Bonding Mechanism and Process Characteristics of Special Polymers Applied in Pelletizing Binders
Abstract
:1. Introduction
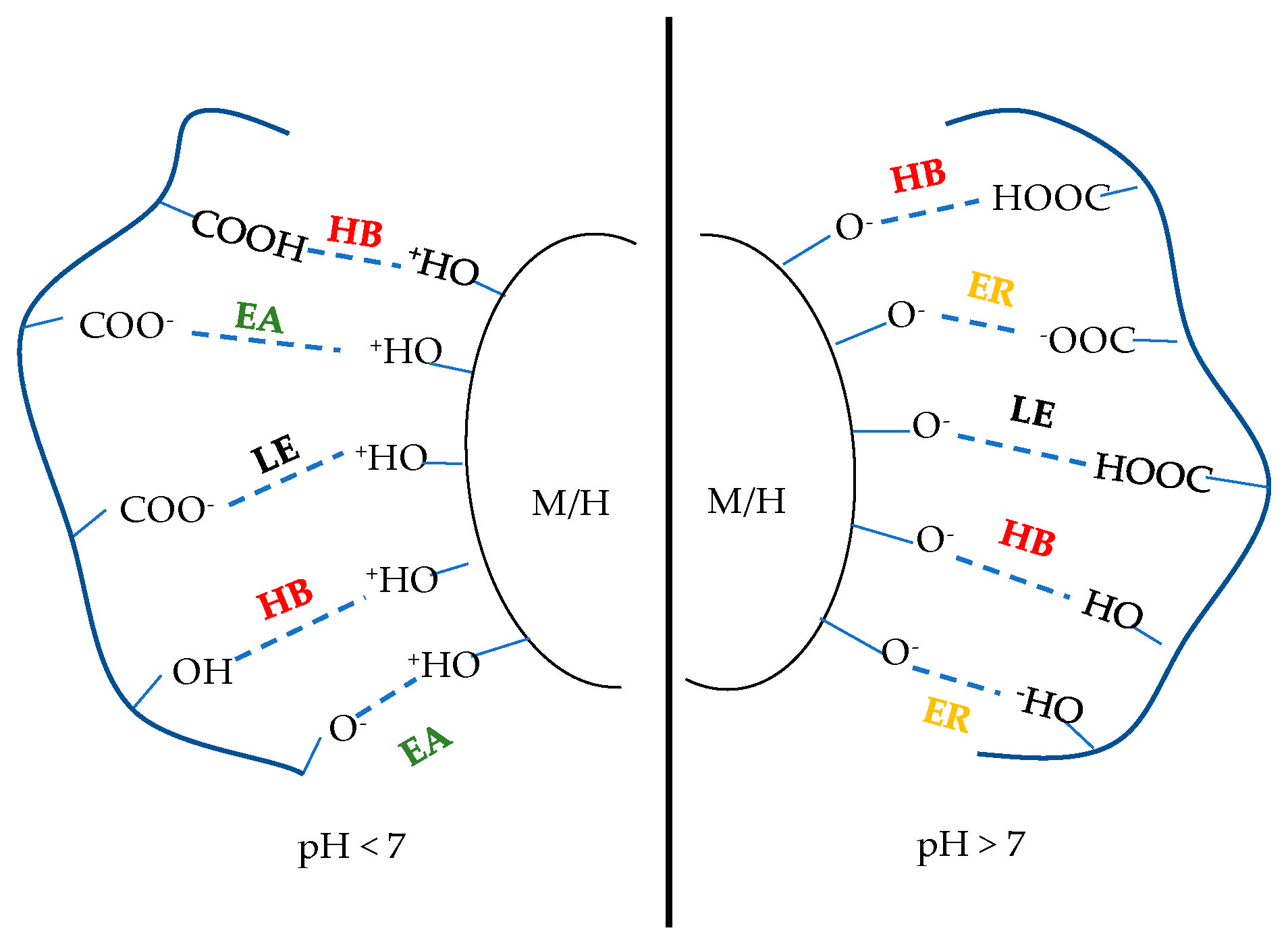
2. The Binding Mechanism of Organic Binder in the Pellets
3. Organic Polymers
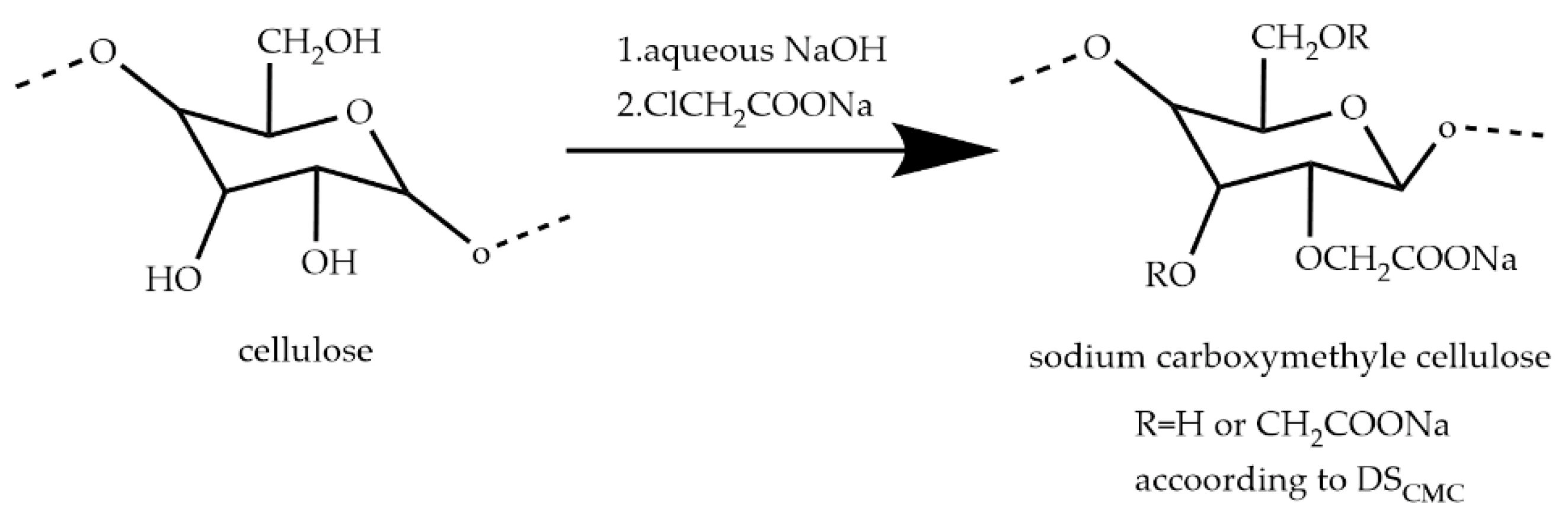
3.1. Carboxymethyl Cellulose (CMC)
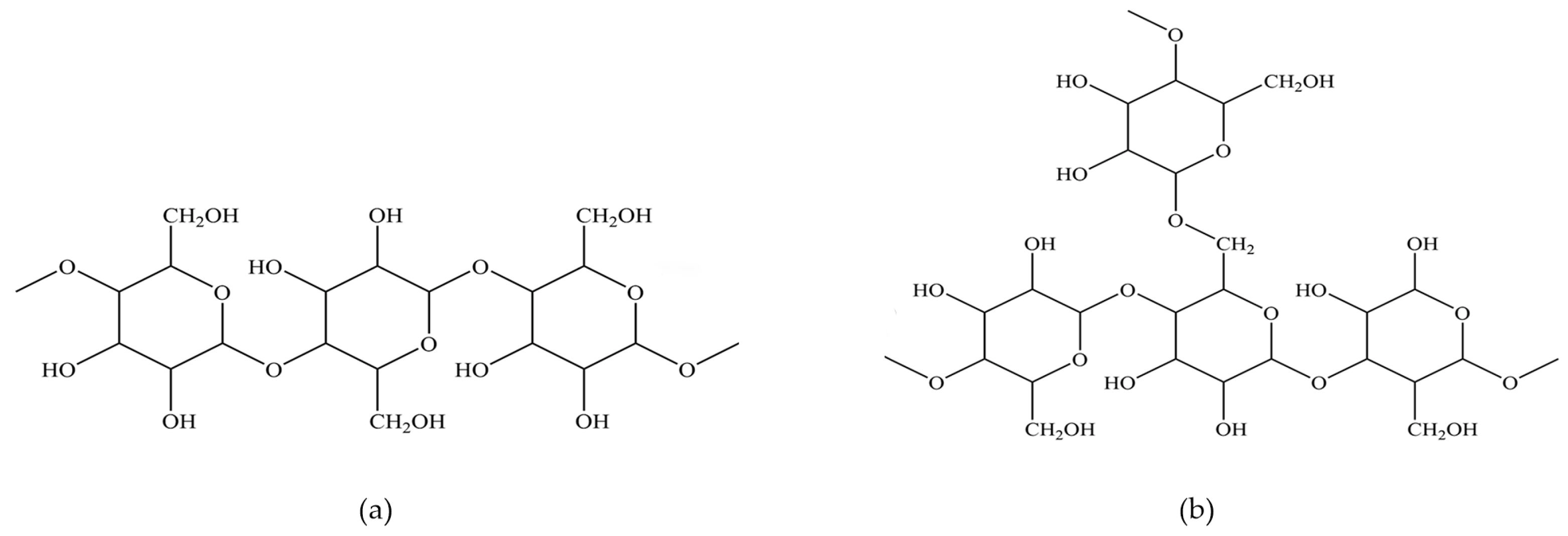
3.1.1. The Interaction between CMC and Minerals
3.1.2. The Application Effect of CMC in the Pellets
| CMC and Other Additives (wt.%) | Green Pellet Drop Number (times/P) | Dry Pellet Compressive Strength (N/P) | Fired Pellet Compressive Strength (N/P) | Ref. | |
|---|---|---|---|---|---|
| CMC1 Dp * = 1825, DS = 0.65 | 0.1 | 4.7 | [33] | ||
| 0.2 | 5.1 | ||||
| 0.3 | 7.8 | ||||
| CMC2 Dp = 560, DS * = 0.51 | 0.1 | 1.3 | |||
| 0.2 | 2.2 | ||||
| 0.3 | 3.1 | ||||
| CMCD Dp = 76, DS = 0.46 | 0.1 | 1.3 | 9.1 | [35] | |
| 0.2 | 2.2 | 9.9 | |||
| CMCS Dp = 552, DS = 0.51 | 0.1 | 3.1 | 11.3 | ||
| 0.2 | 5.1 | 12.9 | |||
| CMCT Dp = 1504, DS = 0.65 | 0.1 | 4.7 | 12.5 | ||
| 0.2 | 7.8 | 13.3 | |||
| 0.15 wt.% CMC | 0 | 4.1 | 56.7 | 2655 | [38] |
| 0.03 wt.% Na2CO3 | 6.6 | 60.75 | 3163.5 | ||
| 0.06 wt.% Na2CO3 | 5.5 | 69.3 | 3136.5 | ||
| 0.03 wt.% NaCl | 5.3 | 49.5 | 3069 | ||
| 0.03 wt.% Na2CO3 + 0.03 wt.% NaCl | 14.7 | 87.75 | 2916 | ||
| 0.04 wt.% CMC | 0 | 2.4 | 15.9 | [43] | |
| 0.02 wt.% NaOH | 2.4 | 19.1 | |||
| 0.02 wt.% TPP | 2.9 | 21.5 | |||
| 0.1 wt.% CMC | 0.25 wt.% Calcined colemanite | 3.0 | 6.7 | 4551.12 | [44] |
| 0.50 wt.% Calcined colemanite | 2.7 | 9.1 | 4557 | ||
| 0.75 wt.% Calcined colemanite | 2.9 | 5.9 | 4968.6 | ||
| 1.00 wt.% Calcined colemanite | 3.0 | 6.1 | 5001.92 | ||
| 0.1 wt.% CMC | 0.50 wt.% boric acid | 3920 | [45] | ||
| 0.75 wt.% boric acid | 4385.5 | ||||
| 1.00 wt.% boric acid | 4434.5 | ||||
3.2. Starch
3.2.1. The Structure and Properties of Starch
3.2.2. The Application Effect of Starch in the Pellets
| Starch and Other Additives (wt.%) | Green Pellet Drop Number (times/P) | Dry Pellet Compressive Strength (N/P) | Fired Pellet Compressive Strength (N/P) | Ref. | |
|---|---|---|---|---|---|
| Corn starch | 0.5 | 4 | 110.25 | 2320.44 | [64] |
| 1.0 | 5 | 90.98 | 1924.13 | ||
| 1.5 | 5 | 97.02 | 1294.78 | ||
| 2.0 | 6 | 81.21 | 886.9 | ||
| 0.145 wt.% 7.5% solubility starch + 0.435 wt.% Bent. | 7.13 | 61.79 | 3731.86 | [63] | |
| 0.333 wt.% 7.5% solubility starch + 0.333 wt.% Bent. | 5.50 | 70.73 | 3648.09 | ||
| 0.435 wt.% 7.5% solubility starch + 0.145 wt.% Bent. | 7.48 | 84.42 | 3812.13 | ||
| 0.66 wt.% 7.5% solubility starch | 6.92 | 82.91 | 3626.42 | ||
| 0.145 wt.% 60% solubility starch + 0.435 wt.% Bent. | 19.90 | 133.97 | 3542.65 | ||
| 0.333 wt.% 60% solubility starch + 0.333 wt.% Bent. | 19.81 | 187.50 | 3750.04 | ||
| 0.435 wt.% 60% solubility starch + 0.145 wt.% Bent. | 20.19 | 267.13 | 3622.58 | ||
| 0.66 wt.% 60% solubility starch | 19.85 | 305.43 | 3786.61 | ||
| 0.1 wt.% Corn starch | 0.25 wt.% Calcined colemanite | 3.0 | 6.7 | 4551.12 | [44] |
| 0.50 wt.% Calcined colemanite | 2.7 | 9.1 | 4557 | ||
| 0.75 wt.% Calcined colemanite | 2.9 | 5.9 | 4968.6 | ||
| 1.00 wt.% Calcined colemanite | 3.0 | 6.1 | 5001.92 | ||
3.3. Humic Acids (HA)
3.3.1. The Interaction between HA/FA and Minerals
3.3.2. The Application Effect of HA and FA in the Pellets
| MHA with Other Additives (wt.%) | Green Pellet Drop Number (times/P) | Dry Pellet Compressive Strength (N/P) | Fired Pellet Compressive Strength (N/P) | Ref. | |
|---|---|---|---|---|---|
| MHA (HA:FA = 7:3) | 0.25 | 8.2 | 57 | 3185.12 | [78] |
| 0.50 | 11.7 | 116 | 3101.43 | ||
| 0.75 | 13.5 | 132 | 2918.16 | ||
| 1.00 | 17.9 | 167 | 2757.51 | ||
| MHA | 0.75 | 3.3 | 2204.61 | [79] | |
| MHA | 0.25 | 5.2 | 2912.39 | [80] | |
| 0.50 | 9.6 | 2813.37 | |||
| 0.75 | 13.7 | 2652.41 | |||
| 1.00 | 18.4 | 2572.64 | |||
| 0.5 wt.% MHA | 4.14 | 131.55 | 3353.67 | [81] | |
| 0.333 wt.% MHA + 0.167 wt.% Ca-Bent.#1 | 5.84 | 149.47 | 3462.63 | ||
| 0.333 wt.% MHA + 0.167 wt.% Ca-Bent.#2 | 9.15 | 160.62 | 3631.51 | ||
3.4. Lignosulfonate
3.4.1. The Interaction between Lignosulfonate and Minerals
3.4.2. The Application Effect of HA and FA in the Pellets
| Lignosulfonate with Additives (wt.%) | Green Pellet Drop Number (times/P) | Dry Pellet Compressive Strength (N/P) | Fired Pellet Compressive Strength (N/P) | Ref. | |
|---|---|---|---|---|---|
| ALS * | 0.1 | 3.4 | 26.55 | 3115 | [38] |
| ALS | 0.2 | 3.5 | 42.30 | ||
| CLS * | 0.24 | 3.1 | 35.10 | 2042.55 | |
| CLS | 0.50 | 5.8 | 122.40 | ||
| SLS | 0.5 | 3.16 | 21.79 | 1977.15 | [89] |
| 1.0 | 4.67 | 41.52 | 2217.64 | ||
| 2.0 | 11.92 | 91.82 | 1452.91 | ||
| 0.75 wt.% CaCO3 | 0.5 wt.% SLS | 4.21 | 23.72 | 2609.58 | |
| 0.75 wt.%SLS | 5.22 | 28.58 | 2734.16 | ||
| 1.0 wt.% SLS | 6.10 | 36.04 | 2701.24 | ||
| 0.75 wt.% Bentonite | 0.5 wt.% SLS | 3.88 | 23.37 | 2639.09 | |
| 0.75 wt.%SLS | 4.88 | 28.42 | 2750.52 | ||
| 1.0 wt.% SLS | 5.67 | 34.44 | 2689.74 | ||
| 0.5 wt.% SLS * | 0.5 wt.% Cu-S | 8 | 49 | 3088.10 | [92] |
| 2.0 wt.% Cu-S | 7 | 50.96 | 2994.89 | ||
| 1 wt.% Cu-S * | 0.5 wt.% SLS | 12.4 | 80.26 | 7708.36 | |
| 1.0 wt.% SLS | 19.4 | 93.20 | 8950.73 | ||
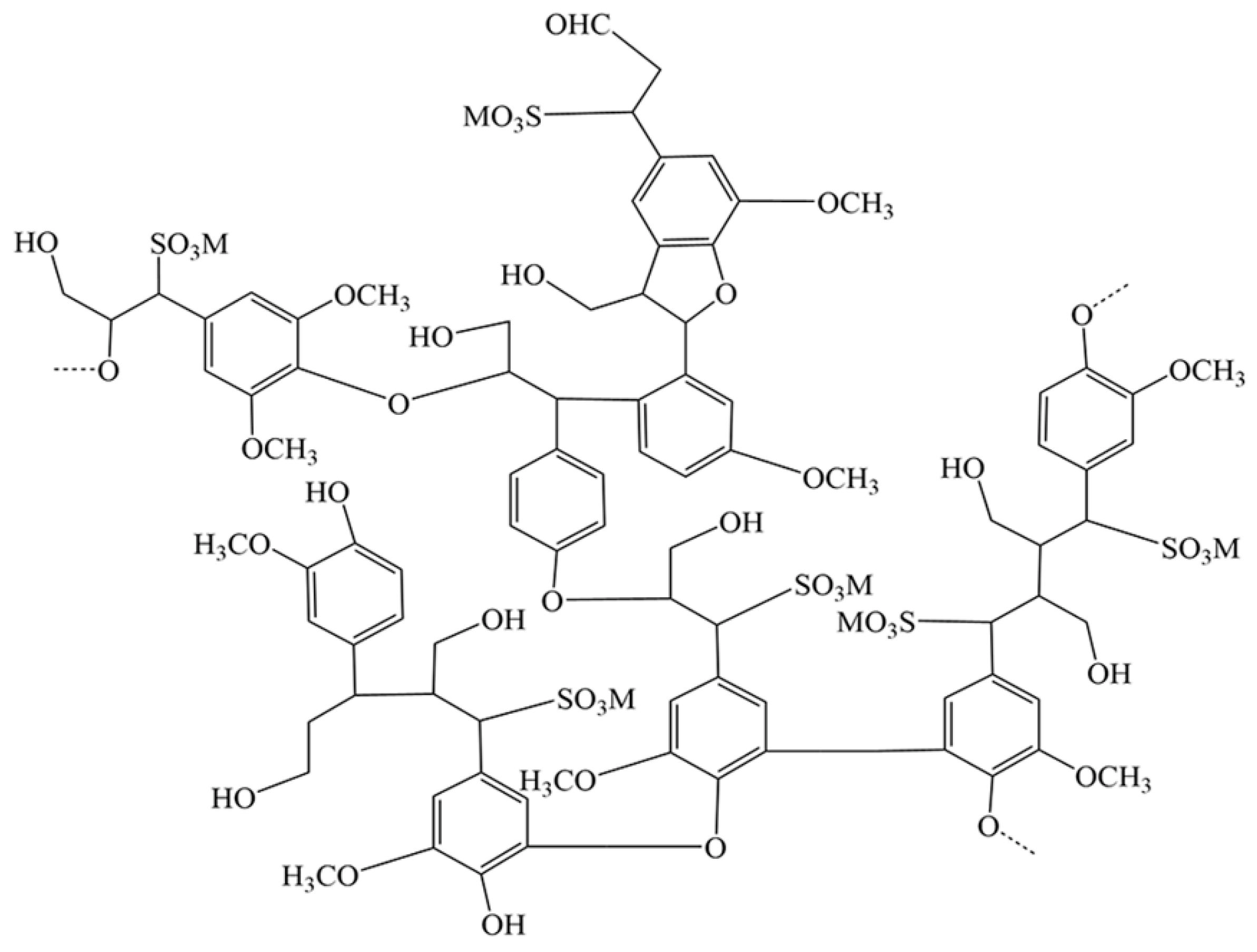
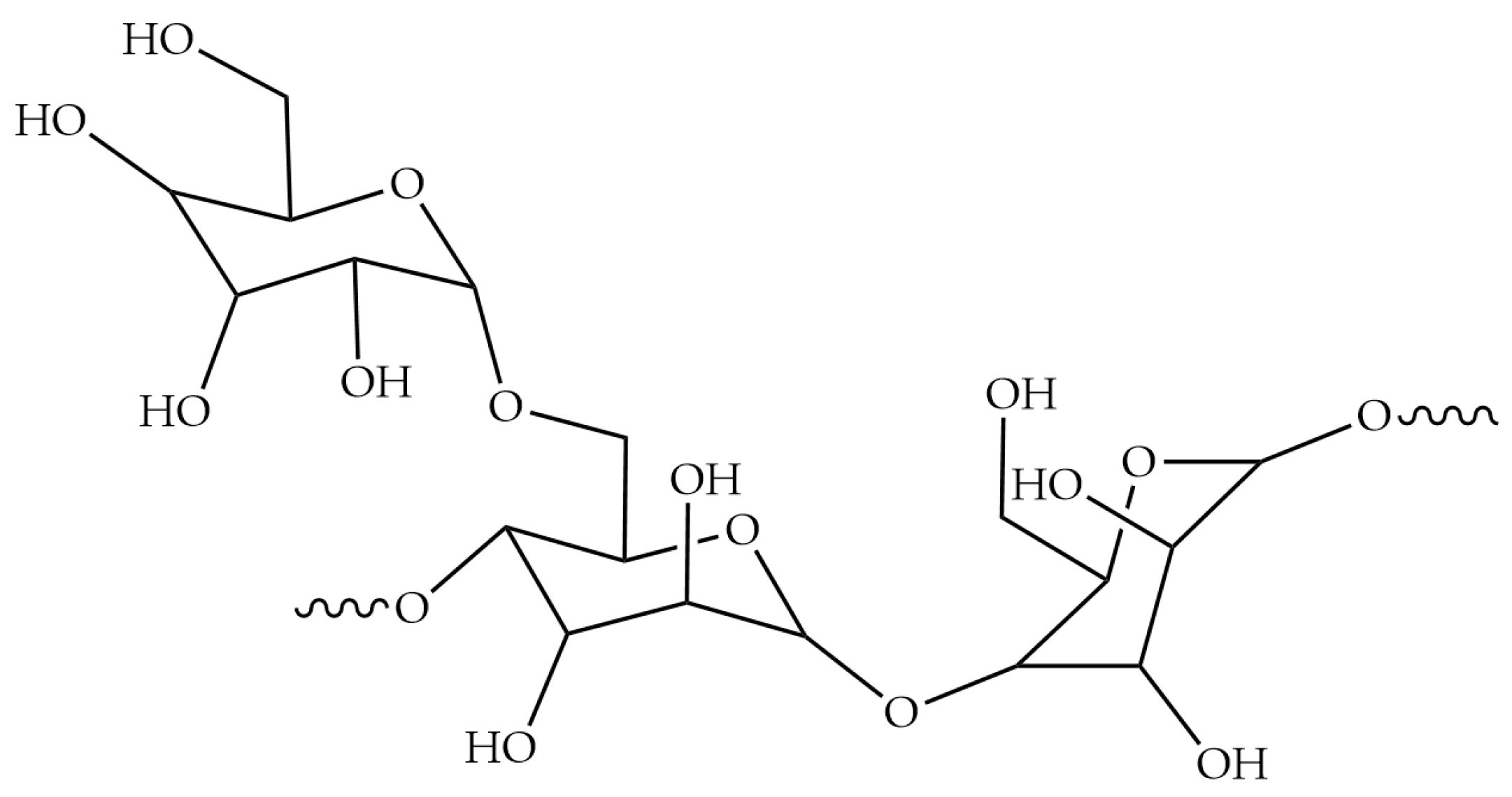
3.5. Guar Gum
3.5.1. The Structure, Properties and Application of Guar Gum
3.5.2. The Interaction between Guar Gum and Minerals
3.6. Polyacrylamide
3.6.1. The Interaction between Polyacrylamide and Minerals
3.6.2. The Application Effect of Polyacrylamide in the Pellets
3.7. Molasses
3.7.1. The Properties and Application of Molasses
3.7.2. The Interaction between Molasses and Minerals
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forsmo, S.P.E.; Apelqvist, A.J.; Björkman, B.M.T.; Samskog, P.O. Binding mechanisms in wet iron ore green pellets with a bentonite binder. Powder Technol. 2006, 169, 147–158. [Google Scholar] [CrossRef]
- Wang, R.-R.; Zhang, J.-L.; Liu, Y.-R.; Liu, Z.-J.; Liu, X.-L.; Li, N.-Y. Effects of an inorganic binder on the strength property of cold-bonded pellets. J. Rev. De Metall. Cah. D Inf. Tech. 2017, 114, 604. [Google Scholar] [CrossRef]
- Fan, X.H.; Gan, M.; Jiang, T.; Chen, X.L.; Yuan, L.-S. Decreasing bentonite dosage during iron ore pelletising. J. Ironmak. Steelmak. 2011, 38, 597–601. [Google Scholar]
- Souza, R.P.D.; Mendonca, C.F.D.; Kater, T. Production of acid iron ore pellet for direct reduction, using an organic binder. Min. Eng. 1984, 36, 1437–1441. [Google Scholar]
- Kater, T.; Steeghs, R. Organic Binders for Iron Ore Pelletizing; Mining Symposium: Duluth, MN, USA, 1984; pp. 1–28. [Google Scholar]
- Claremboux, V.; Kawatra, S.K. Iron ore pelletization: Part III. organic binders. Miner. Process. Extr. Metall. Rev. 2022, 1–17. [Google Scholar] [CrossRef]
- Engelleitner, W.H. Binders: How they work and how to select one. Powder Bulk Eng. 2001, 15, 31–37. [Google Scholar]
- Zhu, D.Q.; Pan, J.; Lu, L.; Holmes, R.J. Iron Ore Pelletization. In Iron Ore; Lu, L., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 435–473. [Google Scholar]
- Kawatra, S.K.; Claremboux, V. Iron ore pelletization: Part II. Inorganic binders. Miner. Process. Extr. Metall. Rev. 2022, 43, 813–832. [Google Scholar] [CrossRef]
- Prusti, P.; Barik, K. Effect of additives concentration on pelletization of high grade hematite. Mater. Today: Proc. 2020, 33, 5373–5377. [Google Scholar] [CrossRef]
- Liu, H.; Xie, B.; Qin, Y.L. Effect of bentonite on the pelleting properties of iron concentrate. J. Chem. 2017, 2017, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Kawatra, S.K.; Claremboux, V. Iron ore pelletization: Part I. fundamentals. Miner. Process. Extr. Metall. Rev. 2021, 1–16. [Google Scholar] [CrossRef]
- Butt, H.J.; Kappl, M. Normal capillary forces. Adv. Colloid Interface Sci. 2009, 146, 48–60. [Google Scholar] [CrossRef]
- Zhu, D.Q.; Pan, J.; Lu, L.M.; Holmes, R.J. Iron Ore Pelletization. In Iron Ore, 2nd ed.; Lu, L., Ed.; Woodhead Publishing: Sawston, UK, 2022; pp. 539–578. [Google Scholar]
- Qiu, G.Z.; Jiang, T.; Fan, X.H.; Zhu, D.Q.; Huang, Z. Effects of binders on balling behaviors of iron ore concentrates. Scand. J. Metall. 2004, 33, 39–46. [Google Scholar] [CrossRef]
- Vermeer, A.W.P.; van Riemsdijk, W.H.; Koopal, L.K. Adsorption of humic acid to mineral particles. 1. specific and electrostatic interactions. Langmuir 1998, 14, 2810–2819. [Google Scholar] [CrossRef]
- Gu, B.; Schmitt, J.; Chen, Z.H.; Liang, L.Y.; McCarthy, J.F. Adsorption and desorption of natural organic matter on iron oxide: Mechanisms and models. J Environ. Sci. Technol. 1994, 28, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Li, P.; Zhou, Y.L.; Han, G.H.; Li, G.H.; Xu, B.; Jiang, T. Adsorption of lignite humic acid onto magnetite particle surface. J. Cent. South Univ. 2012, 19, 1967–1972. [Google Scholar] [CrossRef]
- Yang, K.; Lin, D.; Xing, B. Interactions of humic acid with nanosized inorganic oxides. Langmuir ACS J. Surf. Colloids Surf. A: Physicochem. Eng. Asp. 2009, 25, 3571–3576. [Google Scholar] [CrossRef] [PubMed]
- Casey, L. Organic Binders for Iron Ore Pelletization. Master’s Thesis, Aalto University, Helsinki, Espoo, Finland, 2016. [Google Scholar]
- Qiu, G.Z.; Jiang, T.; Li, H.X.; Wang, D.Z. Functions and molecular structure of organic binders for iron ore pelletization. Colloids Surf. A: Physicochem. Eng. Asp. 2003, 224, 11–22. [Google Scholar] [CrossRef]
- Forsmo, S. Influence of Green Pellet Properties on Pelletizing of Magnetite Iron Ore. Ph.D. Thesis, Luleå Tekniska Universitet, Luleå, Sweden, 2007. [Google Scholar]
- Goetzman, H.; Bleifuss, R.; Engesser, J. Investigation of carboxymethyl cellulose binders for taconite pelletizing. In Proceedings of the SME Annual Meeting, Phoenix, AZ, USA, 25 January 1988; pp. 88–111. [Google Scholar]
- Forsmo, S.P.E.; Samskog, P.O.; Björkman, B.M.T. A study on plasticity and compression strength in wet iron ore green pellets related to real process variations in raw material fineness. Powder Technol. 2008, 181, 321–330. [Google Scholar] [CrossRef]
- Halt, J.A.; Kawatra, S.K. Review of organic binders for iron ore concentrate agglomeration. Min. Metall. Explor. 2014, 31, 73–94. [Google Scholar] [CrossRef]
- Sunde, M. Organic Binder as a Substitute for Bentonite in Ilmenite Pelletization. Master’s Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2012. [Google Scholar]
- Potapova, E.; Yang, X.; Grahn, M.; Holmgren, A.; Forsmo, S.P.E.; Fredriksson, A.; Hedlund, J. The effect of calcium ions, sodium silicate and surfactant on charge and wettability of magnetite. Colloids Surf. A Physicochem. Eng. Asp. 2011, 386, 79–86. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hasan, M.S.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, M.S.; Shiddiky, M.J.A.; Ahmed, M.B. Recent developments of carboxymethyl cellulose. Polymers 2021, 13, 1345. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.Y.; Zhang, H.Z.; Ma, Y.; Song, D.W.; Shi, X.X.; Zhang, L.Q.; Zhou, Y. Polyglutamic acid binder for high-performance lithium-sulfur batteries. Coatings 2022, 12, 1433. [Google Scholar] [CrossRef]
- Pushpamalar, V.; Langford, S.J.; Ahmad, M.; Lim, Y.Y. Optimization of reaction conditions for preparing carboxymethyl cellulose from sago waste. Carbohydr. Polym. 2006, 64, 312–318. [Google Scholar] [CrossRef]
- Hoogendam, C.W.; de Keizer, A.; Cohen Stuart, M.A.; Bijsterbosch, B.H.; Batelaan, J.G.; van der Horst, P.M. Adsorption mechanisms of carboxymethyl cellulose on mineral surfaces. Langmuir 1998, 14, 3825–3839. [Google Scholar] [CrossRef]
- Saha, B.; Patra, A.S.; Mukherjee, A.K.; Paul, I. Interaction and thermal stability of carboxymethyl cellulose on alpha-Fe2O3(001) surface: ReaxFF molecular dynamics simulations study. J. Mol. Graph. Model. 2021, 102, 107787. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-M.; Fan, X.-H.; Chen, X.-L.; Yuan, L.-S.; Huang, X.-X.; Li, X. Interaction mechanism between carboxylmethyl cellulose and iron ore concentrates in iron ore agglomeration. J. Cent. South Univ. 2015, 22, 1241–1246. [Google Scholar] [CrossRef]
- Van Der Merwe, M.; Garbers-Craig, A. Influence of a carboxymethyl cellulose (CMC) binder on the mechanical properties of iron ore pellets. J. South. Afr. Inst. Min. Met. 2017, 117, 337–341. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.-H.; Yang, G.-M.; Chen, X.-L.; He, X.-N.; Huang, X.-X.; Gao, L. Effect of carboxymethyl cellulose on the drying dynamics and thermal cracking performance of iron ore green pellets. Powder Technol. 2014, 267, 11–17. [Google Scholar] [CrossRef]
- De Moraes, S.L.; Lima, J.R.B.D.; Neto, J.B.F.; Fredericci, C.; Saccoccio, E.M. Binding mechanism in green iron ore pellets with an organic binder. Miner. Process. Extr. Metall. Rev. 2020, 41, 247–254. [Google Scholar] [CrossRef]
- Zhao, H.X.; Zhou, F.S.; Evelina, L.M.A.; Liu, J.L.; Zhou, Y. A review on the industrial solid waste application in pelletizing additives: Composition, mechanism and process characteristics. J. Hazard. Mater. 2022, 423, 127056. [Google Scholar] [CrossRef]
- Haas, L.A.; Aldinger, J.A.; Zahl, R.K. Effectiveness of Organic Binders for Iron Ore Pelletization; US Department of the Interior, Bureau of Mines: Pittsburgh, PA, USA, 1989. [Google Scholar]
- Song, W.; Luo, G.P.; Sun, C.C.; Zhang, J.; Zhu, J.G. Effect of K and Na on reduction swelling performance of oxidized roasted briquettes. High Temp. Mater. Process. 2021, 40, 241–252. [Google Scholar] [CrossRef]
- Makara, A.; Smol, M.; Kulczycka, J.; Kowalski, Z. Technological, environmental and economic assessment of sodium tripolyphosphate production-a case study. J. Clean. Prod. 2016, 133, 243–251. [Google Scholar] [CrossRef]
- Wang, X.; Jia, W.H.; Yang, C.R.; He, R.; Jiao, F.; Qin, W.Q.; Cui, Y.F.; Zhang, Z.Q.; Li, W.; Song, H. Innovative application of sodium tripolyphosphate for the flotation separation of scheelite from calcite. Miner. Eng. 2021, 170, 106981. [Google Scholar] [CrossRef]
- Cassola, M.S.; Chaves, A.P. Effect of the addition of organic binders on the behavior of iron ore pellets. KONA Powder Part. J. 1998, 16, 136–142. [Google Scholar] [CrossRef] [Green Version]
- De Moraes, S.L.; Kawatra, S.K. Laboratory study of an organic binder for pelletization of a magnetite concentrate. Min. Metall. Explor. 2010, 27, 148–153. [Google Scholar] [CrossRef]
- Sivrikaya, O.; Arol, A.I. Pelletization of magnetite ore with colemanite added organic binders. Powder Technol. 2011, 210, 23–28. [Google Scholar] [CrossRef]
- Kumar, S.; Suman, S.K. Compressive strength of fired pellets using organic binder: Response surface approach for analyzing the performance. Trans. Indian Inst. Met. 2018, 71, 1629–1634. [Google Scholar] [CrossRef]
- Sivrikaya, O.; Arol, A. Use of Organic Binders and Borates in Pelletizing of Iron Oxides. In Proceedings of the IV International Boron Symposium, Eskişehir, Turkey, 15–17 October 2009. [Google Scholar]
- Sivrikaya, O.; Arol, A.I. Use of boron compounds as binders in iron ore pelletization. Open Miner. Process. J. 2010, 3, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Sivrikaya, O.; Arol, A. The bonding/strengthening mechanism of colemanite added organic binders in iron ore pelletization. Int. J. Miner. Process. 2012, s110–s111, 90–100. [Google Scholar] [CrossRef]
- Guo, H.W.; Bai, J.L.; Zhang, J.L.; Li, H.G. Mechanism of strength improvement of magnetite pellet by adding boron-bearing iron concentrate. J. Iron Steel Res. Int. 2014, 21, 9–15. [Google Scholar] [CrossRef]
- Zhuchkov, V.; Zayakin, O.; Akberdin, A. Prospects for using boron in metallurgy. report Izvestiya. Ferr. Metall. 2021, 64, 471–476. [Google Scholar] [CrossRef]
- Dolitskaya, O.A. Effect of mineral additives on the strengthening of pellets of skarn-type ores. Russ. Metall. 2001, 2001, 230–232. [Google Scholar]
- Malysheva, T.; Chesnokova, G.; Akberdin, A.; Dolitskaya, O. Effect of boron on the quality of iron ore pellets. Russ. Metall. Met. 1996, 1, 1–4. [Google Scholar]
- BeMiller, J.N.; Huber, K.C. Starch. Ullmann’s Encycl. Ind. Chem. 2011, 32, 113–141. [Google Scholar] [CrossRef]
- Whistler, R.L.; Daniel, J.R. Starch. Kirk-Othmer Encycl. Chem. Technol. 2000, 1–18. [Google Scholar] [CrossRef]
- Swinkels, J.J.M. Composition and properties of commercial native starches. Starch-Stärke 1985, 37, 1–5. [Google Scholar] [CrossRef]
- Ratnayake, W.S.; Jackson, D.S. Starch Gelatinization. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2008; Volume 55, pp. 221–268. [Google Scholar]
- Ai, Y.F.; Jane, J.L. Gelatinization and rheological properties of starch. Starch 2015, 67, 213–224. [Google Scholar] [CrossRef]
- Song, X.Y. Effect of storage conditions on the physicochemical characteristics of bilayer edible films based on iron Yam-pea starch blend and corn zein. Coatings 2022, 12, 1524. [Google Scholar] [CrossRef]
- Li, N.N.; Zhou, Z.G.; Wu, F.Q.; Lu, Y.Y.; Jiang, D.Y.; Zhong, L.; Xie, F.W. Development of pH-indicative and antimicrobial films based on polyvinyl alcohol/starch incorporated with ethyl lauroyl arginate and mulberry anthocyanin for active packaging. Coatings 2022, 12, 1392. [Google Scholar] [CrossRef]
- Halt, J.A. Controlling Properties of Agglomerates for Chemical Processes. Ph.D. Thesis, Michigan Technological University, Houghton, MI, USA, 2017. [Google Scholar]
- Halt, J.A.; Kawatra, S.K. Can modified starch be used as a binder for iron ore pellets? Miner. Process. Extr. Metall. Rev. 2017, 38, 73–82. [Google Scholar] [CrossRef]
- McDonald, J. Advances in Alternative Binders for Iron Ore Pellets. Ph.D. Thesis, Michigan Technological University, Houghton, MI, USA, 2017. [Google Scholar]
- McDonald, J.E.D.; Kawatra, S.K. Agglomeration of hematite concentrate by starches. Miner. Process. Extr. Metall. Rev. 2017, 38, 1–6. [Google Scholar] [CrossRef]
- Mishra, B.; Dishwar, R.K.; Omar, R.J.; Mahobia, G.S. Hardening behaviour of pellets prepared from a novel combination of rare multimetallic magnetite ore and binder. Trans. Indian Inst. Met. 2021, 74, 2049–2055. [Google Scholar] [CrossRef]
- Wang, J.; He, Y.Q.; Li, H.; Yu, J.D.; Xie, W.N.; Wei, H. The molecular structure of inner mongolia lignite utilizing XRD, solid state 13C NMR, HRTEM and XPS techniques. Fuel 2017, 203, 764–773. [Google Scholar] [CrossRef]
- Xiang, J.H.; Zeng, F.G.; Li, B.; Zhang, L.; Li, M.F.; Liang, H.Z. Construction of macromolecular structural model of anthracite from Chengzhuang coal mine and its molecular simulation. J. Fuel Chem. Technol. 2013, 41, 391–400. [Google Scholar] [CrossRef]
- Peuravuori, J.; Žbánková, P.; Pihlaja, K. Aspects of structural features in lignite and lignite humic acids. Fuel Process. Technol. 2006, 87, 829–839. [Google Scholar] [CrossRef]
- Schnitzer, M. Humic substances: Chemistry and reactions. In Developments in Soil Science; Schnitzer, M., Khan, S.U., Eds.; Elsevier: Amsterdam, The Netherlands, 1978; Volume 8, pp. 1–64. [Google Scholar]
- Han, G.H.; Huang, Y.; Li, G.H.; Zhang, Y.B.; Jiang, T. Detailed adsorption studies of active humic acid fraction of a new binder on iron ore particles. Miner. Process. Extr. Metall. Rev. 2013, 35, 1–14. [Google Scholar] [CrossRef]
- Han, G.H.; Su, S.P.; Cao, Y.J.; Huang, Y.F.; Song, X.Y. Research on the interaction of humic acid with iron minerals. In Characterization of Minerals, Metals, and Materials 2018; Springer: Cham, Switzerland, 2018; pp. 653–660. [Google Scholar]
- Jiang, T.; Han, G.H.; Zhang, Y.B.; Li, G.H.; Huang, Y.F. A further study on the interaction between one of organic active fractions of the MHA binder and iron ore surface. Int. J. Miner. Process. 2011, 100, 172–178. [Google Scholar] [CrossRef]
- Zhao, H.X.; Zhou, F.S.; Bao, X.C.; Zhou, S.H.; Wei, Z.J.; Long, W.J.; Zhou, Y. A review on the humic substances in pelletizing binders: Preparation, interaction mechanism, and process characteristics. ISIJ Int. 2022, advpub. [Google Scholar] [CrossRef]
- Han, G.H. Relationship between Structure and Performance of Humic Substances Based Binder for Iron Ore Pellets. Ph.D. Thesis, Central South University, Changsha, China, 2013. [Google Scholar]
- Zhou, Y.L.; Zhang, Y.B.; Li, P.; Li, G.H.; Jiang, T. Comparative study on the adsorption interactions of humic acid onto natural magnetite, hematite and quartz: Effect of initial HA concentration. Powder Technol. 2014, 251, 1–8. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Zhang, Y.B.; Li, G.H.; Jiang, T. Effects of metal cations on the fulvic acid (FA) adsorption onto natural iron oxide in iron ore pelletizing process. Powder Technol. 2016, 302, 90–99. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Kawatra, S.K. Humic substance-based binder in iron ore pelletization: A review. Miner. Process. Extr. Metall. Rev. 2017, 38, 321–337. [Google Scholar] [CrossRef]
- Qiu, G.Z.; Jiang, T.; Fa, K.Q.; Zhu, D.Q.; Wang, D.Z. Interfacial characterizations of iron ore concentrates affected by binders. Powder Technol. 2004, 139, 1–6. [Google Scholar] [CrossRef]
- Han, G.H.; Huang, Y.F.; Li, G.H.; Zhang, Y.B.; Zhou, Y.L.; Jiang, T. Optimizing the mass ratio of two organic active fractions in modified humic acid (MHA) binders for iron ore pelletizing. ISIJ Int. 2012, 52, 378–384. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.L.; Zhang, Y.B.; Liu, B.B.; Li, G.H.; Jiang, T. Effect of modified humic acid binder on pelletisation of specularite concentrates. J. Cent. South Univ. 2015, 22, 1247–1255. [Google Scholar] [CrossRef]
- Han, G.H.; Zhang, Y.B.; Jiang, T.; Huang, Y.F.; Li, G.H. Effects of binders on oxidized pellets preparation from vanadium/titanium-bearing magnetite. In 2nd International Symposium on High-Temperature Metallurgical Processing, Hoboken, NJ, USA; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2011; pp. 279–287. [Google Scholar]
- Zhou, Y.L.; Kawatra, S.K. Pelletization using humic substance-based binder. Miner. Process. Extr. Metall. Rev. 2017, 38, 83–91. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Wattanaphan, P.; Kawatra, S.K. Application of modified humic acid (MHA) binder in the pelletizing of fluxed hematite concentrate. Miner. Process. Extr. Metall. Rev. 2017, 38, 126–131. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Lu, M.M.; Su, Z.J.; Wang, J.; Tu, Y.K.; Chen, X.J.; Cao, C.T.; Gu, F.Q.; Liu, S.; Jiang, T. Interfacial reaction between humic acid and Ca-montmorillonite: Application in the preparation of a novel pellet binder. Appl. Clay Sci. 2019, 180, 105177. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- White, J. Top Value-Added Chemicals from Biomass Volume II-Results of Screening for Potential Candidates from Biorefinery Lignin; Biomass Fuels; U.S. Department of Energy Office of Scientific and Technical Information: Richland, WA, USA, 2007; pp. 1–79. [Google Scholar]
- Ruwoldt, J. A critical review of the physicochemical properties of lignosulfonates: Chemical structure and behavior in aqueous solution, at surfaces and interfaces. Surfaces 2020, 3, 42. [Google Scholar] [CrossRef]
- Nanthakumar, B.; Arinaitwe, E.; Pawlik, M. Adsorption of sodium lignosulfonates on hematite. Adsorption 2010, 16, 447–455. [Google Scholar] [CrossRef]
- Stewart, D. Lignin as a base material for materials applications: Chemistry, application and economics. Ind. Crops Prod. 2008, 27, 202–207. [Google Scholar] [CrossRef]
- Ding, B. Study on the Binding Mechanisms Andexperiment of Sodium Lignosulfonate in Pellets. Master’s Thesis, Wuhan University of Science and Technology, Wuhan, China, 2018. [Google Scholar]
- Gorai, B.; Jana, R.K. Premchand, Characteristics and utilisation of copper slag-a review. Resour. Conserv. Recycl. 2003, 39, 299–313. [Google Scholar] [CrossRef]
- Phiri, T.C.; Singh, P.; Nikoloski, A.N. The potential for copper slag waste as a resource for a circular economy: A review-part II. Miner. Eng. 2021, 172, 107150. [Google Scholar] [CrossRef]
- Ammasi, A.; Pal, J. Replacement of bentonite in hematite ore pelletisation using a combination of sodium lignosulphonate and copper smelting slag. Ironmak. Steelmak. 2016, 43, 203–213. [Google Scholar] [CrossRef]
- Thombare, N.; Jha, U.; Mishra, S.; Siddiqui, M.Z. Guar gum as a promising starting material for diverse applications: A review. Int. J. Biol. Macromol. 2016, 88, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.W.; Wang, S.B.; Wang, C.A.; Zhao, F.; Lei, S.; Yi, H.Y.; Guo, J.C. Influence of nanomaterial morphology of guar-gum fracturing fluid, physical and mechanical properties. Carbohydr. Polym. 2020, 234, 115915. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar gum: Processing, properties and food applications—A review. J. Food Sci. Technol. 2014, 51, 409–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.D.; Pawlik, M. Adsorption of guar gum onto quartz from dilute mixed electrolyte solutions. J. Colloid Interface Sci. 2006, 298, 609–614. [Google Scholar] [CrossRef]
- Mhlanga, S.S.; O’Connor, C.T.; McFadzean, B. A study of the relative adsorption of guar onto pure minerals. Miner. Eng. 2012, 36–38, 172–178. [Google Scholar] [CrossRef]
- Lu, S.S.; Yuan, Z.T.; Zhang, C. Binding mechanisms of polysaccharides adsorbing onto magnetite concentrate surface. Powder Technol. 2018, 340, 17–25. [Google Scholar] [CrossRef]
- Xiong, B.; Loss, R.D.; Shields, D.; Pawlik, T.; Hochreiter, R.; Zydney, A.L.; Kumar, M.J.N.C.W. Polyacrylamide degradation and its implications in environmental systems. Clean Water 2018, 1, 1–9. [Google Scholar] [CrossRef]
- Kulicke, W.M.; Kniewske, R.; Klein, J. Preparation, characterization, solution properties and rheological behaviour of polyacrylamide. Prog. Polym. Sci. 1982, 8, 373–468. [Google Scholar] [CrossRef]
- Samsonova, N.S.; Il’chenko, L.G.; Gol’dman, M.M.; Ni, L.P. IR spectroscopic study of the adsorption of polyacrylamide on hematite. J. Appl. Spectrosc. 1975, 23, 963–966. [Google Scholar] [CrossRef]
- Lee, L.T.; Somasundaran, P. Adsorption of polyacrylamide on oxide minerals. Langmuir 1989, 5, 854–860. [Google Scholar] [CrossRef]
- Deng, Y.; Dixon, J.B.; White, G.N.; Loeppert, R.H.; Juo, A.S.R. Bonding between polyacrylamide and smectite. Colloids Surf. A: Physicochem. Eng. Asp. 2006, 281, 82–91. [Google Scholar] [CrossRef]
- Xiang, A.P. Study on Bentonite-Based Composite Binder for Iron Ore Pellets. Master’s Thesis, Anhui University of Technology, Ma’anshan, China, 2020. [Google Scholar]
- Chizhikova, V.M.; Vainshtein, R.M.; Zorin, S.N.; Zainetdinov, T.I.; Zinyagin, G.A.; Shevchenko, A.A. Production of iron ore pellets with an organic binder. Metallurgist 2003, 47, 141–146. [Google Scholar] [CrossRef]
- Wang, C.; Xu, C.Y.; Liu, Z.J.; Wang, Y.Z.; Wang, R.R.; Ma, L.M. Effect of organic binders on the activation and properties of indurated magnetite pellets. Int. J. Miner. Metall. Mater. 2021, 28, 1145–1152. [Google Scholar] [CrossRef]
- Palmonari, A.; Cavallini, D.; Sniffen, C.J.; Fernandes, L.; Holder, P.; Fagioli, L.; Fusaro, I.; Biagi, G.; Formigoni, A.; Mammi, L. Short communication: Characterization of molasses chemical composition. J. Dairy Sci. 2020, 103, 6244–6249. [Google Scholar] [CrossRef]
- Olbrich, H. The molasses. J Biotechnol. -Kempe GmbH 2006, 128, 1–131. [Google Scholar]
- Fahmy, T.Y. Introducing molasses as a new additive in papermaking. Tappi J. 2007, 8, 23–25. [Google Scholar]
- Kolemen, S.; Baran Acarali, N.; Tugrul, N.; Moroydor Derun, E.; Piskin, S. The zinc adsorption study by using orhaneli fly ash, bentonite, and molasses in wastewater. Water Air Soil Pollut. 2012, 224, 1367. [Google Scholar] [CrossRef]
- Zambrano, A.P.; Takano, C.; Mourão, M.B.; Tagusagawa, S.Y. Influence of the Binder on the Mechanical Properties of the Chromite Self-Reducing Pellets. Int. J. Bus. Humanit. Technol. 2013, 3, 99–108. [Google Scholar]
- Ripke, S.J. Advances in Iron Ore Pelletization by Understanding Bonding and Strengthening Mechanisms. Ph.D. Thesis, Michigan Technological University, Houghton, MI, USA, 2002. [Google Scholar]
- Kotta, A.B.; Patra, A.; Kumar, M.; Karak, S.K. Effect of molasses binder on the physical and mechanical properties of iron ore pellets. Int. J. Miner. Metall. Mater. 2019, 26, 41–51. [Google Scholar]
- Srivastava, U.; Kawatra, S.K.; Eisele, T.C. Study of organic and inorganic binders on strength of iron oxide pellets. Metall. Mater. Trans. B 2013, 44, 1000–1009. [Google Scholar] [CrossRef]
- Sah, R.; Dutta, S.K. Effects of binder on the properties of iron ore-coal composite pellets. Miner. Process. Extr. Metall. Rev. 2010, 31, 73–85. [Google Scholar] [CrossRef]
- Eisele, T.C.; Kawatra, S.K. A review of binders in iron ore pelletization. Miner. Process. Extr. Metall. Rev. 2003, 24, 1–90. [Google Scholar] [CrossRef]
| Guar Gum (wt.%) | Green Pellet Drop Number (times/P) | Dry Pellet Compressive Strength (N/P) | Fired Pellet Compressive Strength (N/P) | Ref. | |
|---|---|---|---|---|---|
| Guar Gum | 0.075 | 3.41 | 12.72 | [98] | |
| 0.125 | 7.06 | 27.49 | |||
| 0.200 | 18.44 | 67.71 | |||
| GG211 | 0.1 | 11.9 | 26.55 | 2898 | [38] |
| GG211D | 0.1 | 6.1 | 27.45 | 2056.5 | |
| GG416 | 0.05 | 8.2 | 25.65 | 2979 | |
| 0.1 | 54.6 | 54.45 | 2412 | ||
| PAM with Other Additives (wt.%) | Green Pellet Drop Number (times/P) | Dry Pellet Compressive Strength (N/P) | Fired Pellet Compressive Strength (N/P) | Ref. | |
|---|---|---|---|---|---|
| Superfloc A-150LMW | 0.05 | 3.4 | 4617.76 | [44] | |
| 0.10 | 5.0 | 3971.94 | |||
| Superfloc A-150HMW | 0.005 | 2.2 | 3624.04 | ||
| 0.010 | 2.0 | 3453.52 | |||
| Floform 1049 V | 0.02 | 1.4 | 8 | [105] | |
| 0.03 | 2.1 | 15 | |||
| 0.05 | 5.4 | 25 | |||
| 0.02 wt.% Aloctac | 0.6 wt.% Bentonite | 4.61 | 2977.53 | [106] | |
| 0.8 wt.% Bentonite | 4.83 | 2752.81 | |||
| 1.0 wt.% Bentonite | 8.23 | 2550.56 | |||
| Molasses with Other Additives (wt.%) | Green Pellet Drop Number (times/P) | Dry Pellet Compressive Strength (N/P) | Fired Pellet Compressive Strength (N/P) | Ref. | |
|---|---|---|---|---|---|
| Molasses | 2 | 5.51 | 37.5 | [113] | |
| 4 | 7.71 | 89.58 | |||
| 6 | 9.66 | 233.33 | |||
| 8 | 11.86 | 429.17 | |||
| 3 wt.% Molasses | 3 | 213.15 | 2387.1 | [114] | |
| 2 wt.% Molasses + 2 wt.% CaO | 4.15 | 92.09 | 1799.2 | ||
| Organic Binders | General Dosage (wt.%) | Green and Dry Pellet Compressive | Fired Pellet Compressive Strength |
|---|---|---|---|
| CMC | ≤0.5 | The larger DS and Dp in CMC will produce greater adsorption and cohesion forces with minerals, thus improving the green and dry pellet strength. | The pellet strength decreases with the increase in CMC. Sodium salt and boride improve the strength by lowering the melting point and generating a large amount of liquid phase through Na+ and B2O3, which enhances the solid phase bridging effect. |
| Starch | high solubility starch ≤ 0.5 | The higher the solubility of starch, the greater the dispersion and viscosity, and the higher the green and dry pellet strength. | The pellet strength decreases with the increase in starch. By adding bent. and calcined colemanite, the high temperature will generate sufficient liquid phase to produce a large number of solid-phase bridges. |
| Humic acids | ≥0.5 | The initial concentration of MHA, pH and metal cations significantly affects the adsorption of MHA with minerals, which further affects the green and dry pellet strength. | MHA contains trace amounts of inorganic components that produce a certain amount of liquid phase at high temperatures. The strength does not decrease sharply with the increasing content of MHA. |
| Lignosulfonate | ≥0.5 | Ligninsulfonate contains a lot of SO3-, which has good adsorption with minerals. The high viscosity can improve the green and dry pellet strength. | The high temperature will lead to volatilization. CaCO3, bentonite, and Cu -slag left inside will produce a sufficient solid phase, which can improve the pellet strength. |
| Guar Gum | ≤0.3 | Guar gum has a high viscosity, and mainly relies on hydroxyl and mineral adsorption. Green and dry pellet strength is high. | The contribution of guar gum to the pellet strength is almost small, and will be able to lead to a decrease. Only by maintaining a low level or adding inorganic minerals can we meet the basic production requirements. |
| Polyacrylamide | ≤0.05 | PAM has a variety of internal cathodic and anodic groups and large molecular weights, which have strong adsorption on minerals and can provide sufficient green and dry pellet strength. | The amount of polyacrylamide added to the pellets is usually small and has a less negative effect on the pellet strength. It can meet the basic pellet ore strength requirements. |
| Molasses | ≥1.0 | The high viscosity of molasses adheres to the minerals to form a continuous matrix, which easily generates calcium sucrose with CaO and hardens pellets, thus improving the green and dry pellet strength. | The addition of molasses tends to be higher and has a greater negative impact on the pellet strength, and is usually mixed with CaO. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Zhou, F.; Ma, C.; Wei, Z.; Long, W. Bonding Mechanism and Process Characteristics of Special Polymers Applied in Pelletizing Binders. Coatings 2022, 12, 1618. https://doi.org/10.3390/coatings12111618
Zhao H, Zhou F, Ma C, Wei Z, Long W. Bonding Mechanism and Process Characteristics of Special Polymers Applied in Pelletizing Binders. Coatings. 2022; 12(11):1618. https://doi.org/10.3390/coatings12111618
Chicago/Turabian StyleZhao, Hongxing, Fengshan Zhou, Cunfa Ma, Zhongjin Wei, and Wenjun Long. 2022. "Bonding Mechanism and Process Characteristics of Special Polymers Applied in Pelletizing Binders" Coatings 12, no. 11: 1618. https://doi.org/10.3390/coatings12111618
APA StyleZhao, H., Zhou, F., Ma, C., Wei, Z., & Long, W. (2022). Bonding Mechanism and Process Characteristics of Special Polymers Applied in Pelletizing Binders. Coatings, 12(11), 1618. https://doi.org/10.3390/coatings12111618






