.; Pimentel Filho, N.J.; Bernardes, P.C. Cinnamon essential oil reduces adhesion of food pathogens to polystyrene. Int. Food Res. J. 2019, 26, 1103–1110. [Google Scholar]
- Rota, M.C.; Herrera, A.; Martínez, R.M.; Sotomayor, J.A.; Jordán, M.J. Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control 2008, 19, 681–687. [Google Scholar] [CrossRef]
- Somrani, M.; Inglés, M.C.; Debbabi, H.; Abidi, F.; Palop, A. Garlic, Onion, and Cinnamon Essential Oil Anti-biofilms’ Effect against Listeria monocytogenes. Foods 2020, 9, 567. [Google Scholar] [CrossRef] [PubMed]
- Sarengaowa; Hu, W.Z.; Jiang, A.L.; Xiu, Z.L.; Feng, K. Effect of thyme oil–alginate-based coating on quality and microbial safety of fresh-cut apples. J. Sci. Food Agric. 2018, 98, 2302–2311. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, F.; Jiang, Y.; Chai, Z.; Li, P.; Cheng, Y.; Jing, H.; Leng, X. Synergistic antimicrobial activities of natural essential oils with chitosan film. J. Agric. Food Chem. 2011, 59, 12411–12419. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xu, N.; Liu, R.; Liu, J.; Peng, Y.; Wang, Q. Exogenous proline treatment inhibiting enzymatic browning of fresh-cut potatoes during cold storage. Postharvest Biol. Technol. 2022, 184, 111754. [Google Scholar] [CrossRef]
- Zhao, S.; Han, X.; Liu, B.; Wang, S.; Guan, W.; Wu, Z.; Theodorakis, P.E. Shelf-life prediction model of fresh-cut potato at different storage temperatures. J. Food Eng. 2022, 317, 110867. [Google Scholar] [CrossRef]
- Ji, Y.; Hu, W.; Liao, J.; Jiang, A.; Xiu, Z.; Saren, G.; Guan, Y.; Yang, X.; Feng, K.; Liu, C. Effect of atmospheric cold plasma treatment on antioxidant activities and reactive oxygen species production in postharvest blueberries during storage. J. Sci. Food Agric. 2020, 100, 5586–5595. [Google Scholar] [CrossRef] [PubMed]
- Gómez, P.L.; Salvatori, D.M. Pulsed light treatment of cut apple: Dose effect on color, structure, and microbiological stability. Food Bioprocess Technol. 2012, 5, 2311–2322. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Tabanelli, G.; Montanari, C.; Gardini, F.; Lanciotti, R. Lactic acid bacteria and natural antimicrobials to improve the safety and shelf-life of minimally processed sliced apples and lamb’s lettuce. Food Microbiol. 2015, 47, 74–84. [Google Scholar] [CrossRef]
- Sarengaowa; Hu, W.; Feng, K.; Xiu, Z.; Jiang, A.; Lao, Y. Efficacy of thyme oil-alginate-based coating in reducing foodborne pathogens on fresh-cut apples. Int. J. Food Sci. Technol. 2019, 54, 3128–3137. [Google Scholar] [CrossRef]
- Antunes, M.D.C.; Cavaco, A.M. The use of essential oils for postharvest decay control: A review. Flavour Frag. J. 2010, 25, 351–366. [Google Scholar] [CrossRef]
- Yuan, Y.; Huang, M.; Pang, Y.X.; Yu, F.L.; Chen, C.; Liu, L.W.; Chen, Z.X.; Zhang, Y.B.; Chen, X.L.; Hu, X. Variations in essential oil yield, composition, and antioxidant activity of different plant organs from Blumea balsamifera (L.) DC. at different growth times. Molecules 2016, 21, 1024. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wu, D.; Zhang, Q.; Chen, H.; Li, H.; Han, Q.; Lai, X.; Wang, H.; Wu, Y.; Yuan, J.; et al. Efficacy and mechanism of cinnamon essential oil on inhibition of colletotrichum acutatum isolated from ‘hongyang’ kiwifruit. Front. Microbiol. 2018, 9, 1288. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Burton, D.; Parra, F.; López, J.; Muñoz, P.; Escobar, H.; Parra, C. Antioxidant and antibacterial capacities of origanum vulgare L. essential oil from the arid andean region of Chile and its chemical characterization by GC-MS. Metabolites 2020, 10, 414. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Li, X.; Peng, Y.; He, X.; Pan, S. Anti-oxidant and anti-melanogenic properties of essential oil from peel of Pomelo cv. Guan Xi. Molecules 2019, 24, 242. [Google Scholar] [CrossRef]
- Osaili, T.M.; Hasan, F.; Dhanasekaran, D.K.; Obaid, R.S.; Al-Nabulsi, A.A.; Ayyash, M.; Karam, L.; Savvaidis, I.N.; Holley, R. Effect of active essential oils added to chicken tawook on the behaviour of Listeria monocytogenes, Salmonella spp. and Escherichia coli O157:H7 during storage. Int. J. Food Microbiol. 2021, 337, 108947. [Google Scholar] [CrossRef] [PubMed]
- Kosakowska, O.; Węglarz, Z.; Pióro-Jabrucka, E.; Przybył, J.L.; Kraśniewsk, K.; Gniewosz, M.; Bączek, K. Antioxidant and antibacterial activity of essential oils and hydroethanolic extracts of Greek oregano (O. vulgare L. subsp. hirtum (link) ietswaart) and Common oregano (O. vulgare L. subsp. vulgare). Molecules 2021, 26, 988. [Google Scholar] [PubMed]
- Scollard, J.; McManamon, O.; Schmalenberger, A. Inhibition of Listeria monocytogenes growth on fresh-cut produce with thyme essential oil and essential oil compound verbenone. Postharvest Biol. Technol. 2016, 120, 61–68. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, Y.; Jiang, P.; Quek, S.Y. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Matan, N.; Rimkeeree, H.; Mawson, A.J.; Chompreeda, P.; Haruthaithanasan, V.; Parker, M. Antimicrobial activity of cinnamon and clove oils under modified atmosphere conditions. Int. J. Food Microbiol. 2006, 107, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Di Lorenzo, A.; Izadi, M.; Sobarzo-Sánchez, E.; Daglia, M.; Nabavi, S.M. Antibacterial effects of cinnamon: From farm to food, cosmetic and pharmaceutical industries. Nutrients 2015, 7, 7729–7748. [Google Scholar] [CrossRef] [PubMed]
- Dovene, A.K.; Wang, L.; Bokhary, S.U.F.; Madebo, M.P.; Yonghua, Z.; Jin, P. Effect of cutting styles on quality and antioxidant activity of stored fresh-cut sweet potato (Ipomoea batatas L.) cultivars. Foods 2019, 8, 674. [Google Scholar] [CrossRef] [PubMed]
- Olivas, G.I.; Mattinson, D.S.; Barbosa-Cánovas, G.V. Alginate coatings for preservation of minimally processed ‘Gala’ apples. Postharvest Biol. Technol. 2007, 45, 89–96. [Google Scholar] [CrossRef]
- Ru, Z.H.; Lai, Y.Y.; Xu, C.J.; Li, L. Polyphenol oxidase (PPO) in early stage of browning of phalaenopsis leaf explants. J. Agric. Sci. 2013, 5, 57–64. [Google Scholar] [CrossRef]
- Zhang, D.; Quantick, P.C. Effects of chitosan coating on enzymatic browning and decay during postharvest storage of litchi (Litchi chinensis Sonn.) fruit. Postharvest Biol. Technol. 1997, 12, 195–202. [Google Scholar] [CrossRef]
- Sahraee, S.; Milani, J.M.; Regenstein, J.M.; Kafil, H.S. Protection of foods against oxidative deterioration using edible films and coatings: A review. Food Biosci. 2019, 32, 100451. [Google Scholar] [CrossRef]
- Nea, F.; Kambiré, D.A.; Genva, M.; Tanoh, E.A.; Wognin, E.L.; Martin, H.; Brostaux, Y.; Tomi, F.; Lognay, G.C.; Tonzibo, Z.F.; et al. Composition, seasonal variation, and biological activities of Lantana camara essential oils from Côte d’Ivoire. Molecules 2020, 25, 2400. [Google Scholar] [CrossRef] [PubMed]
- Massolo, J.F.; Concellón, A.; Chaves, A.R.; Vicente, A.R. 1-methylcyclopropene (1-MCP) delays senescence, maintains quality and reduces browning of non-climacteric eggplant (Solanum melongena L.) fruit. Postharvest Biol. Technol. 2011, 59, 10–15. [Google Scholar] [CrossRef]
- Scollard, J.; Francis, G.A.; O’Beirne, D. Some conventional and latent antilisterial effects of essential oils, herbs: Carrot and cabbage in fresh-cut vegetable systems. Postharvest Biol. Technol. 2013, 77, 87–93. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Use of essential oils in bioactive edible coatings: A review. Food Eng. Rev. 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Jackmen, R.L.; Stanley, D.W. Perspectives in the textural evaluation of plant foods. Trends Food Sci. Technol. 1995, 6, 187–194. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, F.; Lai, S.; Wang, H.; Yang, H. Impact of soybean protein isolate-chitosan edible coating on the softening of apricot fruit during storage. LWT-Food Sci. Technol. 2018, 96, 604–611. [Google Scholar] [CrossRef]
- Eshghi, S.; Hashemi, M.; Mohammadi, A.; Badii, F.; Mohammadhoseini, Z.; Ahmadi, K. Effect of nanochitosan-based coating with and without copper loaded on physicochemical and bioactive components of fresh strawberry fruit (Fragaria x ananassa Duchesne) during storage. Food Bioprocess Technol. 2014, 7, 2397–2409. [Google Scholar] [CrossRef]
- Gardesh, A.S.K.; Badii, F.; Hashemi, M.; Ardakani, A.Y.; Maftoonazad, N.; Gorji, A.M. Effect of nanochitosan based coating on climacteric behavior and postharvest shelf-life extension of apple cv Golab Kohanz. LWT-Food Sci. Technol. 2016, 70, 33–40. [Google Scholar] [CrossRef]
- Zhou, R.; Mo, Y.; Li, Y.; Zhao, Y.; Zhang, G.; Hu, Y. Quality and internal characteristics of Huanghua peers (Pyrus pyrifolia Nakai, cv. Huanghua) treated with different kinds of coatings during storage. Postharvest Biol. Technol. 2008, 49, 171–179. [Google Scholar]
- Tamminga, S.K.; Beumer, R.R.; Keijbets, M.J.H.; Kampelmacher, E.M. Microbial spoilage and development of food poisoning bacteria in peeled, completely or partly cooked vacuum- packed potatoes. Arch. Lemensmittelhyg 1978, 29, 215–219. [Google Scholar]
- Doan, C.H.; Davidson, P.M. Microbiology of potatoes and potato products: A review. J. Food Prot. 2000, 63, 668–683. [Google Scholar] [CrossRef]
- Chien, P.; Sheu, F.; Yang, F. Effects of edible chitosan coating on quality and shelf life of sliced mango fruit. J. Food Eng. 2007, 78, 225–229. [Google Scholar] [CrossRef]
- Gonzalez-Aguilar, G.A.; Valenzuela-Soto, E.; Lizardi-Mendoza, J.; Goycoole, F.; Martinez-Tellez, M.A.; Villegas-Ochoa, M.A.; Monroy-Garcia, I.N.; Ayala-Zavala, J.F. Effect of chitosan coating in preventing deterioration and preserving the quality of fresh-cut papaya ‘Maradol’. J. Sci. Food Agric. 2009, 89, 15–23. [Google Scholar] [CrossRef]
- Hernandez-Munoz, P.; Almenar, E.; Ocio, M.J.; Gavara, R. Effect of calcium dips and chitosan coatings on postharvest life of strawberries (Fragaria x ananassa). Postharvest Biol. Technol. 2006, 39, 247–253. [Google Scholar] [CrossRef]
- Devlieghere, F.; Vermeulen, A.; Debevere, J. Chitosan: Antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiol. 2004, 21, 703–714. [Google Scholar] [CrossRef]
- Jung, E.J.; Youn, D.K.; Lee, S.H.; No, H.K.; Ha, J.G.; Prinyawiwatkul, W. Antibacterial activity of chitosans with different degrees of deacetylation and viscosities. Int. J. Food Sci. Technol. 2010, 45, 676–682. [Google Scholar] [CrossRef]
- Feng, K.; Hu, W.Z.; Jiang, A.L.; Sarengaowa; Xu, Y.P.; Ji, Y.R.; Shao, W.J. Growth of Salmonella spp. and Escherichia coli O157:H7 on fresh-cut fruits stored at different temperatures. Foodborne Pathog. Dis. 2017, 14, 510–517. [Google Scholar] [CrossRef] [PubMed]
- IFST. Development and Use of Microbiological Criteria for Foods; Institute of Food Science and Technology: London, UK, 1999; 76p. [Google Scholar]
- Wu, Z.S.; Zhang, M.; Wang, S. Effects of high pressure argon treatments on the quality of fresh-cut apples at cold storage. Food Control 2012, 23, 120–127. [Google Scholar] [CrossRef]
- Zhang, C.L.; Cao, W.; Hung, Y.C.; Li, B.M. Application of electrolyzed oxidizing water in production of radish sprouts to reduce natural microbiota. Food Control 2016, 67, 177–182. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J. Fabrication of bioactive binary composite film based on gelatin/chitosan incorporated with cinnamon essential oil and rutin. Colloids Surf. B 2021, 204, 111830. [Google Scholar] [CrossRef]
- Wendakoon, C.N.; Sakaguchi, M. Inhibition of amino acid decarboxylase activity of Enterobacter aerogenes by active components of spices. J. Food Prot. 1995, 58, 280–283. [Google Scholar] [CrossRef]
- Wu, J.; Sun, X.; Guo, X.; Ge, S.; Zhang, Q. Physicochemical properties, antimicrobial activity and oil release of fish gelatin films incorporated with cinnamon essential oil. Aquac. Fish. 2017, 2, 185–192. [Google Scholar] [CrossRef]
- Haghighi, H.; Biard, S.; Bigi, F.; De Leo, R.; Bedin, E.; Pfeifer, F.; Siesler, H.W.; Licciardello, F.; Pulvirenti, A. Comprehensive characterization of active chitosan gelatin blend films enriched with different essential oils. Food Hydrocoll. 2019, 95, 33–42. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, S.; Duffy, B.; Jaiswal, A.K. Development of essential oil incorporated active film based on biodegradable blends of poly (lactide)/poly (butylene adipate-co-terephthalate) for food packaging application. J. Packag. Technol. Res. 2020, 4, 235–245. [Google Scholar] [CrossRef]

| Essential Oil | Latin Name | Strains Tested (mm) | |||
|---|---|---|---|---|---|
| SA | ST | LM | EC O157:H7 | ||
| Cinnamon | Cinnamomum cassia | 30.47 ± 0.14 b | 26.07 ± 0.63 bc | 26.8 ± 0.18 b | 16.33 ± 0.19 f |
| Oregano | Origanum vulgare | 31.31 ± 0.23 a | 26.87 ± 0.53 b | 30.01 ± 0.25 a | 22.01 ± 0.91 b |
| Clove | Eugenia caryophyllus | 14.76 ± 0.01 f | 15.98 ± 0.51 h | 13.99 ± 0.62 e | 12.43 ± 0.94 g |
| Tea tree | Melaleuca alternifolia | 10.75 ± 0.47 h | 18.65 ± 0.93 f | 12.74 ± 0.04 f | 20.89 ± 0.26 c |
| Pomelo | Citrus maxima (Burm.) Merr. | 21.75 ± 0.30 c | 35.18 ± 0.01 a | 30.05 ± 0.85 a | 27.01 ± 0.99 a |
| Jasmine | Jasminum sambac (L.) Ait. | - | - | - | - |
| Eucalyptus | Eucalyptus globulus | 17.84 ± 0.95 e | 19.86 ± 0.10 e | 12.45 ± 0.37 g | 16.33 ± 0.55 f |
| Rosemary | Rosmarinus officinalis | 9.49 ± 0.99 i | 10.75 ± 0.51 j | 7.75 ± 0.76 h | 12.11 ± 0.75 g |
| Sweet orange | Citrus sinensis (Linn.) Osbeck | 11.99 ± 0.29 g | 17.67 ± 0.51 g | 17.65 ± 0.93 d | 17.49 ± 0.48 d |
| Sea buckthorn pulp | Hippophae rhamnoides L. | - | - | - | - |
| Sweet osmanthus | Osmanthus fragrans (Thunb.) Lour. | - | - | - | - |
| Lavender | Lavandula angustifolia | 8.65 ± 0.54 j | 25.06 ± 0.83 d | - | - |
| Petitgrain | Citurs sinensis L. Osbeck | 7.78 ± 0.52 k | 7.83 ± 0.59 l | - | - |
| Grapefruit | Citrus paradisi Macf. | 8.91 ± 0.45 j | 14.19 ± 0.91 i | 8.13 ± 0.98 g | 8.29 ± 0.01 hi |
| Rose | Rosa rugosa Thunb. | 17.35 ± 0.89 e | 17.7 ± 0.002 g | 12.09 ± 0.87 fg | 8.85 ± 0.05 h |
| Citrus | Citrus reticulata Blanco | - | - | - | - |
| Blumea | Blumea balsamifera | 18.11 ± 0.21 d | 17.97 ± 0.04 g | 20.36 ± 0.17 c | 16.66 ± 0.59 e |
| Valerian | Valeriana officinalis | 10.64 ± 0.84 h | 9.96 ± 0.46 k | 12.41 ± 0.04 f | 7.88 ± 0.82 i |
| Essential Oil | Strains Tested | Concentrations of Essential Oils (μL/mL) | MIC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 5 | 2.5 | 1.25 | 0.625 | 0.313 | 0.156 | 0.078 | 0.039 | 0.020 | |||
| Cinnamon oil | LM | - | - | - | - | - | - | + | + | + | ++ | 0.313 |
| ST | - | - | - | - | - | - | + | + | + | ++ | 0.313 | |
| SA | - | - | - | - | - | - | + | + | + | ++ | 0.313 | |
| EC O157:H7 | - | - | - | - | - | + | + | + | + | ++ | 0.313 | |
| Oregano oil | LM | - | - | - | - | + | ++ | ++ | ++ | ++ | ++ | 1.25 |
| ST | - | - | - | - | - | + | + | ++ | ++ | ++ | 0.625 | |
| SA | - | - | - | - | + | + | ++ | ++ | ++ | ++ | 1.25 | |
| EC O157:H7 | - | - | - | - | + | ++ | ++ | ++ | ++ | ++ | 1.25 | |
| Pomelo peel oil | LM | - | - | - | + | + | + | + | ++ | ++ | ++ | 2.5 |
| ST | - | - | - | + | + | + | ++ | ++ | ++ | 1.25 | ||
| SA | - | - | - | + | + | + | + | ++ | ++ | ++ | 2.5 | |
| EC O157:H7 | - | - | - | + | + | + | + | ++ | ++ | ++ | 2.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarengaowa; Wang, L.; Liu, Y.; Yang, C.; Feng, K.; Hu, W. Screening of Essential Oils and Effect of a Chitosan-Based Edible Coating Containing Cinnamon Oil on the Quality and Microbial Safety of Fresh-Cut Potatoes. Coatings 2022, 12, 1492. https://doi.org/10.3390/coatings12101492
Sarengaowa, Wang L, Liu Y, Yang C, Feng K, Hu W. Screening of Essential Oils and Effect of a Chitosan-Based Edible Coating Containing Cinnamon Oil on the Quality and Microbial Safety of Fresh-Cut Potatoes. Coatings. 2022; 12(10):1492. https://doi.org/10.3390/coatings12101492
Chicago/Turabian StyleSarengaowa, Liying Wang, Yumeng Liu, Chunmiao Yang, Ke Feng, and Wenzhong Hu. 2022. "Screening of Essential Oils and Effect of a Chitosan-Based Edible Coating Containing Cinnamon Oil on the Quality and Microbial Safety of Fresh-Cut Potatoes" Coatings 12, no. 10: 1492. https://doi.org/10.3390/coatings12101492
APA StyleSarengaowa, Wang, L., Liu, Y., Yang, C., Feng, K., & Hu, W. (2022). Screening of Essential Oils and Effect of a Chitosan-Based Edible Coating Containing Cinnamon Oil on the Quality and Microbial Safety of Fresh-Cut Potatoes. Coatings, 12(10), 1492. https://doi.org/10.3390/coatings12101492






