Influence of Dental Implant Surface Modifications on Osseointegration and Biofilm Attachment
Abstract
:1. Introduction
2. Sandblasting and Acid Etching Methodologies
3. Plasma Spraying
4. Metal Ions Implantation
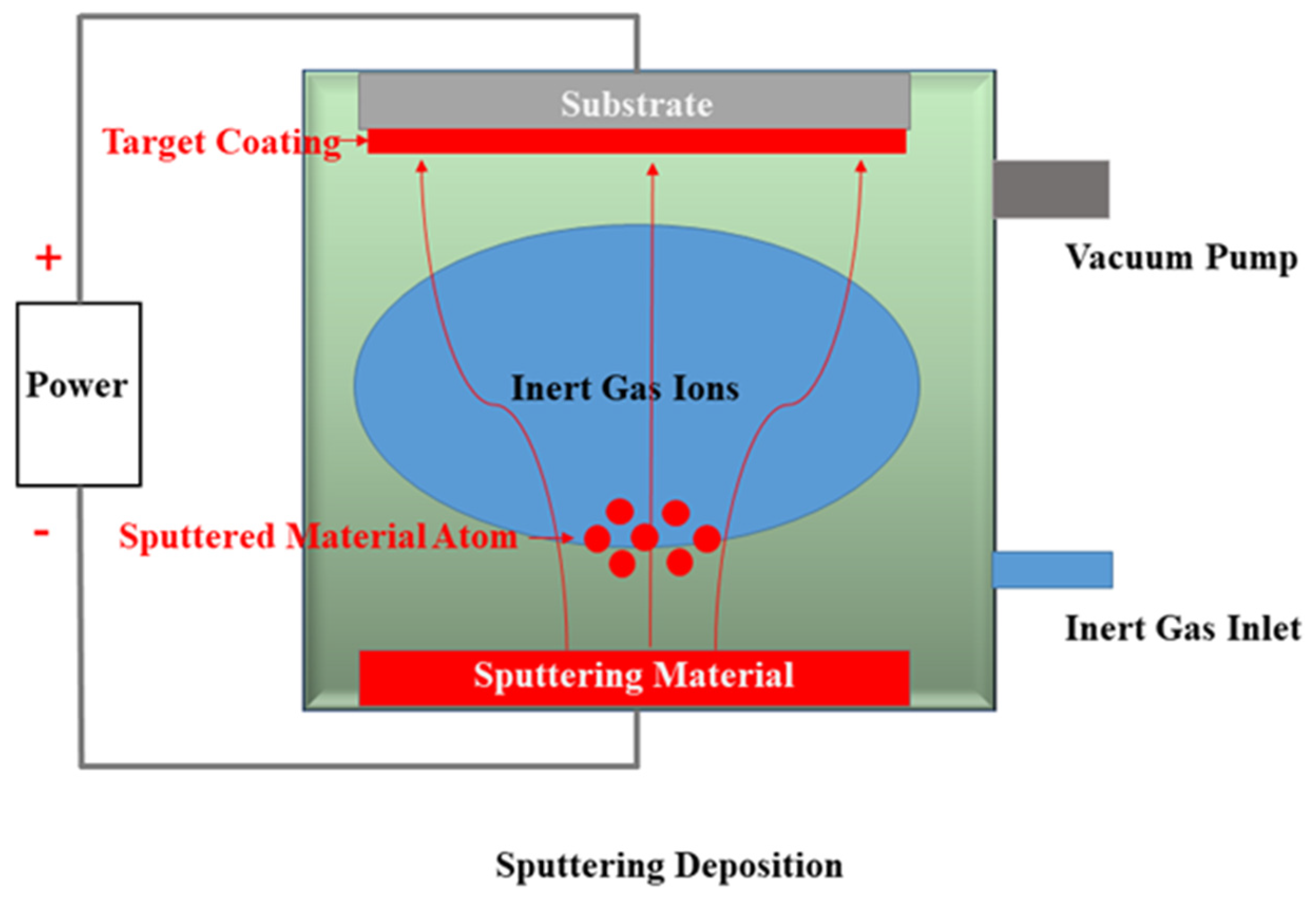
5. Sputter-Deposition
6. Selective Laser Melting (SLM)
7. Anodic Oxidation
8. Micro-Arc Oxidation
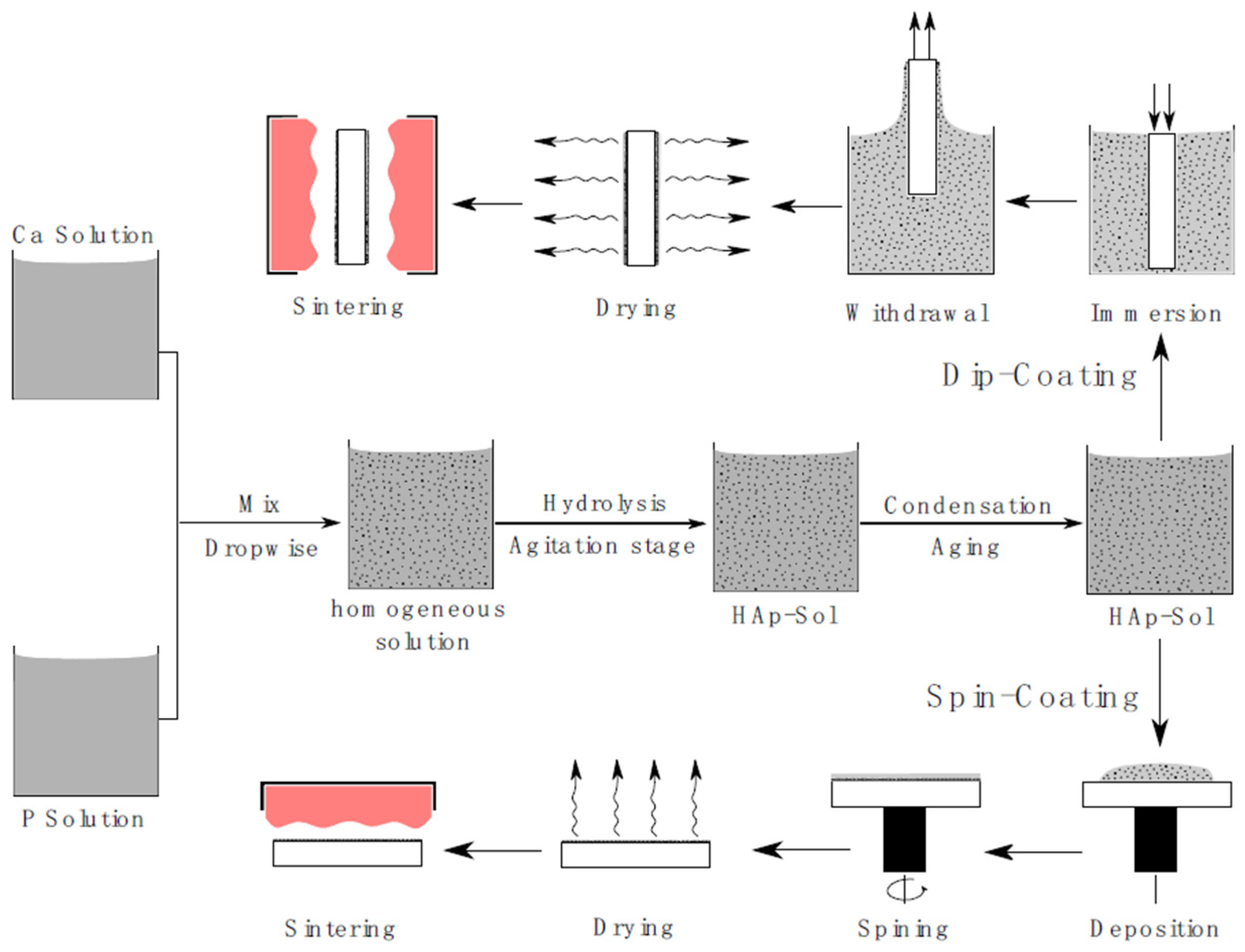
9. Sol-Gel Coating
10. Alkaline Heat Treatment
11. Acid-Alkali Treatment
12. Layer-by-Layer Self-Assembly Technique
13. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Howe, M.-S.; Keys, W.; Richards, D. Long-term (10-year) dental implant survival: A systematic review and sensitivity meta-analysis. J. Dent. 2019, 84, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, H.S.; Jansen, J.A. The development and future of dental implants. Dent. Mater. J. 2020, 39, 167–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsigarida, A.; Chochlidakis, K.; Fraser, D.; Lampraki, E.; Einarsdottir, E.R.; Barmak, A.B.; Papaspyridakos, P.; Ercoli, C. Peri-Implant Diseases and Biologic Complications at Implant-Supported Fixed Dental Prostheses in Partially Edentulous Patients. J. Prosthodont. Off. J. Am. Coll. Prosthodont. 2020, 29, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Brånemark, P.I.; Hansson, H.A.; Lindström, J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [Green Version]
- Do, T.A.; Le, H.S.; Shen, Y.W.; Huang, H.L.; Fuh, L.J. Risk Factors related to Late Failure of Dental Implant-A Systematic Review of Recent Studies. Int. J. Environ. Res. Public Health 2020, 17, 3931. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Manoil, D. Microbial Community-Driven Etiopathogenesis of Peri-Implantitis. J. Dent. Res. 2021, 100, 21–28. [Google Scholar] [CrossRef]
- Butera, A.; Pascadopoli, M.; Pellegrini, M.; Gallo, S.; Zampetti, P.; Scribante, A. Oral Microbiota in Patients with Peri-Implant Disease: A Narrative Review. Appl. Sci. 2022, 12, 3250. [Google Scholar] [CrossRef]
- Renvert, S.; Persson, G.R.; Pirih, F.Q.; Camargo, P.M. Peri-implant health, peri-implant mucositis, and peri-implantitis: Case definitions and diagnostic considerations. J. Periodontol. 2018, 89 (Suppl. 1), S304–S312. [Google Scholar] [CrossRef]
- Romanos, G.E.; Weitz, D. Therapy of peri-implant diseases. Where is the evidence? J. Evid. Based Dent. Pract. 2012, 12 (Suppl. 3), 204–248. [Google Scholar] [CrossRef]
- Sahrmann, P.; Gilli, F.; Wiedemeier, D.B.; Attin, T.; Schmidlin, P.R.; Karygianni, L. The Microbiome of Peri-Implantitis: A Systematic Review and Meta-Analysis. Microorganisms 2020, 8, 661. [Google Scholar] [CrossRef]
- Kasemo, B. Biocompatibility of titanium implants: Surface science aspects. J. Prosthet. Dent. 1983, 49, 832–837. [Google Scholar] [CrossRef]
- Katou, F.; Andoh, N.; Motegi, K.; Nagura, H. Immuno-inflammatory responses in the tissue adjacent to titanium miniplates used in the treatment of mandibular fractures. J. Cranio Maxillofac. Surg. 1996, 24, 155–162. [Google Scholar] [CrossRef]
- Wang, K. The use of titanium for medical applications in the USA. Mater. Sci. Eng. A 1996, 213, 134–137. [Google Scholar] [CrossRef]
- Guglielmotti, M.B.; Renou, S.; Cabrini, R.L. A histomorphometric study of tissue interface by laminar implant test in rats. Int. J. Oral Maxillofac. Implant. 1999, 14, 565–570. [Google Scholar]
- Hashim, D.; Cionca, N.; Courvoisier, D.S.; Mombelli, A. A systematic review of the clinical survival of zirconia implants. Clin. Oral Investig. 2016, 20, 1403–1417. [Google Scholar] [CrossRef] [Green Version]
- Cionca, N.; Hashim, D.; Mombelli, A. Zirconia dental implants: Where are we now, and where are we heading? Periodontology 2000 2017, 73, 241–258. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontology 2000 2017, 73, 22–40. [Google Scholar] [CrossRef]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface Modifications and Their Effects on Titanium Dental Implants. BioMed Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef] [Green Version]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef]
- Subramani, K.; Jung, R.E.; Molenberg, A.; Hammerle, C.H. Biofilm on dental implants: A review of the literature. Int. J. Oral Maxillofac. Implant. 2009, 24, 616–626. [Google Scholar]
- Cervino, G.; Fiorillo, L.; Iannello, G.; Santonocito, D.; Risitano, G.; Cicciù, M. Sandblasted and Acid Etched Titanium Dental Implant Surfaces Systematic Review and Confocal Microscopy Evaluation. Materials 2019, 12, 1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibata, Y.; Tanimoto, Y. A review of improved fixation methods for dental implants. Part I: Surface optimization for rapid osseointegration. J. Prosthodont. Res. 2015, 59, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Stenberg, T. Prospective 10-year cohort study based on a randomized controlled trial (RCT) on implant-supported full-arch maxillary prostheses. Part 1: Sandblasted and acid-etched implants and mucosal tissue. Clin. Implant Dent. Relat. Res. 2012, 14, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Gottlow, J.; Dard, M.; Kjellson, F.; Obrecht, M.; Sennerby, L. Evaluation of a new titanium-zirconium dental implant: A biomechanical and histological comparative study in the mini pig. Clin. Implant Dent. Relat. Res. 2012, 14, 538–545. [Google Scholar] [CrossRef]
- Zhan, X.; Li, S.; Cui, Y.; Tao, A.; Wang, C.; Li, H.; Zhang, L.; Yu, H.; Jiang, J.; Li, C. Comparison of the osteoblastic activity of low elastic modulus Ti-24Nb-4Zr-8Sn alloy and pure titanium modified by physical and chemical methods. Mater. Sci. Eng. C 2020, 113, 111018. [Google Scholar] [CrossRef]
- Xie, H.; Shen, S.; Qian, M.; Zhang, F.; Chen, C.; Tay, F.R. Effects of Acid Treatment on Dental Zirconia: An In Vitro Study. PLoS ONE 2015, 10, e0136263. [Google Scholar] [CrossRef] [Green Version]
- An, N.; Rausch-fan, X.; Wieland, M.; Matejka, M.; Andrukhov, O.; Schedle, A. Initial attachment, subsequent cell proliferation/viability and gene expression of epithelial cells related to attachment and wound healing in response to different titanium surfaces. Dent. Mater. 2012, 28, 1207–1214. [Google Scholar] [CrossRef]
- Roehling, S.; Astasov-Frauenhoffer, M.; Hauser-Gerspach, I.; Braissant, O.; Woelfler, H.; Waltimo, T.; Kniha, H.; Gahlert, M. In Vitro Biofilm Formation on Titanium and Zirconia Implant Surfaces. J. Periodontol. 2017, 88, 298–307. [Google Scholar] [CrossRef]
- Becker, S.T.; Beck-Broichsitter, B.E.; Rossmann, C.M.; Behrens, E.; Jochens, A.; Wiltfang, J. Long-term Survival of Straumann Dental Implants with TPS Surfaces: A Retrospective Study with a Follow-up of 12 to 23 Years. Clin. Implant Dent. Relat. Res. 2016, 18, 480–488. [Google Scholar] [CrossRef]
- De Bruyn, H.; Christiaens, V.; Doornewaard, R.; Jacobsson, M.; Cosyn, J.; Jacquet, W.; Vervaeke, S. Implant surface roughness and patient factors on long-term peri-implant bone loss. Periodontology 2000 2017, 73, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.A.; Wilson, T.G., Jr.; Weber, H.P. The ITI Dental Implant System. Compendium 1994, 15, 526. [Google Scholar] [CrossRef] [PubMed]
- Claes, L.; Hutzschenreuter, P.; Pohler, O. The dependence of the removal torque of a leg screw a screw surface and implantation time (author’s transl). Arch. Orthop. Trauma. Surg. 1976, 85, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Chappuis, V.; Buser, R.; Brägger, U.; Bornstein, M.M.; Salvi, G.E.; Buser, D. Long-term outcomes of dental implants with a titanium plasma-sprayed surface: A 20-year prospective case series study in partially edentulous patients. Clin. Implant Dent. Relat. Res. 2013, 15, 780–790. [Google Scholar] [CrossRef]
- Urban, R.M.; Jacobs, J.J.; Tomlinson, M.J.; Gavrilovic, J.; Black, J.; Peoc’h, M. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J. Bone Jt. Surg. Am. Vol. 2000, 82, 457–476. [Google Scholar] [CrossRef]
- Roccuzzo, M.; Bunino, M.; Prioglio, F.; Bianchi, S.D. Early loading of sandblasted and acid-etched (SLA) implants: A prospective split-mouth comparative study. Clin. Oral Implant. Res. 2001, 12, 572–578. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Kolmas, J.; Groszyk, E.; Kwiatkowska-Różycka, D. Substituted hydroxyapatites with antibacterial properties. BioMed Res. Int. 2014, 2014, 178123. [Google Scholar] [CrossRef] [Green Version]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [Green Version]
- Murugan, R.; Ramakrishna, S. Development of nanocomposites for bone grafting. Compos. Sci. Technol. 2005, 65, 2385–2406. [Google Scholar] [CrossRef]
- Arias, J.L.; Mayor, M.B.; Pou, J.; Leng, Y.; León, B.; Pérez-Amor, M. Micro- and nano-testing of calcium phosphate coatings produced by pulsed laser deposition. Biomaterials 2003, 24, 3403–3408. [Google Scholar] [CrossRef]
- Davison, N.L.; Su, J.; Yuan, H.; van den Beucken, J.J.; de Bruijn, J.D.; Barrère-de Groot, F. Influence of surface microstructure and chemistry on osteoinduction and osteoclastogenesis by biphasic calcium phosphate discs. Eur. Cells Mater. 2015, 29, 314–329. [Google Scholar] [CrossRef] [PubMed]
- Ducheyne, P.; Radin, S.; Heughebaert, M.; Heughebaert, J.C. Calcium phosphate ceramic coatings on porous titanium: Effect of structure and composition on electrophoretic deposition, vacuum sintering and in vitro dissolution. Biomaterials 1990, 11, 244–254. [Google Scholar] [CrossRef]
- Jansen, J.A.; Wolke, J.G.; Swann, S.; Van der Waerden, J.P.; de Groot, K. Application of magnetron sputtering for producing ceramic coatings on implant materials. Clin. Oral Implant. Res. 1993, 4, 28–34. [Google Scholar] [CrossRef]
- Russell, S.W.; Luptak, K.A.; Suchicital, C.T.A.; Alford, T.L.; Pizziconi, V.B. Chemical and Structural Evolution of Sol-Gel-Derived Hydroxyapatite Thin Films under Rapid Thermal Processing. J. Am. Ceram. Soc. 1996, 79, 837–842. [Google Scholar] [CrossRef]
- Yoshinari, M.; Ohtsuka, Y.; Dérand, T. Thin hydroxyapatite coating produced by the ion beam dynamic mixing method. Biomaterials 1994, 15, 529–535. [Google Scholar] [CrossRef]
- Munting, E. The contributions and limitations of hydroxyapatite coatings to implant fixation: A histomorphometric study of load bearing implants in dogs. Int. Orthop. 1996, 20, 1–6. [Google Scholar] [CrossRef]
- Albrektsson, T. Hydroxyapatite-coated implants: A case against their use. J. Oral Maxillofac. Surg. 1998, 56, 1312–1326. [Google Scholar] [CrossRef]
- Wheeler, S.L. Eight-year clinical retrospective study of titanium plasma-sprayed and hydroxyapatite-coated cylinder implants. Int. J. Oral Maxillofac. Implant. 1996, 11, 340–350. [Google Scholar] [CrossRef]
- Baltag, I.; Watanabe, K.; Kusakari, H.; Taguchi, N.; Miyakawa, O.; Kobayashi, M.; Ito, N. Long-term changes of hydroxyapatite-coated dental implants. J. Biomed. Mater. Res. 2000, 53, 76–85. [Google Scholar] [CrossRef]
- Capello, W.N.; D’Antonio, J.A.; Feinberg, J.R.; Manley, M.T. Hydroxyapatite-coated total hip femoral components in patients less than fifty years old. Clinical and radiographic results after five to eight years of follow-up. J. Bone Jt. Surg. 1997, 79, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Rouhfar, L.; Beirne, O.R. Survival of hydroxyapatite-coated implants: A meta-analytic review. J. Oral Maxillofac. Surg. 2000, 58, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- López-Valverde, N.; Flores-Fraile, J.; Ramírez, J.M.; Sousa, B.M.; Herrero-Hernández, S.; López-Valverde, A. Bioactive Surfaces vs. Conventional Surfaces in Titanium Dental Implants: A Comparative Systematic Review. J. Clin. Med. 2020, 9, 2047. [Google Scholar] [CrossRef]
- Ke, D.; Robertson, S.F.; Dernell, W.S.; Bandyopadhyay, A.; Bose, S. Effects of MgO and SiO2 on Plasma-Sprayed Hydroxyapatite Coating: An in Vivo Study in Rat Distal Femoral Defects. ACS Appl. Mater. Interfaces 2017, 9, 25731–25737. [Google Scholar] [CrossRef]
- Brohede, U.; Forsgren, J.; Roos, S.; Mihranyan, A.; Engqvist, H.; Strømme, M. Multifunctional implant coatings providing possibilities for fast antibiotics loading with subsequent slow release. J. Mater. Sci. Mater. Med. 2009, 20, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Govindan, R.; Girija, E.K. Drug loaded phosphate glass/hydroxyapatite nanocomposite for orthopedic applications. J. Mater. Chem. B 2014, 2, 5468–5477. [Google Scholar] [CrossRef]
- Laurent, F.; Bignon, A.; Goldnadel, J.; Chevalier, J.; Fantozzi, G.; Viguier, E.; Roger, T.; Boivin, G.; Hartmann, D. A new concept of gentamicin loaded HAP/TCP bone substitute for prophylactic action: In vitro release validation. J. Mater. Sci. Mater. Med. 2008, 19, 947–951. [Google Scholar] [CrossRef]
- Baro, M.; Sánchez, E.; Delgado, A.; Perera, A.; Evora, C. In vitro-in vivo characterization of gentamicin bone implants. J. Control. Release 2002, 83, 353–364. [Google Scholar] [CrossRef]
- Ratier, A.; Gibson, I.R.; Best, S.M.; Freche, M.; Lacout, J.L.; Rodriguez, F. Setting characteristics and mechanical behaviour of a calcium phosphate bone cement containing tetracycline. Biomaterials 2001, 22, 897–901. [Google Scholar] [CrossRef]
- Luginbuehl, V.; Ruffieux, K.; Hess, C.; Reichardt, D.; von Rechenberg, B.; Nuss, K. Controlled release of tetracycline from biodegradable beta-tricalcium phosphate composites. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 92, 341–352. [Google Scholar] [CrossRef]
- Takechi, M.; Miyamoto, Y.; Ishikawa, K.; Nagayama, M.; Kon, M.; Asaoka, K.; Suzuki, K. Effects of added antibiotics on the basic properties of anti-washout-type fast-setting calcium phosphate cement. J. Biomed. Mater. Res. 1998, 39, 308–316. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, G.; Yu, X.; Wang, T.; Xi, Y.; Tang, Z. Porous deproteinized bovine bone scaffold with three-dimensional localized drug delivery system using chitosan microspheres. Biomed. Eng. Online 2015, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.; Liu, L.; Fan, K.; Cai, Y.; Yao, J. Ibuprofen loaded porous calcium phosphate nanospheres for skeletal drug delivery system. J. Mater. Sci. 2012, 47, 1054–1058. [Google Scholar] [CrossRef]
- Otsuka, M.; Matsuda, Y.; Suwa, Y.; Fox, J.L.; Higuchi, W.I. A novel skeletal drug-delivery system using self-setting calcium phosphate cement. 4. Effects of the mixing solution volume on the drug-release rate of heterogeneous aspirin-loaded cement. J. Pharm. Sci. 1994, 83, 259–263. [Google Scholar] [CrossRef]
- Dong, H.; Liu, H.; Zhou, N.; Li, Q.; Yang, G.; Chen, L.; Mou, Y. Surface Modified Techniques and Emerging Functional Coating of Dental Implants. Coatings 2020, 10, 1012. [Google Scholar] [CrossRef]
- Fielding, G.A.; Roy, M.; Bandyopadhyay, A.; Bose, S. Antibacterial and biological characteristics of silver containing and strontium doped plasma sprayed hydroxyapatite coatings. Acta Biomater. 2012, 8, 3144–3152. [Google Scholar] [CrossRef] [Green Version]
- Vu, A.A.; Robertson, S.F.; Ke, D.; Bandyopadhyay, A.; Bose, S. Mechanical and biological properties of ZnO, SiO2, and Ag2O doped plasma sprayed hydroxyapatite coating for orthopaedic and dental applications. Acta Biomater. 2019, 92, 325–335. [Google Scholar] [CrossRef]
- Qi, S.; Wu, J.; Xu, Y.; Zhang, Y.; Wang, R.; Li, K.; Xu, Y. Chemical Stability and Antimicrobial Activity of Plasma-Sprayed Cerium Oxide-Incorporated Calcium Silicate Coating in Dental Implants. Implant Dent. 2019, 28, 564–570. [Google Scholar] [CrossRef]
- Wu, C.; Ramaswamy, Y.; Liu, X.; Wang, G.; Zreiqat, H. Plasma-sprayed CaTiSiO5 ceramic coating on Ti-6Al-4V with excellent bonding strength, stability and cellular bioactivity. J. R. Soc. Interface 2009, 6, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Lampé, I.; Beke, D.; Biri, S.; Csarnovics, I.; Csík, A.; Dombrádi, Z.; Hajdu, P.; Hegedűs, V.; Rácz, R.; Varga, I.; et al. Investigation of silver nanoparticles on titanium surface created by ion implantation technology. Int. J. Nanomed. 2019, 14, 4709–4721. [Google Scholar] [CrossRef] [Green Version]
- AshaRani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ma, J.; Wang, D.; Liu, X.; Li, H.; Zhou, L.; Liang, C.; Wang, H. Self-adjusting antibacterial properties of Ag-incorporated nanotubes on micro-nanostructured Ti surfaces. Biomater. Sci. 2019, 7, 4075–4087. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lin, D.; Zhu, L. Effects of water chemistry on the dissolution of ZnO nanoparticles and their toxicity to Escherichia coli. Environ. Pollut. 2013, 173, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Su, P.; Chen, S.; Wang, N.; Ma, Y.; Liu, Y.; Wang, J.; Zhang, Z.; Li, H.; Webster, T.J. Synthesis of TiO2 nanotubes with ZnO nanoparticles to achieve antibacterial properties and stem cell compatibility. Nanoscale 2014, 6, 9050–9062. [Google Scholar] [CrossRef]
- Memarzadeh, K.; Sharili, A.S.; Huang, J.; Rawlinson, S.C.; Allaker, R.P. Nanoparticulate zinc oxide as a coating material for orthopedic and dental implants. J. Biomed. Mater. Res. Part A 2015, 103, 981–989. [Google Scholar] [CrossRef]
- Abdulkareem, E.H.; Memarzadeh, K.; Allaker, R.P.; Huang, J.; Pratten, J.; Spratt, D. Anti-biofilm activity of zinc oxide and hydroxyapatite nanoparticles as dental implant coating materials. J. Dent. 2015, 43, 1462–1469. [Google Scholar] [CrossRef]
- Yao, S.; Feng, X.; Lu, J.; Zheng, Y.; Wang, X.; Volinsky, A.A.; Wang, L.-N. Antibacterial activity and inflammation inhibition of ZnO nanoparticles embedded TiO2 nanotubes. Nanotechnology 2018, 29, 244003. [Google Scholar] [CrossRef]
- Li, K.; Xie, Y.; You, M.; Huang, L.; Zheng, X. Plasma sprayed cerium oxide coating inhibits H2O2-induced oxidative stress and supports cell viability. J. Mater. Sci. Mater. Med. 2016, 27, 100. [Google Scholar] [CrossRef]
- Li, X.; Qi, M.; Sun, X.; Weir, M.D.; Tay, F.R.; Oates, T.W.; Dong, B.; Zhou, Y.; Wang, L.; Xu, H.H. Surface treatments on titanium implants via nanostructured ceria for antibacterial and anti-inflammatory capabilities. Acta Biomater. 2019, 94, 627–643. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, Y.; Jiang, P.; Wang, G.; Zhang, J. Highly dispersed CeO₂ on TiO₂ nanotube: A synergistic nanocomposite with superior peroxidase-like activity. ACS Appl. Mater. Interfaces 2015, 7, 6451–6461. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dong, H.; Liu, J.; Qin, G.; Chen, D.; Zhang, E. In vivo antibacterial property of Ti-Cu sintered alloy implant. Mater. Sci. Eng. C 2019, 100, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, J.; Versace, D.L.; Abbad-Andallousi, S.; Pires, R.; Azevedo, C.; Cénédese, P.; Dubot, P. Antibacterial properties of nanostructured Cu-TiO2 surfaces for dental implants. Biomater. Sci. 2017, 5, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Long, J.D.; Ostrikov, K.N.; Lu, J.H.; Diong, C.H. RF magnetron sputtering deposition of bioactive Ca-P-based coatings on Ti-6Al-4V alloy. IEEE Trans. Plasma Sci. 2002, 30, 118–119. [Google Scholar] [CrossRef]
- Yang, Q.; Seo, D.Y.; Zhao, L.R.; Zeng, X.T. Erosion resistance performance of magnetron sputtering deposited TiAlN coatings. Surf. Coat. Technol. 2004, 188, 168–173. [Google Scholar] [CrossRef]
- Yi, P.; Peng, L.; Huang, J. Multilayered TiAlN films on Ti6Al4V alloy for biomedical applications by closed field unbalanced magnetron sputter ion plating process. Mater. Sci. Eng. C 2016, 59, 669–676. [Google Scholar] [CrossRef]
- Bartolomeu, F.; Buciumeanu, M.; Pinto, E.; Alves, N.; Silva, F.S.; Carvalho, O.; Miranda, G. Wear behavior of Ti6Al4V biomedical alloys processed by selective laser melting, hot pressing and conventional casting—ScienceDirect. Trans. Nonferrous Met. Soc. China 2017, 27, 829–838. [Google Scholar] [CrossRef]
- Qin, J.; Yang, D.; Maher, S.; Lima-Marques, L.; Zhou, Y.; Chen, Y.; Atkins, G.J.; Losic, D. Micro- and nano-structured 3D printed titanium implants with a hydroxyapatite coating for improved osseointegration. J. Mater. Chem. B 2018, 6, 3136–3144. [Google Scholar] [CrossRef]
- Hu, X.; Xu, R.; Yu, X.; Chen, J.; Wan, S.; Ouyang, J.; Deng, F. Enhanced antibacterial efficacy of selective laser melting titanium surface with nanophase calcium phosphate embedded to TiO2 nanotubes. Biomed. Mater. 2018, 13, 045015. [Google Scholar] [CrossRef]
- Shin, Y.; Lee, S. Self-organized regular arrays of anodic TiO2 nanotubes. Nano Lett. 2008, 8, 3171–3173. [Google Scholar] [CrossRef]
- Li, Y.; Li, B.; Song, Y.; Ma, A.; Li, C.; Zhang, X.; Li, H.; Zhang, Q.; Zhang, K. Improved osteoblast adhesion and osseointegration on TiO2 nanotubes surface with hydroxyapatite coating. Dent. Mater. J. 2019, 38, 278–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, P.; Mao, F.; He, F.; Han, Y.; Li, H.; Chen, J.; Wei, S. Screening the optimal hierarchical micro/nano pattern design for the neck and body surface of titanium implants. Colloids Surf. B Biointerfaces 2019, 178, 515–524. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, T.-T.; Ao, H.-Y.; Yang, S.-B.; Wang, Y.-G.; Lin, W.-T.; Yu, Z.-F. In vivo evaluation of the anti-infection potential of gentamicin-loaded nanotubes on titania implants. Int. J. Nanomed. 2016, 11, 2223–2234. [Google Scholar] [CrossRef] [Green Version]
- Wassmann, T.; Kreis, S.; Behr, M.; Buergers, R. The influence of surface texture and wettability on initial bacterial adhesion on titanium and zirconium oxide dental implants. Int. J. Implant Dent. 2017, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Salas, B.; Beltrán-Partida, E.; Castillo-Uribe, S.; Curiel-Álvarez, M.; Zlatev, R.; Stoytcheva, M.; Montero-Alpírez, G.; Vargas-Osuna, L. In Vitro Assessment of Early Bacterial Activity on Micro/Nanostructured Ti6Al4V Surfaces. Molecules 2017, 22, 832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podporska-Carroll, J.; Panaitescu, E.; Quilty, B.; Wang, L.; Menon, L.; Pillai, S.C. Antimicrobial properties of highly efficient photocatalytic TiO2 nanotubes. Appl. Catal. B Environ. 2015, 176, 70–75. [Google Scholar] [CrossRef]
- Fathi, M.; Akbari, B.; Taheriazam, A. Antibiotics drug release controlling and osteoblast adhesion from Titania nanotubes arrays using silk fibroin coating. Mater. Sci. Eng. C 2019, 103, 109743. [Google Scholar] [CrossRef]
- Guan, M.; Chen, Y.; Wei, Y.; Song, H.; Gao, C.; Cheng, H.; Li, Y.; Huo, K.; Fu, J.; Xiong, W. Long-lasting bactericidal activity through selective physical puncture and controlled ions release of polydopamine and silver nanoparticles-loaded TiO2 nanorods in vitro and in vivo. Int. J. Nanomed. 2019, 14, 2903–2914. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Lee, I.S.; Cui, F.Z.; Choi, S.H. The biocompatibility of nanostructured calcium phosphate coated on micro-arc oxidized titanium. Biomaterials 2008, 29, 2025–2032. [Google Scholar] [CrossRef]
- Ribeiro, A.; Oliveira, F.; Boldrini, L.; Leite, P.; Falagan-Lotsch, P.; Linhares, A.; Zambuzzi, W.; Fragneaud, B.; Campos, A.; Gouvêa, C.; et al. Micro-arc oxidation as a tool to develop multifunctional calcium-rich surfaces for dental implant applications. Mater. Sci. Eng. C 2015, 54, 196–206. [Google Scholar] [CrossRef]
- Giordano, C.; Visai, L.; Pedeferri, M.P.; Chiesa, R.; Cigada, A. Antibacterial treatments ontitanium forimplantology. Biomed. Pharmacother. 2006, 60, 472. [Google Scholar] [CrossRef]
- Wang, Y.M.; Jiang, B.L.; Lei, T.Q.; Guo, L.X. Microarc oxidation coatings formed on Ti6Al4V in Na2SiO3 system solution: Microstructure, mechanical and tribological properties. Surf. Coat. Technol. 2006, 201, 82–89. [Google Scholar] [CrossRef]
- Shimabukuro, M. Antibacterial Property and Biocompatibility of Silver, Copper, and Zinc in Titanium Dioxide Layers Incorporated by One-Step Micro-Arc Oxidation: A Review. Antibiotics 2020, 9, 716. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, X.; Bai, L.; Hang, R.; Huang, X.; Qin, L.; Yao, X.; Tang, B. Antibacterial ability and osteogenic activity of porous Sr/Ag-containing TiO2 coatings. Biomed. Mater. 2016, 11, 045008. [Google Scholar] [CrossRef]
- Wu, C.; Ramaswamy, Y.; Gale, D.; Yang, W.; Xiao, K.; Zhang, L.; Yin, Y.; Zreiqat, H. Novel sphene coatings on Ti-6Al-4V for orthopedic implants using sol-gel method. Acta Biomater. 2008, 4, 569–576. [Google Scholar] [CrossRef]
- Chai, C.S.; Gross, K.A.; Ben-Nissan, B. Critical ageing of hydroxyapatite sol-gel solutions. Biomaterials 1998, 19, 2291–2296. [Google Scholar] [CrossRef]
- Costa, D.O.; Dixon, S.J.; Rizkalla, A.S. One- and three-dimensional growth of hydroxyapatite nanowires during sol-gel-hydrothermal synthesis. ACS Appl. Mater. Interfaces 2012, 4, 1490–1499. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate coatings on magnesium and its biodegradable alloys. Acta Biomater. 2014, 10, 2919–2934. [Google Scholar] [CrossRef]
- Jaafar, A.; Hecker, C.; Árki, P.; Joseph, Y. Sol-Gel Derived Hydroxyapatite Coatings for Titanium Implants: A Review. Bioengineering 2020, 7, 127. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.-T.; Jamshidi, A. Hydrothermal processing of hydroxyapatite nanoparticles—A Taguchi experimental design approach. J. Cryst. Growth 2012, 361, 73–84. [Google Scholar] [CrossRef]
- Kang, K.; Lim, H.; Yun, K.D.; Park, S.; Jeong, C.; Lee, K. Effect of Viscosities on the Surface Morphology and Crystallographic Properties of Hydroxyapatite Coated Titanium Dioxide Nanotubes. J. Nanosci. Nanotechnol. 2015, 15, 5310–5313. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, C.Z.; Wang, D.G. Effect of heating rate on structure of HA coating prepared by sol–gel. Surf. Eng. 2013, 25, 131–135. [Google Scholar] [CrossRef]
- Zhang, J. Biocompatibility and Anti-Bacterial Activity of Zn-Containing HA/TiO2 Hybrid Coatings on Ti Substrate. J. Hard Tissue Biol. 2013, 22, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Batebi, K.; Abbasi Khazaei, B.; Afshar, A. Characterization of sol-gel derived silver/fluor-hydroxyapatite composite coatings on titanium substrate. Surf. Coat. Technol. 2018, 352, 522–528. [Google Scholar] [CrossRef]
- Bi, Q.; Song, X.; Chen, Y.; Zheng, Y.; Yin, P.; Lei, T. Zn-HA/Bi-HA biphasic coatings on Titanium: Fabrication, characterization, antibacterial and biological activity. Colloids Surf. B Biointerfaces 2020, 189, 110813. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Kong, Y.M.; Bae, C.J.; Noh, Y.J.; Kim, H.E. Sol-gel derived fluor-hydroxyapatite biocoatings on zirconia substrate. Biomaterials 2004, 25, 2919–2926. [Google Scholar] [CrossRef]
- Tredwin, C.J.; Young, A.M.; Georgiou, G.; Shin, S.H.; Kim, H.W.; Knowles, J.C. Hydroxyapatite, fluor-hydroxyapatite and fluorapatite produced via the sol-gel method. Optimisation, characterisation and rheology. Dent. Mater. 2013, 29, 166–173. [Google Scholar] [CrossRef]
- Marquis, R.E. Antimicrobial actions of fluoride for oral bacteria. Can. J. Microbiol. 1995, 41, 955–964. [Google Scholar] [CrossRef]
- Kokubo, T.; Yamaguchi, S. Bioactive titanate layers formed on titanium and its alloys by simple chemical and heat treatments. Open Biomed. Eng. J. 2015, 9, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Bright, R.; Hayles, A.; Wood, J.; Ninan, N.; Palms, D.; Visalakshan, R.M.; Burzava, A.; Brown, T.; Barker, D.; Vasilev, K. Bio-Inspired Nanostructured Ti-6Al-4V Alloy: The Role of Two Alkaline Etchants and the Hydrothermal Processing Duration on Antibacterial Activity. Nanomaterials 2022, 12, 1140. [Google Scholar] [CrossRef]
- Zhang, E.; Liu, C. Effect of surface treatments on the surface morphology, corrosion property, and antibacterial property of Ti-10Cu sintered alloy. Biomed. Mater. 2015, 10, 045009. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.B.; Liu, Q.; De Wijn, J.R.; De Groot, K.; Cui, F.Z. Preparation of bioactive microporous titanium surface by a new two-step chemical treatment. J. Mater. Sci. Mater. Med. 1998, 9, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhu, X.; Liang, K.; Ding, J.; Xiang, Z.; Fan, H.; Zhang, X. Osteoinduction of porous titanium: A comparative study between acid-alkali and chemical-thermal treatments. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 95, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Braem, A.; Van Mellaert, L.; Mattheys, T.; Hofmans, D.; De Waelheyns, E.; Geris, L.; Anné, J.; Schrooten, J.; Vleugels, J. Staphylococcal biofilm growth on smooth and porous titanium coatings for biomedical applications. J. Biomed. Mater. Res. Part A 2014, 102, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Li, X.; Yao, Y.; Zhou, J.; Cao, S.; Zhang, X.; Jian, Y.; Zhao, K. Effect of acid-alkali treatment on serum protein adsorption and bacterial adhesion to porous titanium. J. Mater. Sci. Mater. Med. 2022, 33, 20. [Google Scholar] [CrossRef]
- Rawtani, D.; Agrawal, Y.K. Emerging Strategies and Applications of Layer-by-Layer Self-Assembly. Nanobiomedicine 2014, 1, 8. [Google Scholar] [CrossRef]
- de Villiers, M.M.; Otto, D.P.; Strydom, S.J.; Lvov, Y.M. Introduction to nanocoatings produced by layer-by-layer (LbL) self-assembly. Adv. Drug Deliv. Rev. 2011, 63, 701–715. [Google Scholar] [CrossRef]
- Cui, W.; Li, J.; Decher, G. Self-Assembled Smart Nanocarriers for Targeted Drug Delivery. Adv. Mater. 2016, 28, 1302–1311. [Google Scholar] [CrossRef]
- Shi, Q.; Qian, Z.; Liu, D.; Liu, H. Surface Modification of Dental Titanium Implant by Layer-by-Layer Electrostatic Self-Assembly. Front. Physiol. 2017, 8, 574. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Cai, K.; Luo, Z.; Zhang, Y.; Li, L.; Lai, M.; Hou, Y.; Huang, Y.; Li, J.; Ding, X.; et al. Regulation of the differentiation of mesenchymal stem cells in vitro and osteogenesis in vivo by microenvironmental modification of titanium alloy surfaces. Biomaterials 2012, 33, 3515–3528. [Google Scholar] [CrossRef]
- Huang, L.; Luo, Z.; Hu, Y.; Shen, X.; Li, M.; Li, L.; Zhang, Y.; Yang, W.; Liu, P.; Cai, K. Enhancement of local bone remodeling in osteoporotic rabbits by biomimic multilayered structures on Ti6Al4V implants. J. Biomed. Mater. Res. Part A 2016, 104, 1437–1451. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.-H.; Liu, L.; Shen, J.-W.; Peel, S.; Yang, G.-L.; Zhao, S.-F.; He, F.-M. Influence of multilayer rhBMP-2 DNA coating on the proliferation and differentiation of MC3T3-E1 cells seeded on roughed titanium surface. J. Biomed. Mater. Res. Part A 2012, 100, 2766–2774. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, W.; Song, X.; Yang, C.; Gao, S.; Klausen, L.H.; Dong, M.; Kjems, J. Chitosan/siRNA functionalized titanium surface via a layer-by-layer approach for in vitro sustained gene silencing and osteogenic promotion. Int. J. Nanomed. 2015, 10, 2335–2346. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zheng, Z.; Cao, Z.; Zhuang, L.; Xu, Y.; Liu, X.; Xu, Y.; Gong, Y. Enhancing proliferation and osteogenic differentiation of HMSCs on casein/chitosan multilayer films. Colloids Surf. B Biointerfaces 2016, 141, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Yoshinari, M.; Toyama, T.; Hayakawa, T.; Ohkubo, C. Effects of a multilayered DNA/protamine coating on titanium implants on bone responses. J. Biomed. Mater. Res. Part A 2016, 104, 1500–1509. [Google Scholar] [CrossRef]
- Zhong, X.; Song, Y.; Yang, P.; Wang, Y.; Jiang, S.; Zhang, X.; Li, C. Titanium Surface Priming with Phase-Transited Lysozyme to Establish a Silver Nanoparticle-Loaded Chitosan/Hyaluronic Acid Antibacterial Multilayer via Layer-by-Layer Self-Assembly. PLoS ONE 2016, 11, e0146957. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Chen, Z.; Yang, X.; Cen, L.; Zhang, X.; Gao, P. Layer-by-layer self-assembly of minocycline-loaded chitosan/alginate multilayer on titanium substrates to inhibit biofilm formation. J. Dent. 2014, 42, 1464–1472. [Google Scholar] [CrossRef]
- Pérez-Anes, A.; Gargouri, M.; Laure, W.; Van Den Berghe, H.; Courcot, E.; Sobocinski, J.; Tabary, N.; Chai, F.; Blach, J.-F.; Addad, A.; et al. Bioinspired Titanium Drug Eluting Platforms Based on a Poly-β-cyclodextrin-Chitosan Layer-by-Layer Self-Assembly Targeting Infections. ACS Appl. Mater. Interfaces 2015, 7, 12882–12893. [Google Scholar] [CrossRef]
| Surface Modifications | Implant Brand |
|---|---|
| SLA | SLA Straumann® (Straumann Institute, Basel, Switzerland), Friadent Plus® (Dentsply Friadent, Mannheim, Germany), Promote® (Camlog, Basel, Switzerland), Ankylos® (Dentsply Friadent, Mannheim, Germany) |
| Anodic oxidation | TiUnite® (Nobel Biocare, Gothenburg, Sweden) |
| Plasma spraying | Bonefit® (Straumann Institute, Waldenburg, Switzerland), IMZ-TPS® (Dentsply Friadent, Mannhein, Germany), ITI-TPS® (Straumann Institute, Waldenburg, Germany), Steri-Oss-TPS® (Nobel Biocare, Yorba Linda, CA, USA) |
| Surface Modifications | Publication Time (Year) | Numbers |
|---|---|---|
| SLA | 2011 | 1 |
| 2012 | 2 | |
| 2014 | 1 | |
| 2015 | 1 | |
| 2016 | 1 | |
| 2019 | 2 | |
| 2020 | 1 | |
| Plasma Spraying | 1976 | 1 |
| 1990 | 1 | |
| 1992 | 1 | |
| 1993 | 1 | |
| 1994 | 4 | |
| 1996 | 2 | |
| 1997 | 1 | |
| 1998 | 2 | |
| 2000 | 1 | |
| 2001 | 2 | |
| 2002 | 1 | |
| 2003 | 1 | |
| 2005 | 1 | |
| 2006 | 1 | |
| 2008 | 1 | |
| 2009 | 2 | |
| 2010 | 3 | |
| 2011 | 2 | |
| 2012 | 1 | |
| 2013 | 1 | |
| 2014 | 2 | |
| 2015 | 3 | |
| 2016 | 1 | |
| 2017 | 2 | |
| 2019 | 2 | |
| 2020 | 2 | |
| Metal ions implantation | 2009 | 1 |
| 2011 | 1 | |
| 2012 | 1 | |
| 2014 | 1 | |
| 2015 | 3 | |
| 2016 | 1 | |
| 2017 | 1 | |
| 2018 | 1 | |
| 2019 | 4 | |
| Sputter-deposition | 2002 | 1 |
| 2004 | 1 | |
| 2015 | 1 | |
| Selective laser melting | 2017 | 1 |
| 2018 | 3 | |
| Anodic oxidation | 2008 | 1 |
| 2015 | 1 | |
| 2016 | 1 | |
| 2017 | 2 | |
| 2018 | 1 | |
| 2019 | 3 | |
| Micro-arc oxidation | 2005 | 1 |
| 2006 | 1 | |
| 2008 | 1 | |
| 2015 | 1 | |
| 2020 | 1 | |
| Sol-gel coating | 1995 | 1 |
| 1998 | 1 | |
| 2005 | 1 | |
| 2008 | 1 | |
| 2012 | 2 | |
| 2013 | 2 | |
| 2014 | 1 | |
| 2015 | 1 | |
| 2016 | 1 | |
| 2018 | 1 | |
| 2019 | 1 | |
| 2020 | 1 | |
| Alkaline heat treatment | 2015 | 2 |
| 2022 | 1 | |
| Acid-alkali treatment | 1998 | 1 |
| 2010 | 1 | |
| 2014 | 1 | |
| Layer-by-Layer self-assembly technique | 2011 | 1 |
| 2012 | 2 | |
| 2014 | 2 | |
| 2015 | 3 | |
| 2016 | 4 | |
| 2017 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, W.; Fang, S.; Zhong, Q.; Qi, S. Influence of Dental Implant Surface Modifications on Osseointegration and Biofilm Attachment. Coatings 2022, 12, 1654. https://doi.org/10.3390/coatings12111654
Han W, Fang S, Zhong Q, Qi S. Influence of Dental Implant Surface Modifications on Osseointegration and Biofilm Attachment. Coatings. 2022; 12(11):1654. https://doi.org/10.3390/coatings12111654
Chicago/Turabian StyleHan, Wen, Shuobo Fang, Qun Zhong, and Shengcai Qi. 2022. "Influence of Dental Implant Surface Modifications on Osseointegration and Biofilm Attachment" Coatings 12, no. 11: 1654. https://doi.org/10.3390/coatings12111654
APA StyleHan, W., Fang, S., Zhong, Q., & Qi, S. (2022). Influence of Dental Implant Surface Modifications on Osseointegration and Biofilm Attachment. Coatings, 12(11), 1654. https://doi.org/10.3390/coatings12111654







