Corrosion Behavior of SiO2-Al2O3 Glass Composite Coating on TC4 in Marine Environment
Abstract
1. Introduction
2. Experimental
2.1. Coating Preparation
2.2. Corrosion Test Simulating Marine Environment
2.3. Characterization
3. Results
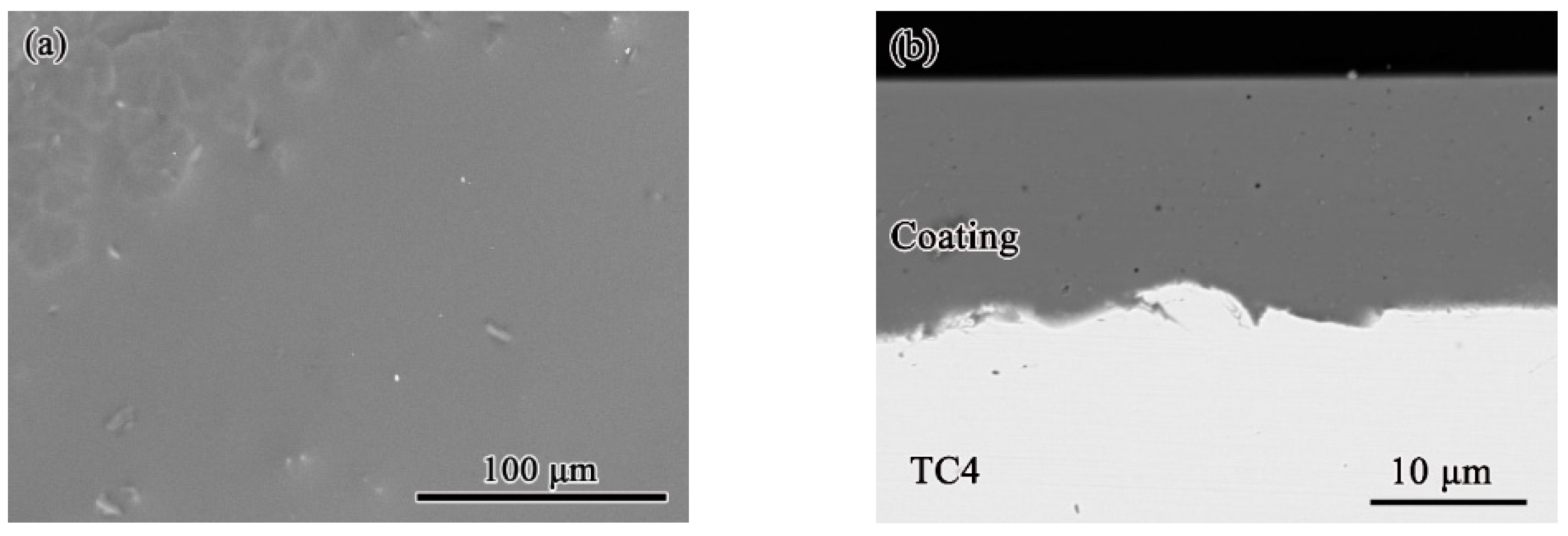
3.1. Initial Microstructure
3.2. Corrosion Kinetics
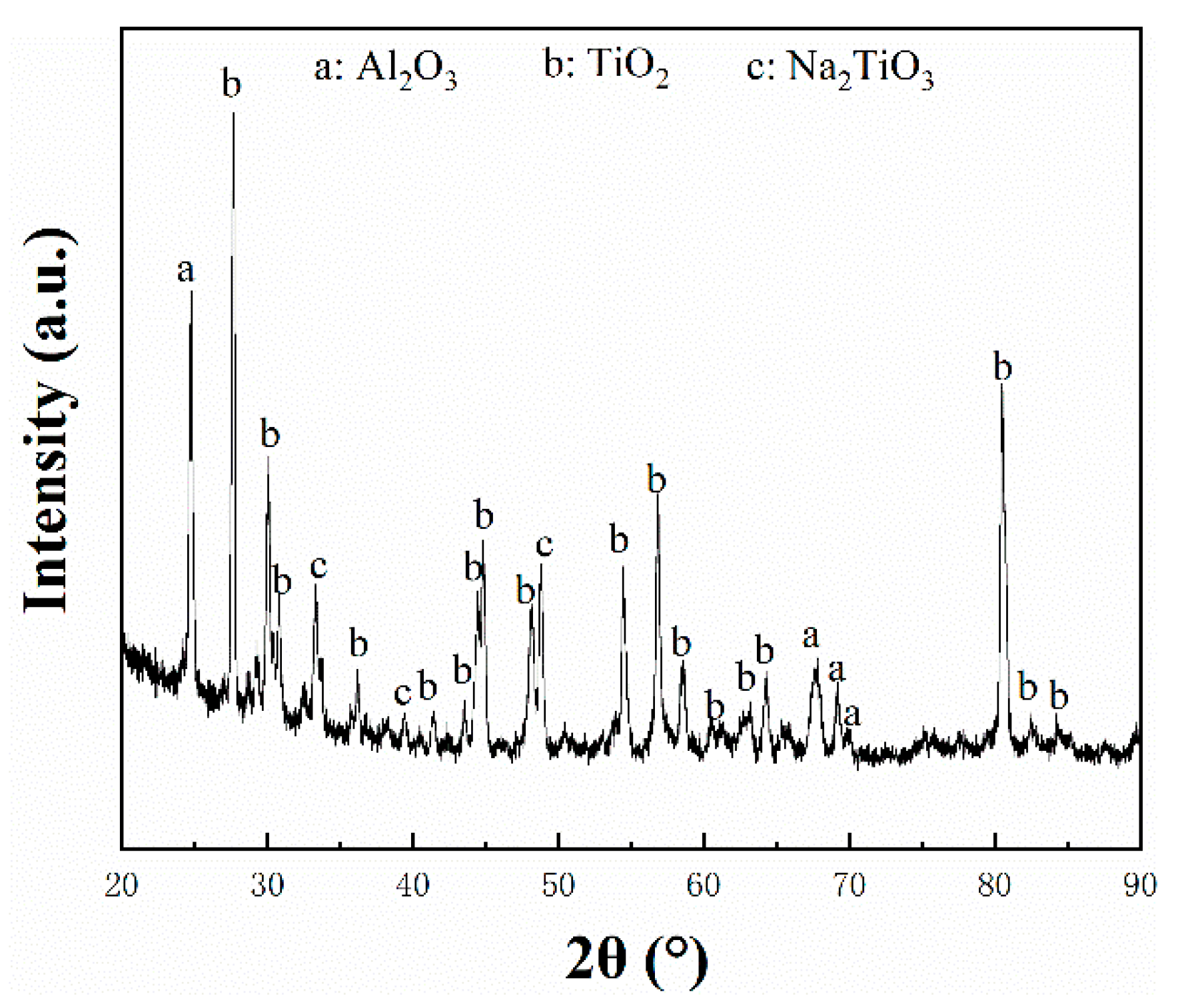
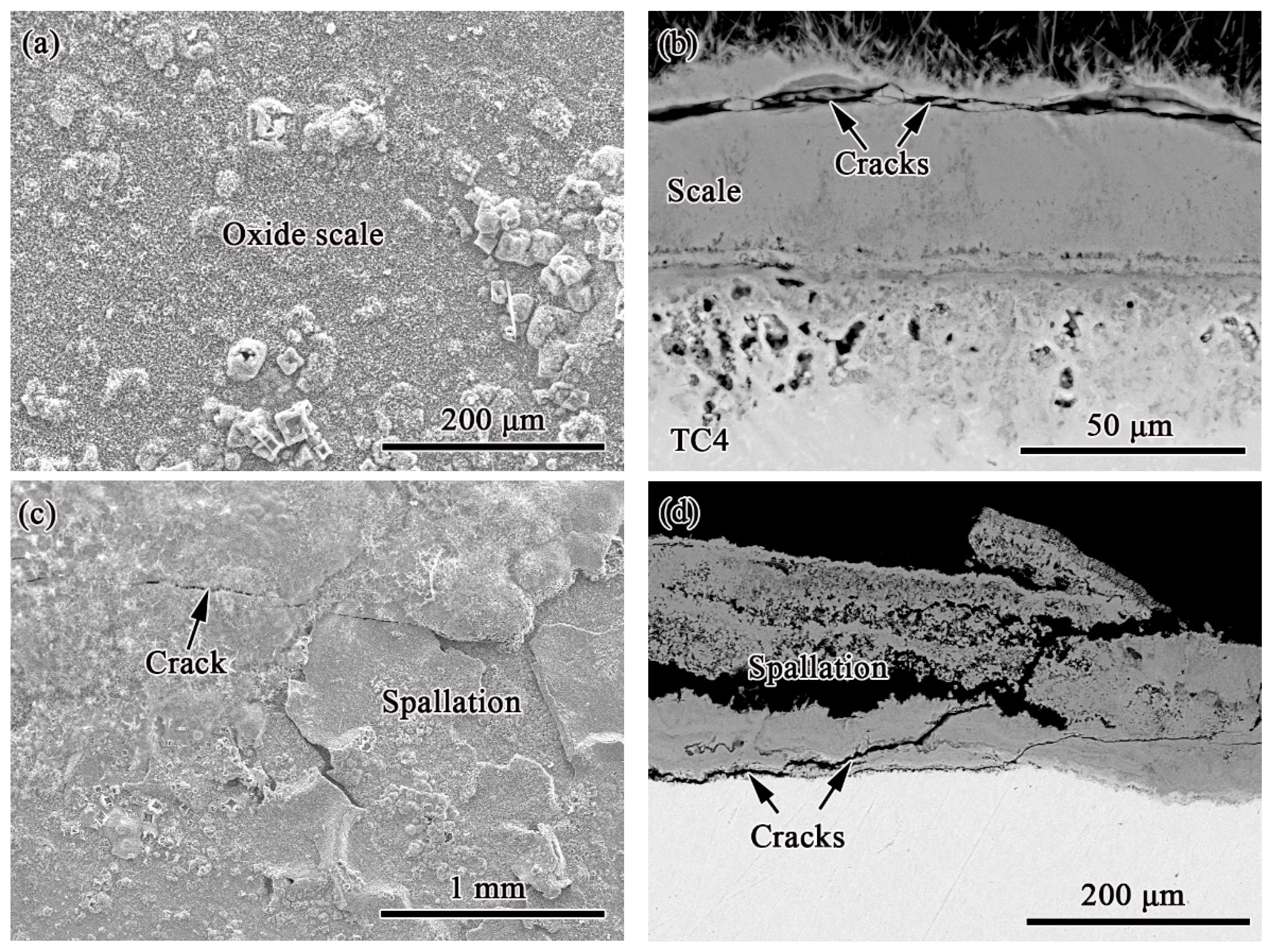
3.3. Corrosion Products in Marine Environment
4. Discussion
4.1. Effect of Ti on Self-Sustainable Oxychlorination in Marine Environment
4.2. Effect of SiO2-Al2O3 Glass Composite Coating on Self-Sustainable Oxychlorination Inhibition
5. Conclusions
- The bare TC4 exhibited serious corrosion due to self-sustainable oxychlorination process;
- The SiO2-Al2O3 glass composite coating showed superior corrosion resistance in marine environment because the self-sustainable oxychlorination reaction is inhibited effectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ciszak, C.; Popa, I.; Brossard, J.M.; Monceau, D.; Chevalier, S. NaCl induced corrosion of Ti-6Al-4V alloy at high temperature. Corros. Sci. 2016, 110, 91–104. [Google Scholar] [CrossRef]
- Fan, L.; Liu, L.; Cui, Y.; Cao, M.; Yu, Z.F.; Oguzie, E.E.; Li, Y.; Wang, F.H. Effect of streaming water vapor on the corrosion behavior of Ti60 alloy under a solid NaCl deposit in water vapor at 600 °C. Corros. Sci. 2019, 160, 108177. [Google Scholar] [CrossRef]
- Li, W.B.; Xie, Y.; Li, D.K.; Hu, J.R.; Chen, H.D. Dew point corrosion of air preheater and preventive measures. Hunan Electr. Power 2016, 1, 3–9. [Google Scholar]
- Li, W.B.; Xie, Y.; Liu, Y.L.; Wu, T.Q.; Long, Y. Study on corrosion resistance of NiTi memory alloy butterfly gasket. Hunan Electr. Power 2017, 37, 29–32. [Google Scholar]
- Wan, D.; Qi, F.; Zhou, H.Y.; Zhao, M.; Duan, X.J.; Huang, Y.Q. Analysis of a typical fault of 10 kV power cable cluster burning. Hunan Electr. Power 2020, 40, 84–89. [Google Scholar]
- Lütjering, G. Influence of processing on microstructure and mechanical properties of (α + β) titanium alloys. Mater. Sci. Eng. A 1998, 243, 32–45. [Google Scholar] [CrossRef]
- Evans, W.J. Optimising mechanical properties in alpha + beta titanium alloys. Mater. Sci. Eng. A 1998, 243, 89–96. [Google Scholar] [CrossRef]
- Semiatin, S.L.; Seetharaman, V.; Weiss, I. Hot workability of titanium and titanium aluminide alloys—An overview. Mater. Sci. Eng. A 1998, 243, 1–24. [Google Scholar] [CrossRef]
- Wenga, T.; Chen, G.; Ma, W.; Yan, B. Study on corrosion kinetics of 310H in different simulated MSW combustion environment. The influence of SO2 and H2O on NaCl assisted corrosion. Corros. Sci. 2019, 154, 254–267. [Google Scholar] [CrossRef]
- Cao, M.; Liu, L.; Yu, Z.; Fan, L.; Ying, L.; Wang, F. Studies on the corrosion behavior of Fe-20Cr alloy in NaCl solution spray at 600 °C. Corros. Sci. 2018, 133, 165–177. [Google Scholar] [CrossRef]
- Meng, B.; Wang, J.L.; Yang, L.L.; Chen, M.H.; Zhu, S.L.; Wang, F.H. On the rumpling mechanism in nanocrystalline coatings: Improved by reactive magnetron sputtering with oxygen. J. Mater. Sci. Technol. 2023, 132, 69–80. [Google Scholar] [CrossRef]
- Meng, B.; Wang, J.L.; Yang, L.L.; Zhu, L.J.; Chen, M.H.; Zhu, S.L.; Wang, F.H. Influence of fuel combustion on the corrosion behavior of pipeline steels in fire flooding technology. NPJ Mater. Degrad. 2022, 21, 6–16. [Google Scholar] [CrossRef]
- Yang, L.L.; Zhou, Z.H.; Yang, R.Z.; Wang, J.L.; Chen, M.H.; Qiao, Y.X.; Zhu, S.L.; Wang, F.H. Effect of Al and Cr on the oxidation behavior of nanocrystalline coatings at 1050 °C. Corros. Sci. 2022, 200, 110191. [Google Scholar] [CrossRef]
- Huang, D.; Qiao, Y.X.; Yang, L.L.; Wang, J.L.; Chen, M.H.; Zhu, S.L.; Wang, F.H. Effect of shot peening of substrate surface on cyclic oxidation behavior of sputtered nanocrystalline coating. Acta Metall. Sin. 2022. [Google Scholar] [CrossRef]
- Zaghloul, M.Y.M.; Zaghloul, M.M.Y.; Zaghloul, M.M.Y. Developments in polyester composite materials—An in-depth review on natural fibres and nano fillers. Compos. Struct. 2021, 278, 114698. [Google Scholar] [CrossRef]
- Zaghloul, M.M.Y.; Mohamed, Y.S.; El-Gamal, H. Fatigue and tensile behaviors of fiber-reinforced thermosetting composites embedded with nanoparticles. J. Compos. Mater. 2019, 53, 709–718. [Google Scholar] [CrossRef]
- Zaghloul, M.M.Y.; Zaghloul, M.Y.M.; Zaghloul, M.M.Y. Experimental and modeling analysis of mechanical-electrical behaviors of polypropylene composites filled with graphite and MWCNT fillers. Polym. Test. 2017, 63, 467–474. [Google Scholar] [CrossRef]
- Zaghloul, M.M.Y.M. Mechanical properties of linear low-density polyethylene fire-retarded with melamine polyphosphate. J. Appl. Polym. Sci. 2018, 135, 46770. [Google Scholar] [CrossRef]
- Zaghloul, M.M.Y.; Zaghloul, M.M.Y. Influence of flame retardant magnesium hydroxide on the mechanical properties of high density polyethylene composites. J. Reinf. Plast. Compos. 2017, 36, 1802–1816. [Google Scholar] [CrossRef]
- Fuseini, M.; Zaghloul, M.M.Y. Investigation of Electrophoretic Deposition of PANI Nano fibers as a Manufacturing Technology for corrosion protection. Prog. Org. Coat. 2022, 171, 107015. [Google Scholar] [CrossRef]
- Li, R.; Wang, S.; Zhou, D.; Pu, J.; Yu, M.; Guo, W. A new insight into the NaCl-induced hot corrosion mechanism of TiN coatings at 500 °C. Corros. Sci. 2020, 174, 108794. [Google Scholar] [CrossRef]
- Li, R.; Wang, S.; Pu, J.; Zhou, D.; Yu, M.; Wei, Y.; Guo, W. Study of NaCl-induced hot-corrosion behavior of TiN single-layer and TiN/Ti multilayer coatings at 500 °C. Corros. Sci. 2021, 192, 109838. [Google Scholar] [CrossRef]
- Marco, J.F.; Agudelo, A.C.; Gancedo, J.R.; Hanzel, D. Corrosion resistance of single TiN Layers, Ti/TiN bilayers and Ti/TiN/Ti/TiN multilayers on iron under a salt fog spray (phohesion) test: An evaluation by XPS. Surf. Interface Anal. 1999, 27, 71–75. [Google Scholar] [CrossRef]
- Herranen, M.; Wiklund, U.; Carlsson, J.-O.; Hogmark, S. Corrosion behaviour of Ti/TiN multilayer coated tool steel. Surf. Coat. Technol. 1998, 99, 191–196. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, G.; Li, Z.; Ruan, H.; Yuan, J.; Wang, L.; Ke, P.; Wang, A. Corrosion mechanism of Ti2AlC MAX phase coatings under the synergistic effects of water vapor and solid NaCl at 600 °C. Corros. Sci. 2021, 192, 109788. [Google Scholar] [CrossRef]
- Liao, Y.; Zhang, B.; Chen, M.; Feng, M.; Wang, J.; Zhu, S.; Wang, F. Self-healing metal-enamel composite coating and its protection for TiAl alloy against oxidation under thermal shock in NaCl solution. Corros. Sci. 2020, 167, 108526. [Google Scholar] [CrossRef]
- Yu, Z.D.; Chen, M.H.; Chen, K.; Xie, D.B.; Zhu, S.L.; Wang, F.H. Corrosion of enamel with and without CaF2 in molten aluminum at 750 °C. Corros. Sci. 2019, 148, 228–236. [Google Scholar] [CrossRef]
- Chen, K.; Chen, M.H.; Yu, Z.D.; Wang, Q.C.; Li, X.X.; Zhu, S.L.; Wang, F.H. Corrosion of SiO2-B2O3-Al2O3-CaF2-R2O (R = Na and K) enamels with different content of ZrO2 in H2SO4 and NaOH solutions. Ceram. Int. 2019, 45, 14958–14967. [Google Scholar] [CrossRef]
- Wu, M.Y.; Chen, M.H.; Zhu, S.L.; Wang, F.H. Protection mechanism of enamel-alumina composite coatings on a Cr-rich nickel-based superalloy against high-temperature oxidation. Surf. Coat. Technol. 2016, 285, 57–67. [Google Scholar] [CrossRef]
- Li, W.B.; Chen, K.; Liu, L.L.; Yang, Y.F.; Zhu, S.L. Effect of SiO2-Al2O3 glass composite coating on the oxidation behavior of Ti60 alloy. Materials 2020, 13, 5085. [Google Scholar] [CrossRef] [PubMed]
- Barin, I. Thermochemical Data of Pure Substance, 3rd ed.; Wiley-VCH: Weinheim, Germany, 1995. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Feng, M.; Liu, L.; Zhang, J.; Hu, B.; Cheng, Q. Corrosion Behavior of SiO2-Al2O3 Glass Composite Coating on TC4 in Marine Environment. Coatings 2022, 12, 1503. https://doi.org/10.3390/coatings12101503
Li W, Feng M, Liu L, Zhang J, Hu B, Cheng Q. Corrosion Behavior of SiO2-Al2O3 Glass Composite Coating on TC4 in Marine Environment. Coatings. 2022; 12(10):1503. https://doi.org/10.3390/coatings12101503
Chicago/Turabian StyleLi, Wenbo, Min Feng, Lanlan Liu, Jun Zhang, Bo Hu, and Qiao Cheng. 2022. "Corrosion Behavior of SiO2-Al2O3 Glass Composite Coating on TC4 in Marine Environment" Coatings 12, no. 10: 1503. https://doi.org/10.3390/coatings12101503
APA StyleLi, W., Feng, M., Liu, L., Zhang, J., Hu, B., & Cheng, Q. (2022). Corrosion Behavior of SiO2-Al2O3 Glass Composite Coating on TC4 in Marine Environment. Coatings, 12(10), 1503. https://doi.org/10.3390/coatings12101503




