Amorphous Carbon Coatings with Different Metal and Nonmetal Dopants: Influence of Cathode Modification on Laser-Arc Evaporation and Film Deposition
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Coating Properties and Deposition Rate
3.2. Cathode Erosion and Arc Spots
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anders, A. Cathodic Arcs: From Fractal Spots to Energetic Condensation; Springer: New York, NY, USA, 2008; ISBN 9780387791074. [Google Scholar]
- Schultrich, B. Tetrahedrally Bonded Amorphous Carbon Films I: Basics, Structure and Preparation; Springer: Berlin, Germany, 2018; ISBN 978-3-662-55925-3. [Google Scholar]
- Bewilogua, K.; Hofmann, D. History of diamond-like carbon films—From first experiments to worldwide applications. Surf. Coat. Technol. 2014, 242, 214–225. [Google Scholar] [CrossRef]
- Kaulfuss, F.; Weihnacht, V.; Zawischa, M.; Lorenz, L.; Makowski, S.; Hofmann, F.; Leson, A. Effect of Energy and Temperature on Tetrahedral Amorphous Carbon Coatings Deposited by Filtered Laser-Arc. Materials 2021, 14, 2176. [Google Scholar] [CrossRef] [PubMed]
- Vetter, J. 60 years of DLC coatings: Historical highlights and technical review of cathodic arc processes to synthesize various DLC types, and their evolution for industrial applications. Surf. Coat. Technol. 2014, 257, 213–240. [Google Scholar] [CrossRef]
- Kandah, M.; Meunier, J.-L. Vacuum arc cathode spot movement on various kinds of graphite cathodes. Plasma Sources Sci. Technol. 1996, 5, 349–355. [Google Scholar] [CrossRef]
- Fraunhofer-Gesellschaft zur Föderung der angewandten Forschung e.V. Offenlegungsschrift. Verfahren zur Bearbeitung von Oberflächen einer Beschichtung aus Hartem Kohlenstoff. Patent DE102006010916A1, 1 March 2006.
- Kandah, M.; Meunier, J.-L. Study of microdroplet generation from vacuum arcs on graphite cathodes. J. Vac. Sci. Technol. A 1995, 13, 2444–2450. [Google Scholar] [CrossRef]
- Kandah, M.; Meunier, J.-L. Erosion Study on Graphite Cathodes Using Pulsed Vacuum Arcs. IEEE Trans. Plasma Sci. 1996, 24, 523–527. [Google Scholar] [CrossRef]
- Donnet, C.; Erdemir, A. (Eds.) Tribology of Diamond-Like Carbon Films: Fundamentals and Applications; Springer: Berlin, Germany, 2008; Available online: https://link.springer.com/book/10.1007/978-0-387-49891-1 (accessed on 5 January 2022).
- Sánchez-López, J.C.; Fernández, A. Doping and Alloying Effects on DLC Coatings. In Tribology of Diamond-Like Carbon Films: Fundamentals and Applications; Donnet, C., Erdemir, A., Eds.; Springer: Berlin, Germany, 2008; pp. 311–338. [Google Scholar]
- Zia, A.W.; Zhou, Z.; Li, L.K.-Y. Structural mechanical and tribological characteristics of DLC coatings: Chapter 7. In Nanomaterials-Based Coatings: Fundamentals and Applications; Nguyen Tri, P., Rtimi, S., Ouellet Plamondon, C.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 171–194. ISBN 978-0-12-815884-5. [Google Scholar]
- Zhang, C.; Liu, Y.; Yang, Z.; Chen, L.; Qiao, S. Cathode spot movement on a continuous carbon fiber reinforced Cu matrix composite in vacuum. Vacuum 2013, 93, 45–49. [Google Scholar] [CrossRef]
- Polster, M. Abscheidung von dotierten Kohlenstoffschichten mit dem laserinduzierten Vakuumbogen. Ph.D. Thesis, Westsächsiche Hochschule Zwickau, Zwickau, Germany, 2002. [Google Scholar]
- Chen, J.S.; Lau, S.P.; Chen, G.Y.; Sun, Z.; Li, Y.J.; Tay, B.K.; Chai, J.W. Deposition of iron containing amorphous carbon films by filtered cathodic vacuum arc technique. Diam. Relat. Mater. 2001, 10, 2018–2023. [Google Scholar] [CrossRef]
- Jang, Y.-J.; Kang, Y.-J.; Kitazume, K.; Umehara, N.; Kim, J. Mechanical and electrical properties of micron-thick nitrogen-doped tetrahedral amorphous carbon coatings. Diam. Relat. Mater. 2016, 69, 121–126. [Google Scholar] [CrossRef]
- Kim, J.-I.; Jang, Y.-J.; Kim, J.; Kim, J. Effects of silicon doping on low-friction and high-hardness diamond-like carbon coating via filtered cathodic vacuum arc deposition. Sci. Rep. 2021, 11, 3529. [Google Scholar] [CrossRef]
- Pasaja, N.; Sansongsiri, S.; Intarasiri, S.; Vilaithong, T.; Anders, A. Mo-containing ta-C deposited by dual filtered cathodic vacuum arc with selective pulsed bias voltage. Nuclear Instr. Methods Phys. Res. 2007, B259, 867–870. [Google Scholar] [CrossRef] [Green Version]
- Chhowalla, M.; Yin, Y.; Amaratunga, G.A.J.; McKenzie, D.R.; Frauenheim, T. Highly tetrahedral amorphous carbon films with low stress. Appl. Phys. Lett. 1996, 69, 2344–2346. [Google Scholar] [CrossRef]
- Anders, S.; Anders, A.; Yu, K.M.; Yao, X.Y.; Brown, I.G. On the macroparticle flux from vacuum arc cathode spots. IEEE Trans. Plasma Sci. 1993, 21, 440–446. [Google Scholar] [CrossRef]
- Daalder, J.E. Erosion and the origin of charged and neutral species in vacuum arcs. J. Phys. D 1975, 8, 1647. [Google Scholar] [CrossRef]
- Daalder, J.E. Components of cathode erosion in vacuum arcs. J. Phys. D 1976, 9, 2379–2397. [Google Scholar] [CrossRef]
- Daalder, J.E. Cathode Spots and Vacuum Arcs. Physica C 1981, 104, 91–106. [Google Scholar] [CrossRef]
- Davis, W.D.; Miller, H.C. Analysis of the Electrode Products Emitted by dc Arcs in a Vacuum Ambient. J. Appl. Phys. 1969, 40, 2212–2221. [Google Scholar] [CrossRef]
- Kimblin, C.W. Erosion and ionization in the cathode spot regions of vacuum arcs. J. Appl. Phys. 1973, 44, 3074–3081. [Google Scholar] [CrossRef]
- Kimblin, C.W. Cathode spot erosion and ionization phenomena in the transition from vacuum to atmospheric pressure arcs. J. Appl. Phys. 1974, 45, 5235–5244. [Google Scholar] [CrossRef]
- Koch, A.W.; Nürnberg, A.W.; Behrisch, R. Investigation of Vacuum Arcs on Graphite Cathodes. J. Nucl. Mat. 1984, 122–123, 1437–1439. [Google Scholar] [CrossRef]
- Sethuraman, S.K.; Chatterton, P.A.; Barrault, M.R. A Study of the Erosion Rate of Vacuum Arcs in a transverse Magnetic Field. J. Nucl. Mat. 1982, 111–112, 510–516. [Google Scholar] [CrossRef]
- Chhowalla, M.; Weiler, M.; Davis, C.A.; Kleinsorge, B.; Amaratunga, G.A.J. Deposition of smooth tetrahedral amorphous carbon thin films using a cathodic arc without a macroparticle filter. Appl. Phys. Lett. 1995, 67, 894–896. [Google Scholar] [CrossRef]
- Anders, A.; Oks, E.M.; Yushkov, G.Y.; Savkin, K.P.; Brown, Y.; Nikolaev, A.G. Determination of the specific ion erosion of the vacuum arc cathode by measuring the total ion current from the discharge plasma. Tech. Phys. 2006, 51, 1311–1315. [Google Scholar] [CrossRef]
- Chhowalla, M.; Yin, Y.; Amaratunga, G.A.J.; McKenzie, D.R.; Frauenheim, T. Boronated tetrahedral amorphous carbon (ta-C:B). Diam. Relat. Mater. 1997, 6, 207–211. [Google Scholar] [CrossRef]
- Kautek, W.; Pentzien, S.; Conradi, A.; Krüger, J.; Brzezinka, K.-W. Pulsed-laser deposition and boron-blending of diamond-like carbon (DLC)thin films. Appl. Surface Sci. 1996, 106, 158–165. [Google Scholar] [CrossRef]
- Kleinsorge, B.; Ilie, A.; Chhowalla, M.; Fukarek, W.; Milnne, W.I.; Robertson, J. Electrical and optical properties of boronated tetrahedrally bonded amorphous carbon (ta-C:B). Diam. Relat. Mater. 1998, 7, 472–476. [Google Scholar] [CrossRef]
- Krauser, J.; Nix, A.-K.; Gehrke, H.-G.; Hofsäss, H.; Trautmann, C.; Weidinger, A. Conductivity enhancement of ion tracks in tetrahedral amorphous carbon by doping with N, B, Cu and Fe. Nucl. Instr. Methods Phys. Res. 2012, B272, 280–283. [Google Scholar] [CrossRef]
- Panwar, O.S.; Khan, M.A.; Satyanarayana, B.S.; Kumar, S. Properties of boron and phosphorous incorporated tetrahedral amorphous carbon films grown using filtered cathodic vacuum arc process. Appl. Surf. Sci. 2010, 256, 4383–4390. [Google Scholar] [CrossRef]
- Ghadai, R.K.; Das, S.; Kumar, D.; Mondal, S.C.; Swain, B.P. Correlation between structural and mechanical properties of silicon doped DLC thin films. Diam. Relat. Mater. 2018, 82, 25–32. [Google Scholar] [CrossRef]
- Hilbert, J. Understanding and Improving The Environmental Dependent Tribology and Thermal Stability of Hydrogenated Amorphous Carbon by Using Silicon and Oxygen as Dopants. Ph.D. Thesis, University of Pennsylvania, Pennsylvania, PA, USA, 2018. [Google Scholar]
- Chen, J.S.; Lau, S.P.; Sun, Z.; Chen, G.Y.; Li, Y.J.; Tay, B.K.; Chai, J.W. Metal-containing amorphous carbon films for hydrophobic application. Thin Solid Films 2001, 398, 110–115. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Lee, K.-R.; Wang, A. Effect of metal doping on structural characteristics of amorphous carbon system: A first-principles study. Thin Solid Films 2016, 607, 67–72. [Google Scholar] [CrossRef]
- Ray, S.C.; Pong, W.F.; Papakonstantinou, P. Iron, nitrogen and silicon doped diamond like carbon (DLC) thin films: A comparative study. Thin Solid Films 2016, 610, 42–47. [Google Scholar] [CrossRef]
- Wang, L.L.; Wang, R.Y.; Yan, S.J.; Zhang, R.; Yang, B.; Zhang, Z.D.; Huang, Z.H.; Fu, D.J. Structure and properties of Mo-containing diamond-like carbon films produced by ion source assisted cathodic arc ion-plating. Appl. Surf. Sci. 2013, 286, 109–114. [Google Scholar] [CrossRef]
- Krülle, T.; Peritsch, P.; Kaulfuß, F.; Bui Thi, Y.; Zawischa, M.; Weihnacht, V. Investigation of surface defects on doped and undoped carbon coatings deposited by Laser Arc Technology using an optical surface quantification method. In Jahrbuch Oberflächentechnik 2021; Sörgel, T., Ed.; Eugen G. Leuze Verlag KG: Bad Saulgau, Germany, 2022. [Google Scholar]
- Widany, J. Density-Functional Tight-Bindung Calculations on the Straucture of Complex Boron Nitride Systems. Ph.D. Thesis, TU Chemnitz, Chemnitz, Germany, 1997. [Google Scholar]
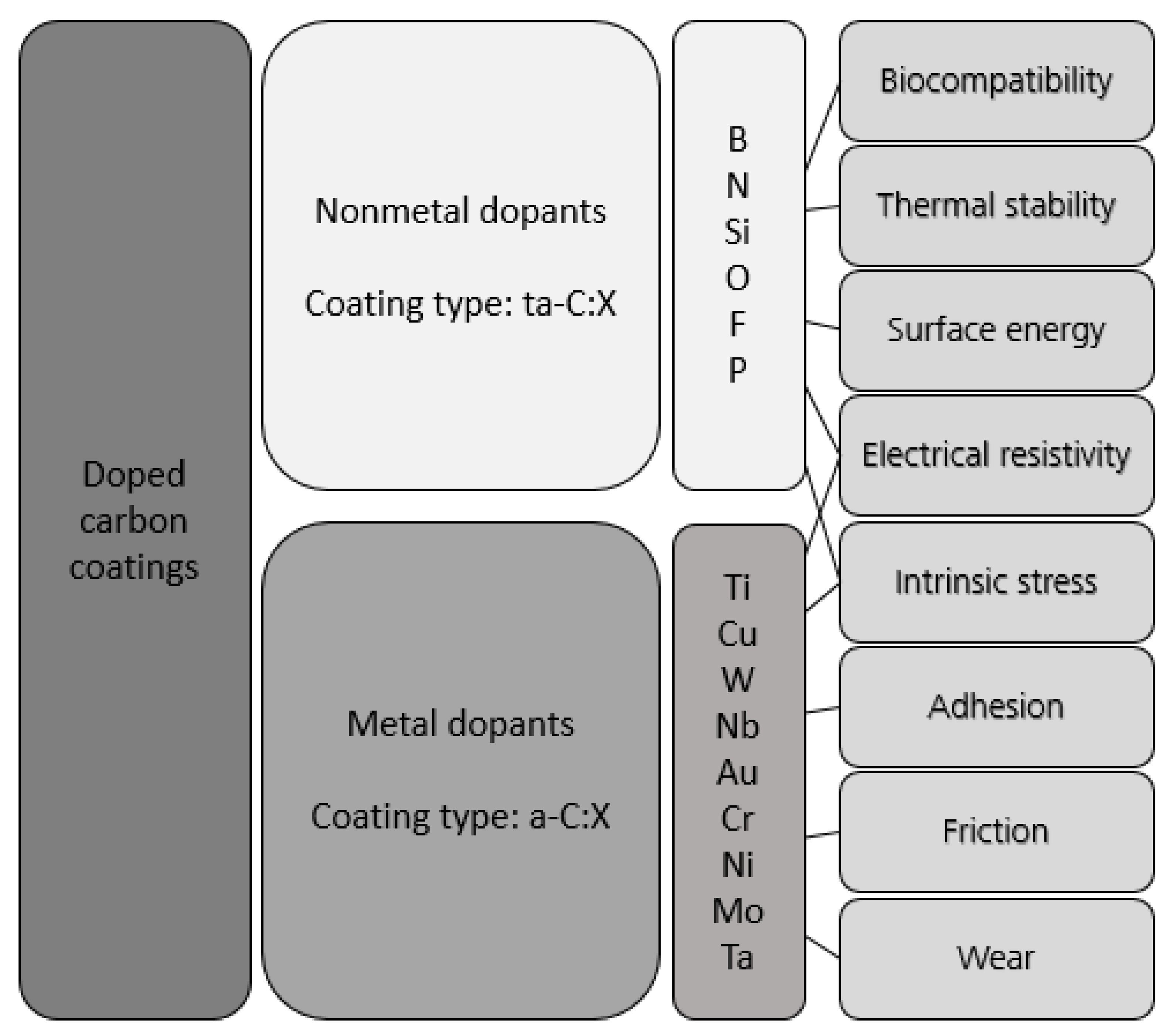
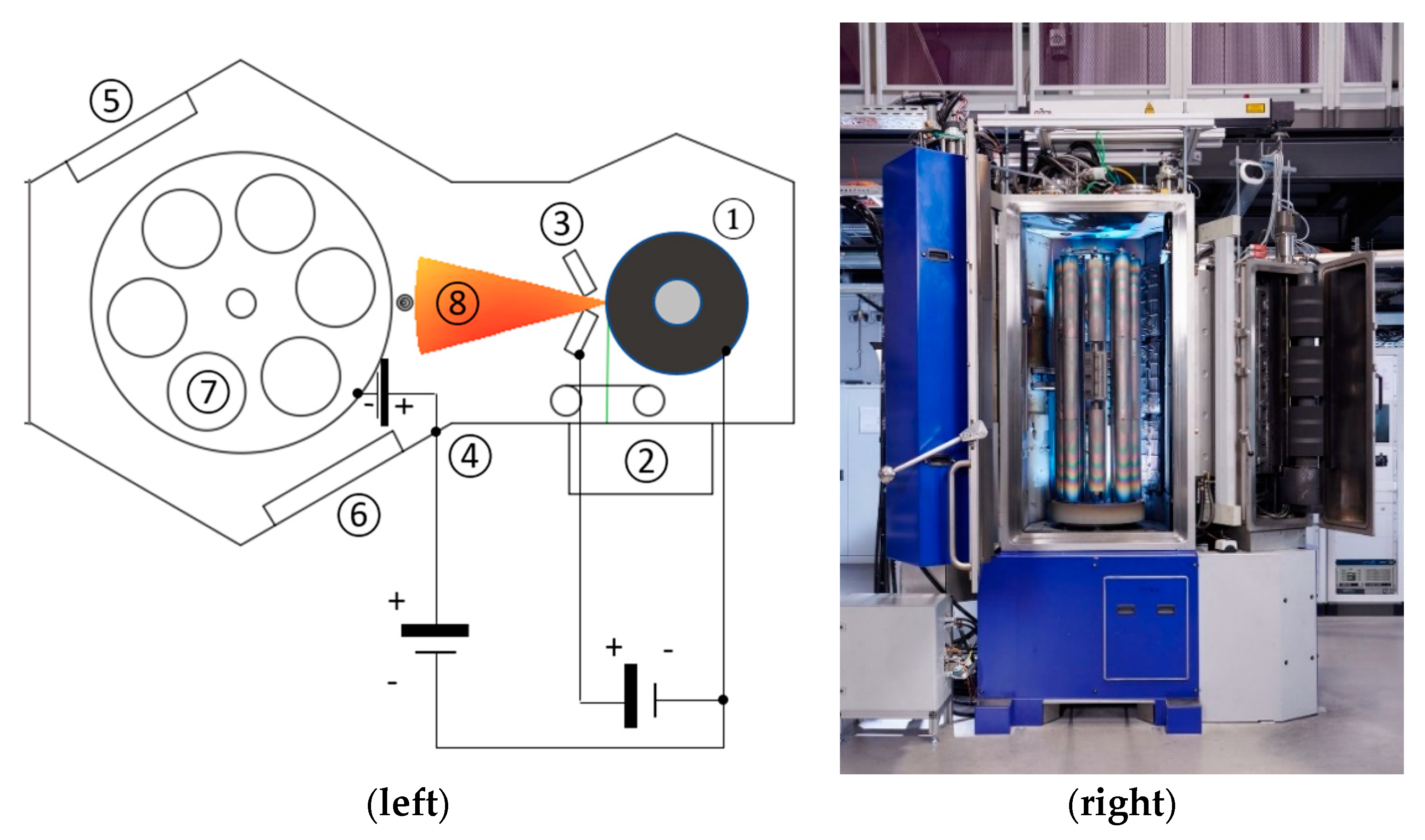

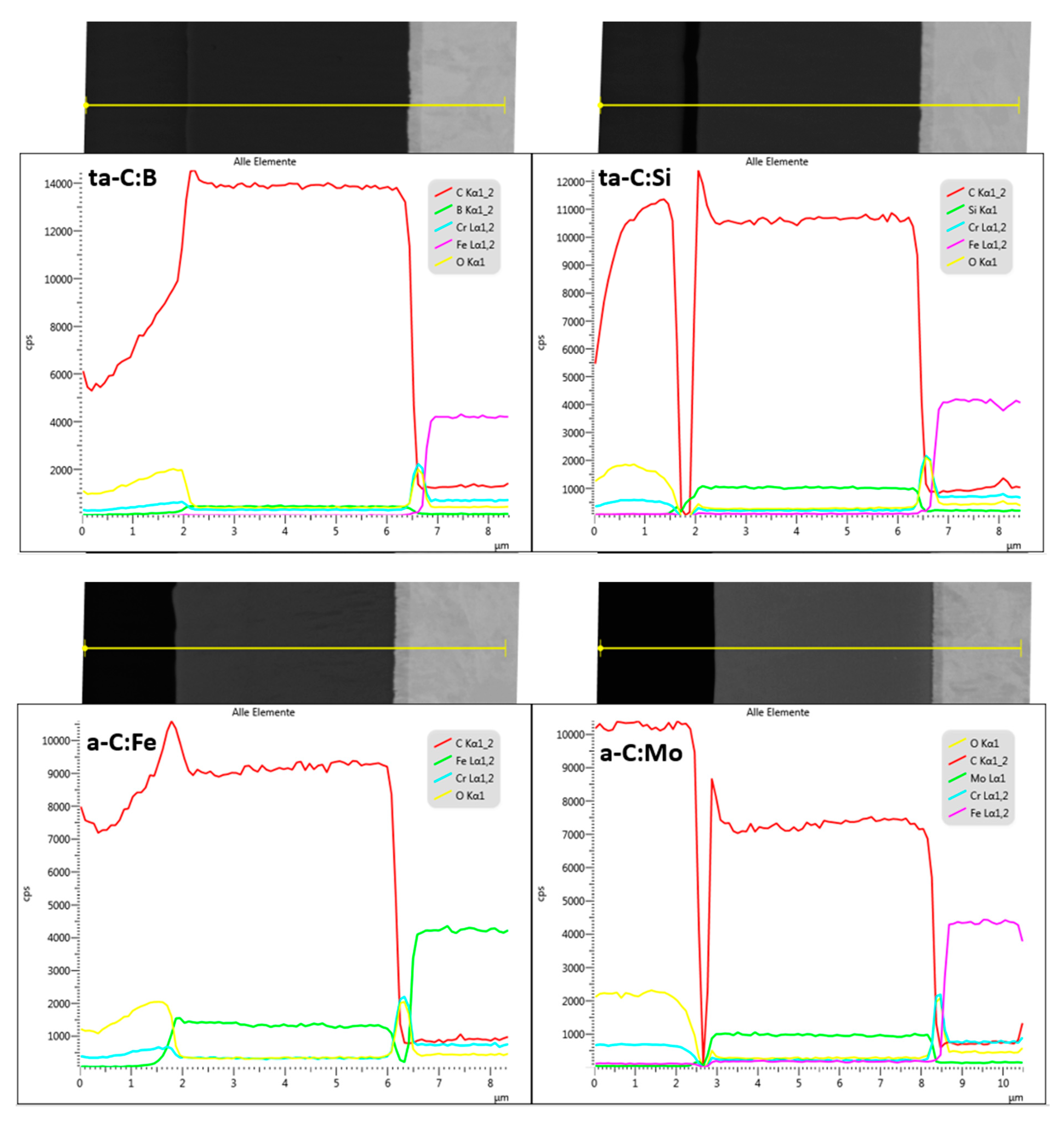

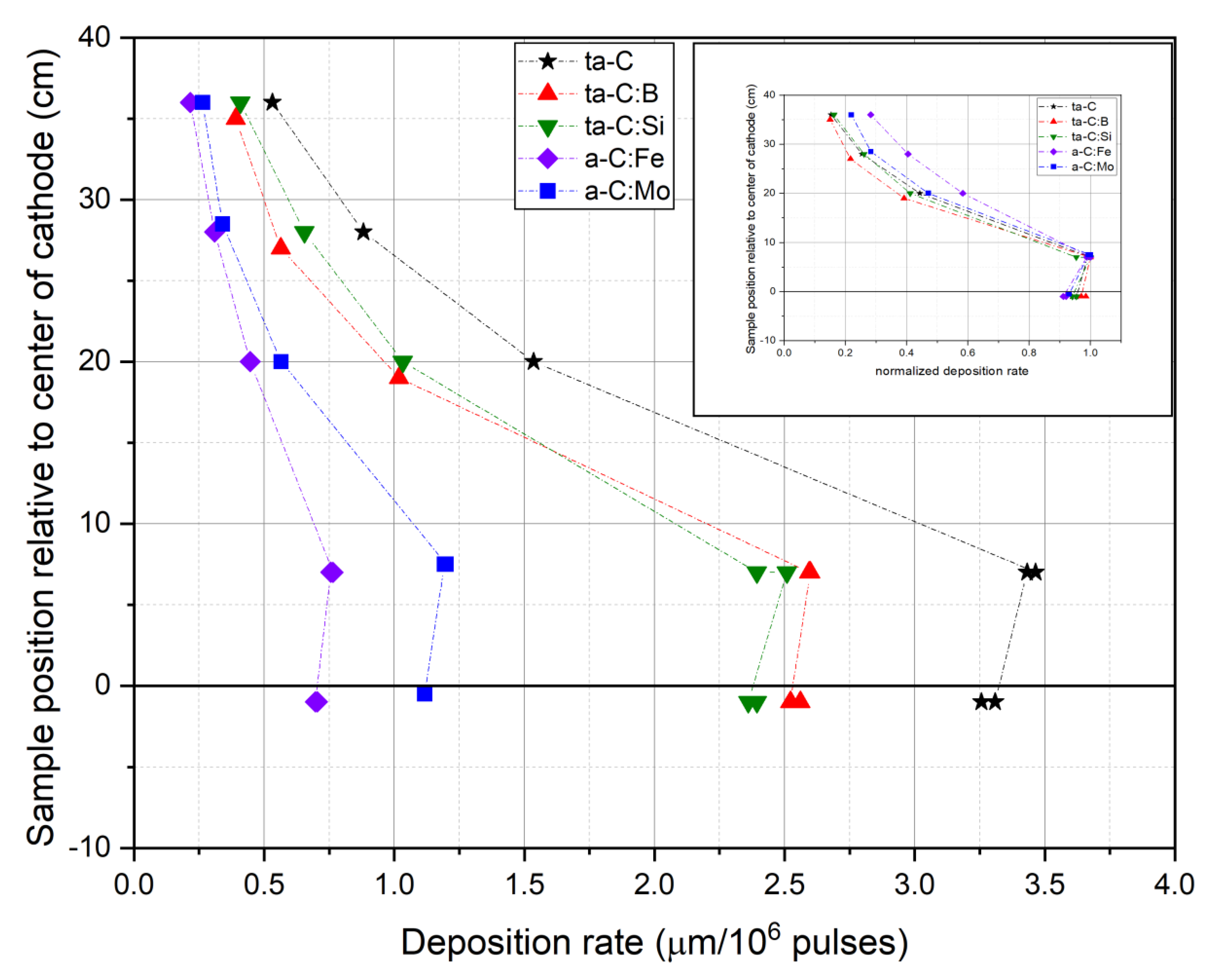
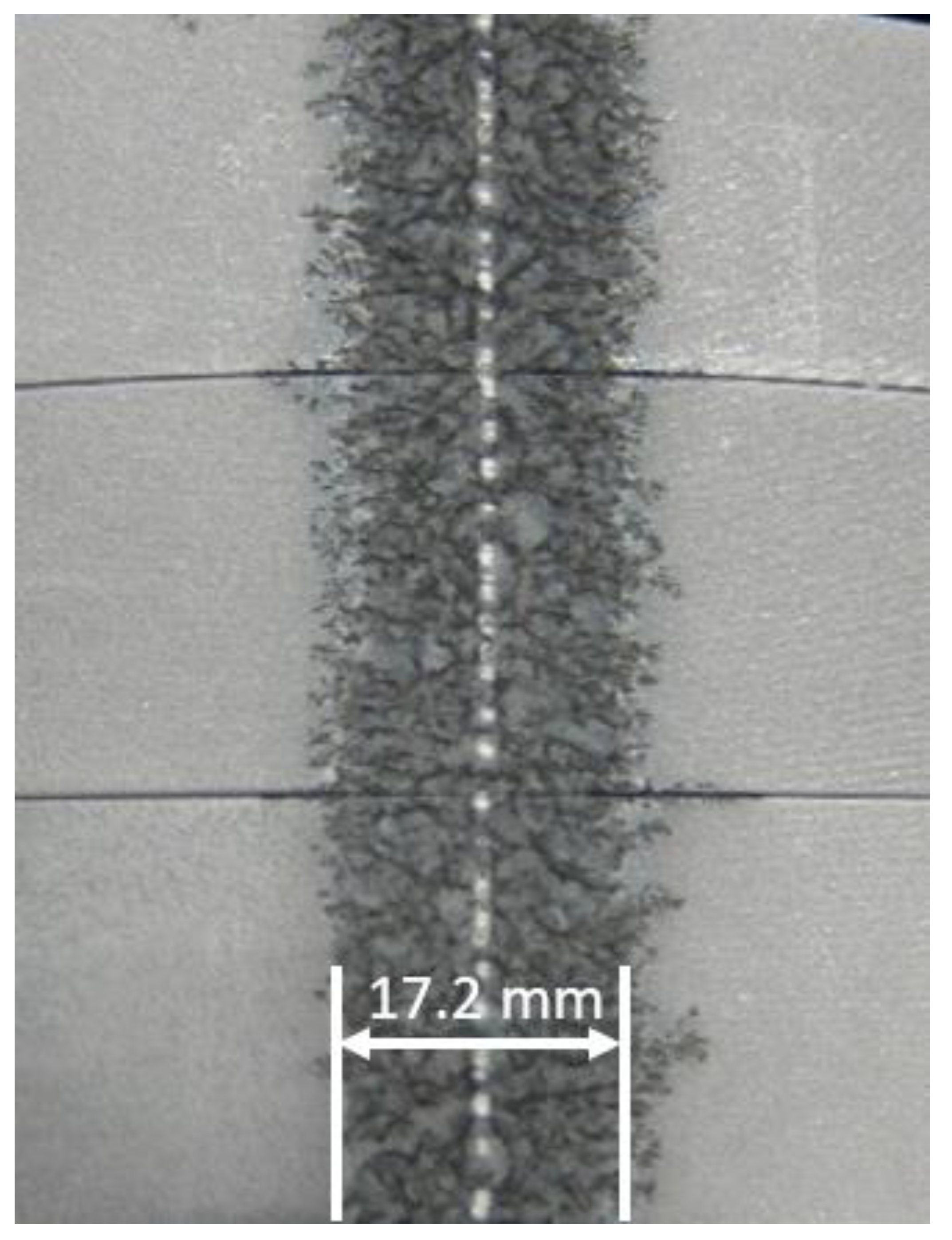
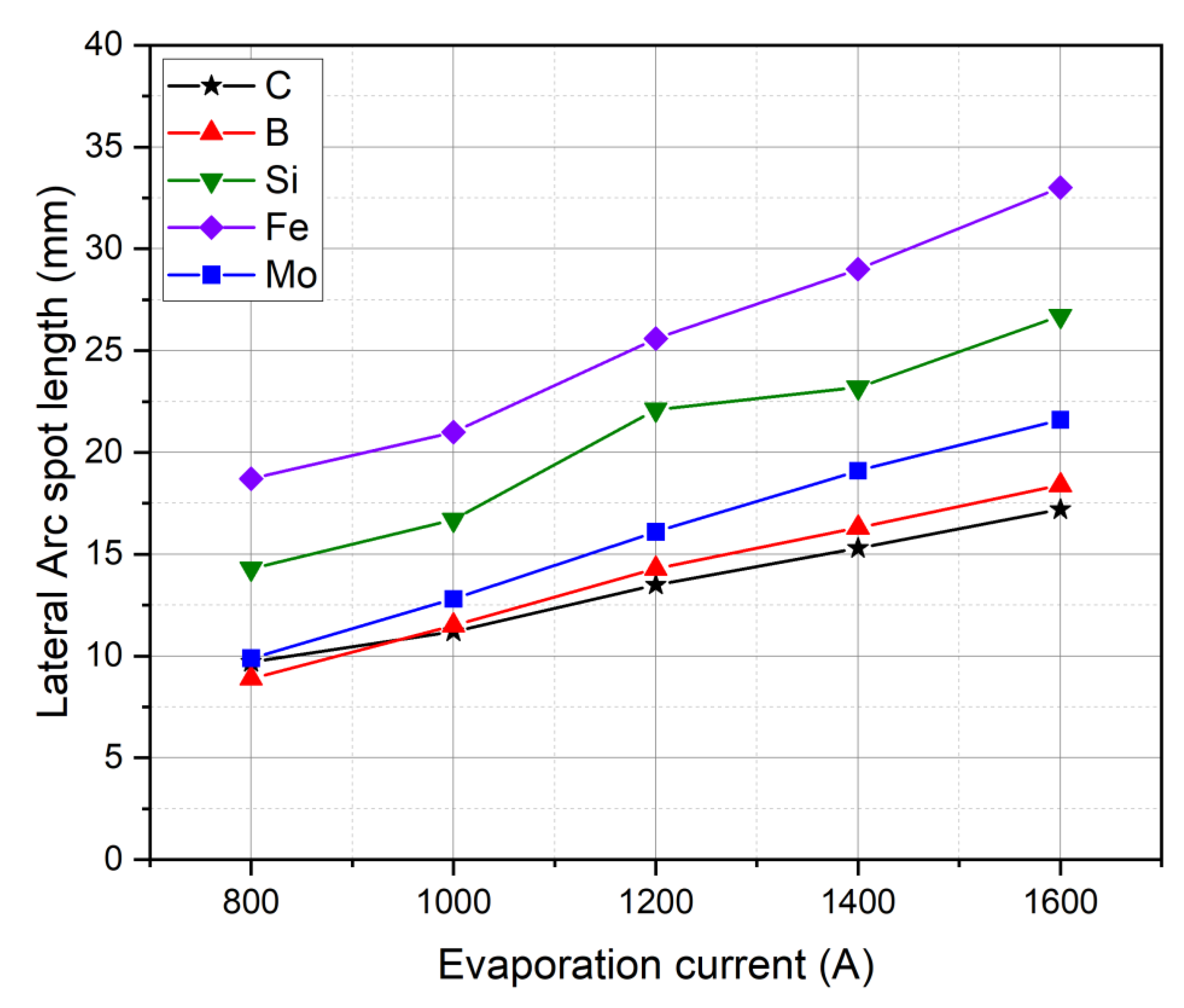
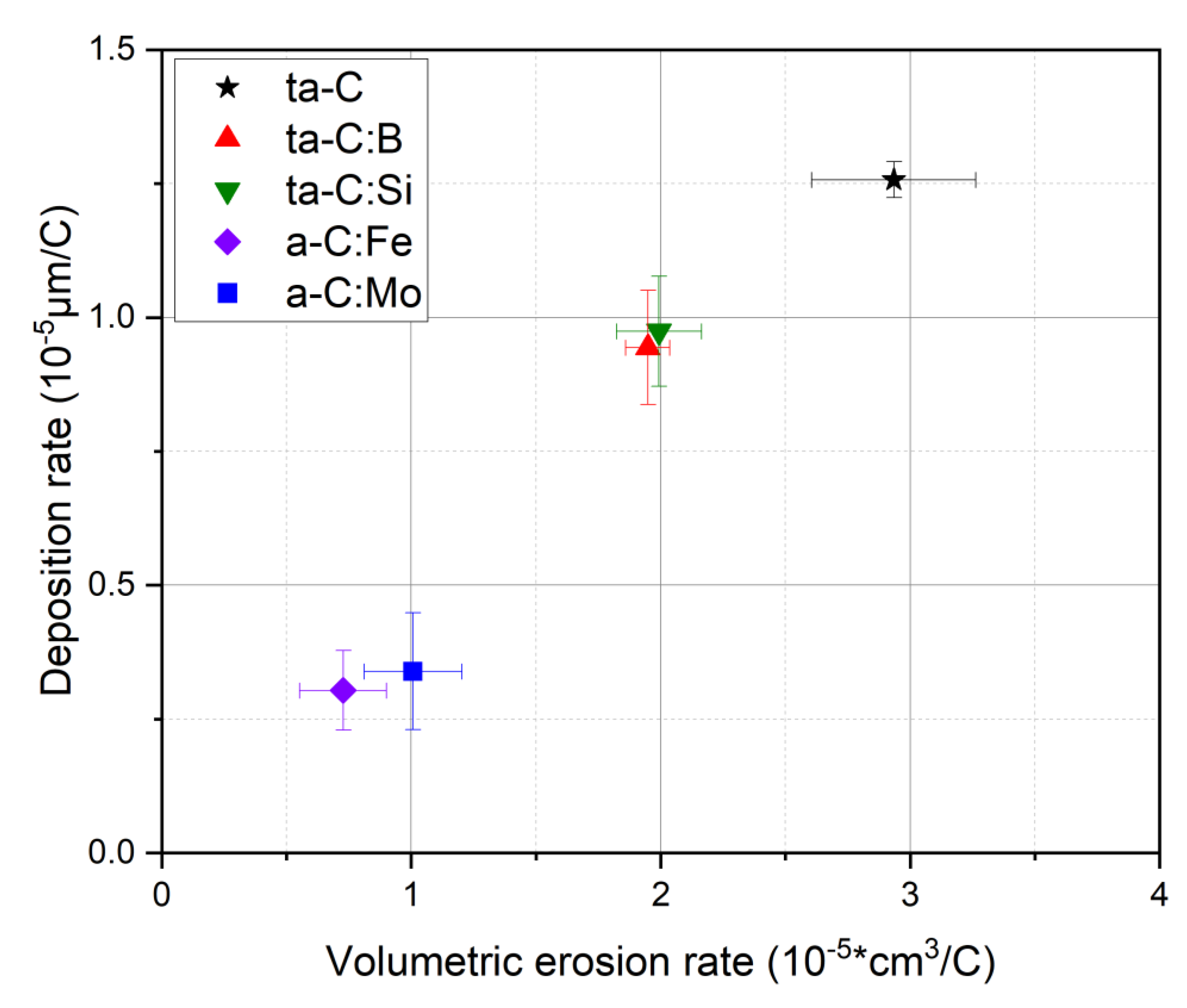
| Dopant | Intended Effect of the Dopant | Reference |
|---|---|---|
| Boron (B) | Reduction of intrinsic stress while maintaining high hardness | [19,31,32,33,34,35] |
| Silicon (Si) | Increase in thermal stability, surface smoothening | [36,37] |
| Iron (Fe) | Reduction of intrinsic stress, change of wetting properties | [15,38,39,40] |
| Molybdenum (Mo) | Reduction of intrinsic stress, reduction of electrical resistivity, improved wear behavior | [18,41] |
| Target | Resulting Coating | Amount of Dopant (at.%) | Coating Thickness d (µm) | Deposition Rate Dr (µm × 10−6 Pulses) | Indentation Hardness HIT (GPa) | Young’s Modulus EIT (GPa) |
|---|---|---|---|---|---|---|
| C (pure) | ta-C | - | 4.9 ± 0.4 | 3.3 | 52.5 ± 0.9 | 541 ± 15 |
| Nonmetal dopants | ||||||
| C-B | ta-C:B | 5.0 | 4.2 ± 0.3 | 2.5 | 50.9 ± 0.5 | 530 ± 9 |
| C-Si | ta-C:Si | 5.7 | 4.3 ± 0.4 | 2.4 | 44.8 ± 0.2 | 497 ± 5 |
| Metal dopants | ||||||
| C-Fe | a-C:Fe | 10.3 | 4.0 ± 0.4 | 0.7 | 14.4 ± 0.1 | 168 ± 4 |
| C-Mo | a-C:Mo | 7.4 | 4.9 ± 0.5 | 1.2 | 25.2 ± 0.3 | 323 ± 5 |
| Material | Atomic Mass | Cohesive Energy (eV/atom) |
|---|---|---|
| Iron (Fe) | 56 | 4.28 [1] |
| Silicon (Si) | 28 | 4.63 [1] |
| Molybdenum (Mo) | 96 | 6.82 [1] |
| Carbon (C) | 12 | 7.37 [1] |
| Boron (B) | 11 | ~8 [43] |
| Target | Calculated Density of Target Material (g/cm3) | Gravimetric Erosion Rate Er,g (µg/C) | Volumetric Erosion Rate Er,v (10−5 cm3/C) |
|---|---|---|---|
| C (pure) | 1.85 ± 0.01 | 47.7 ± 3.1 | 2.9 ± 0.3 |
| Nonmetal dopants | |||
| C-B | 1.83 ± 0.01 | 34.1 ± 1.7 | 1.9 ± 0.1 |
| C-Si | 1.95 ± 0.06 | 36.5 ± 1.9 | 2.0 ± 0.2 |
| Metal dopants | |||
| C-Fe | 2.44 ± 0.01 | 17.0 ± 4.1 | 0.7 ± 0.2 |
| C-Mo | 2.62 ± 0.02 | 27.2 ± 6.6 | 1.0 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krülle, T.; Kaulfuß, F.; Weihnacht, V.; Hofmann, F.; Kirsten, F. Amorphous Carbon Coatings with Different Metal and Nonmetal Dopants: Influence of Cathode Modification on Laser-Arc Evaporation and Film Deposition. Coatings 2022, 12, 188. https://doi.org/10.3390/coatings12020188
Krülle T, Kaulfuß F, Weihnacht V, Hofmann F, Kirsten F. Amorphous Carbon Coatings with Different Metal and Nonmetal Dopants: Influence of Cathode Modification on Laser-Arc Evaporation and Film Deposition. Coatings. 2022; 12(2):188. https://doi.org/10.3390/coatings12020188
Chicago/Turabian StyleKrülle, Tim, Frank Kaulfuß, Volker Weihnacht, Falko Hofmann, and Florian Kirsten. 2022. "Amorphous Carbon Coatings with Different Metal and Nonmetal Dopants: Influence of Cathode Modification on Laser-Arc Evaporation and Film Deposition" Coatings 12, no. 2: 188. https://doi.org/10.3390/coatings12020188
APA StyleKrülle, T., Kaulfuß, F., Weihnacht, V., Hofmann, F., & Kirsten, F. (2022). Amorphous Carbon Coatings with Different Metal and Nonmetal Dopants: Influence of Cathode Modification on Laser-Arc Evaporation and Film Deposition. Coatings, 12(2), 188. https://doi.org/10.3390/coatings12020188







