Surface Conductivity and Preferred Orientation of TiN Film for Ti Bipolar Plate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Deposition of TiN Films
2.2. Characterization of TiN Films
2.3. Measurement of Interfacial Contact Resistance
2.4. Electrochemical Measurement
2.5. Computational Method
3. Results and Discussion
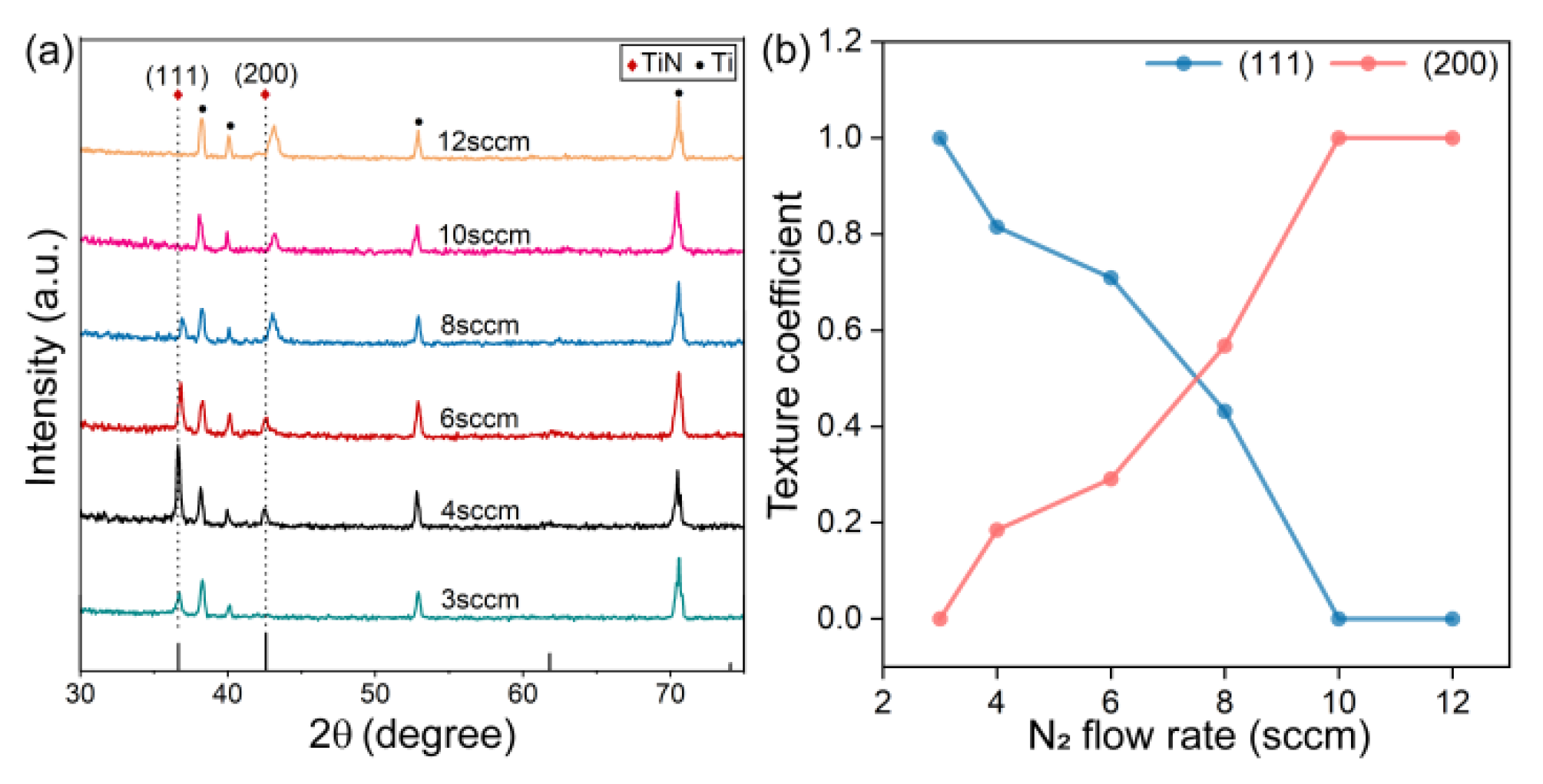
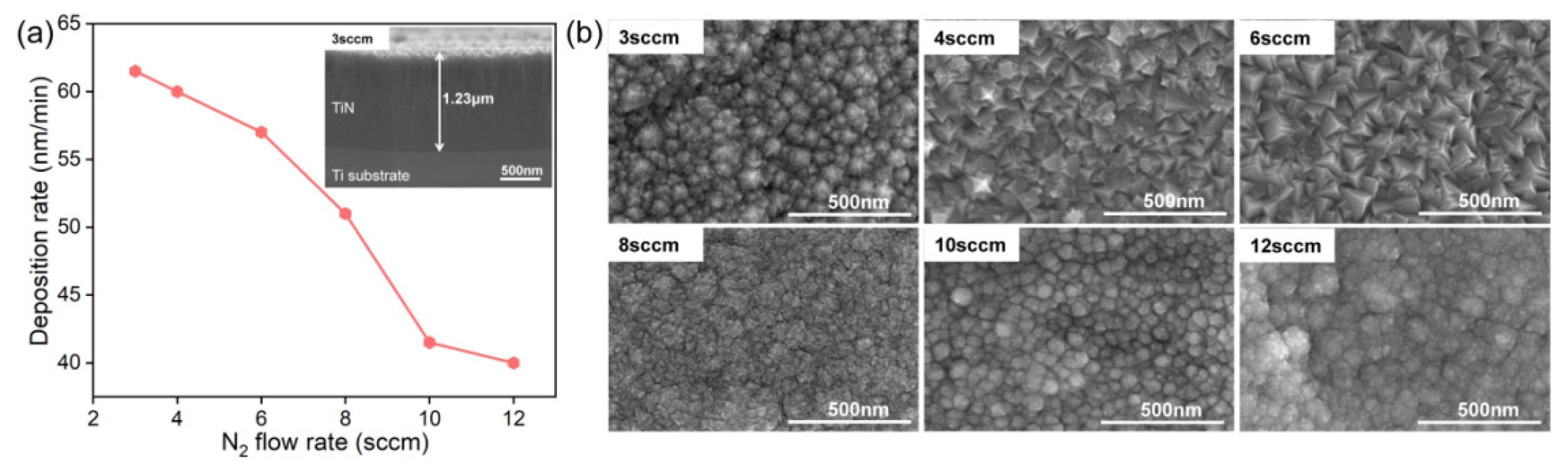
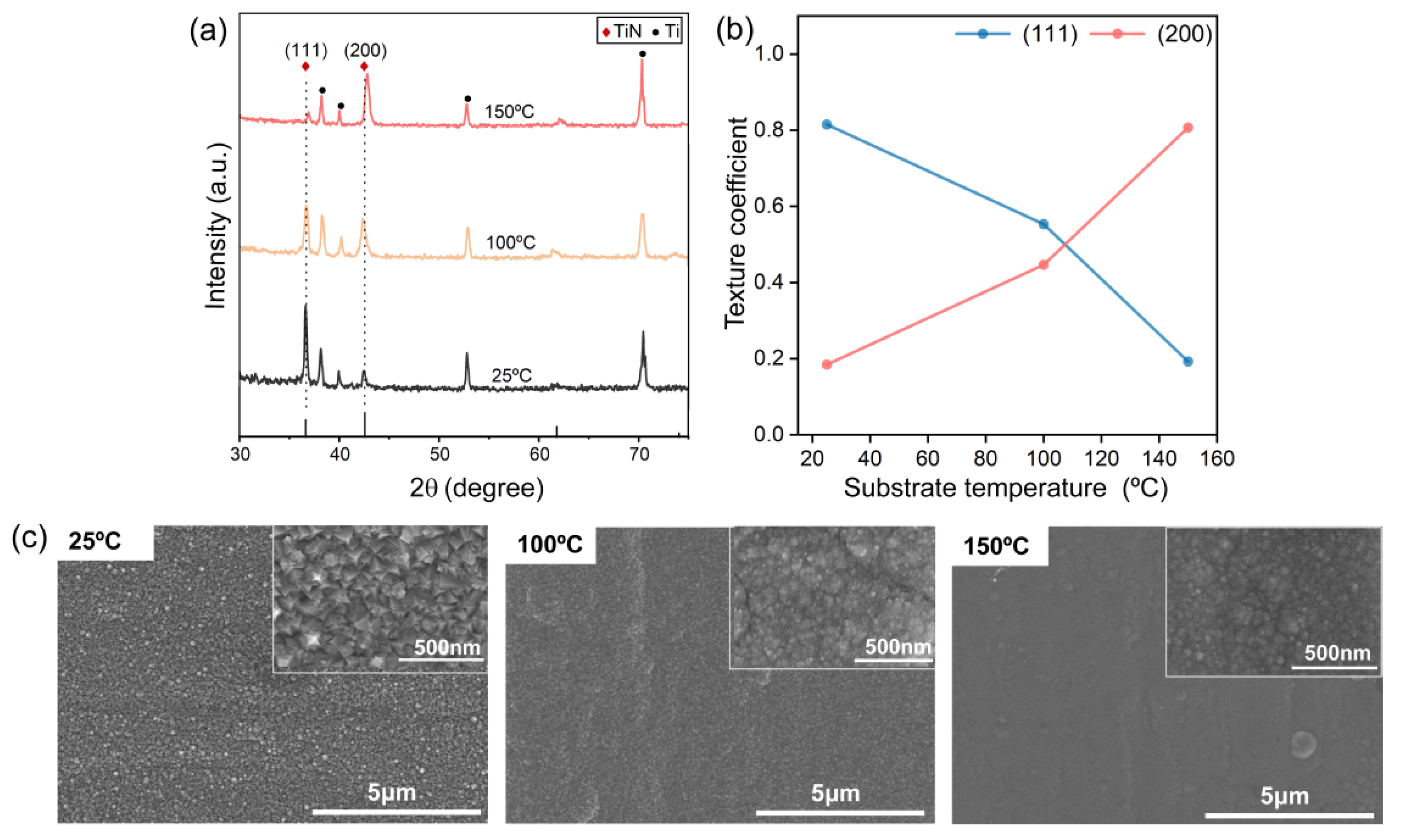
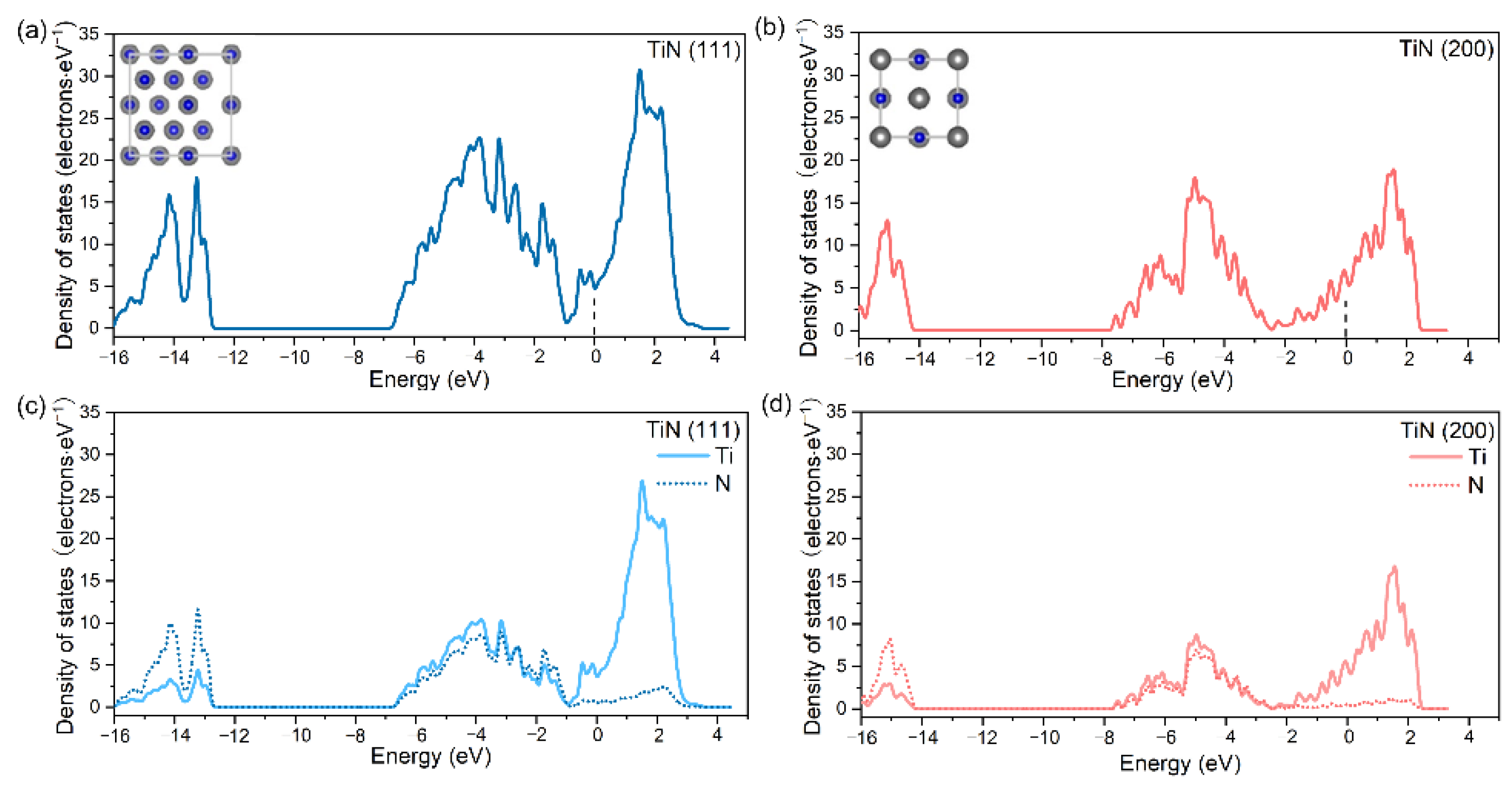
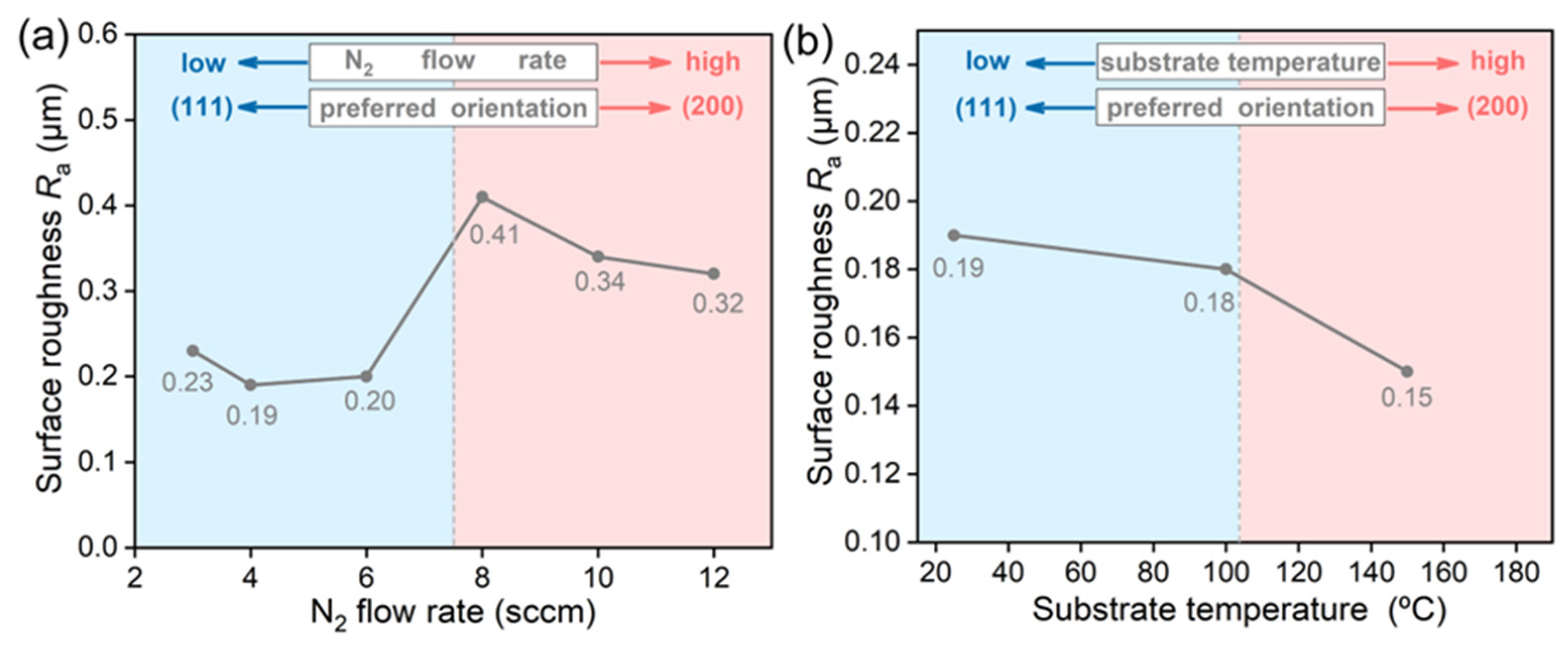
3.1. Characterization of TiN Films
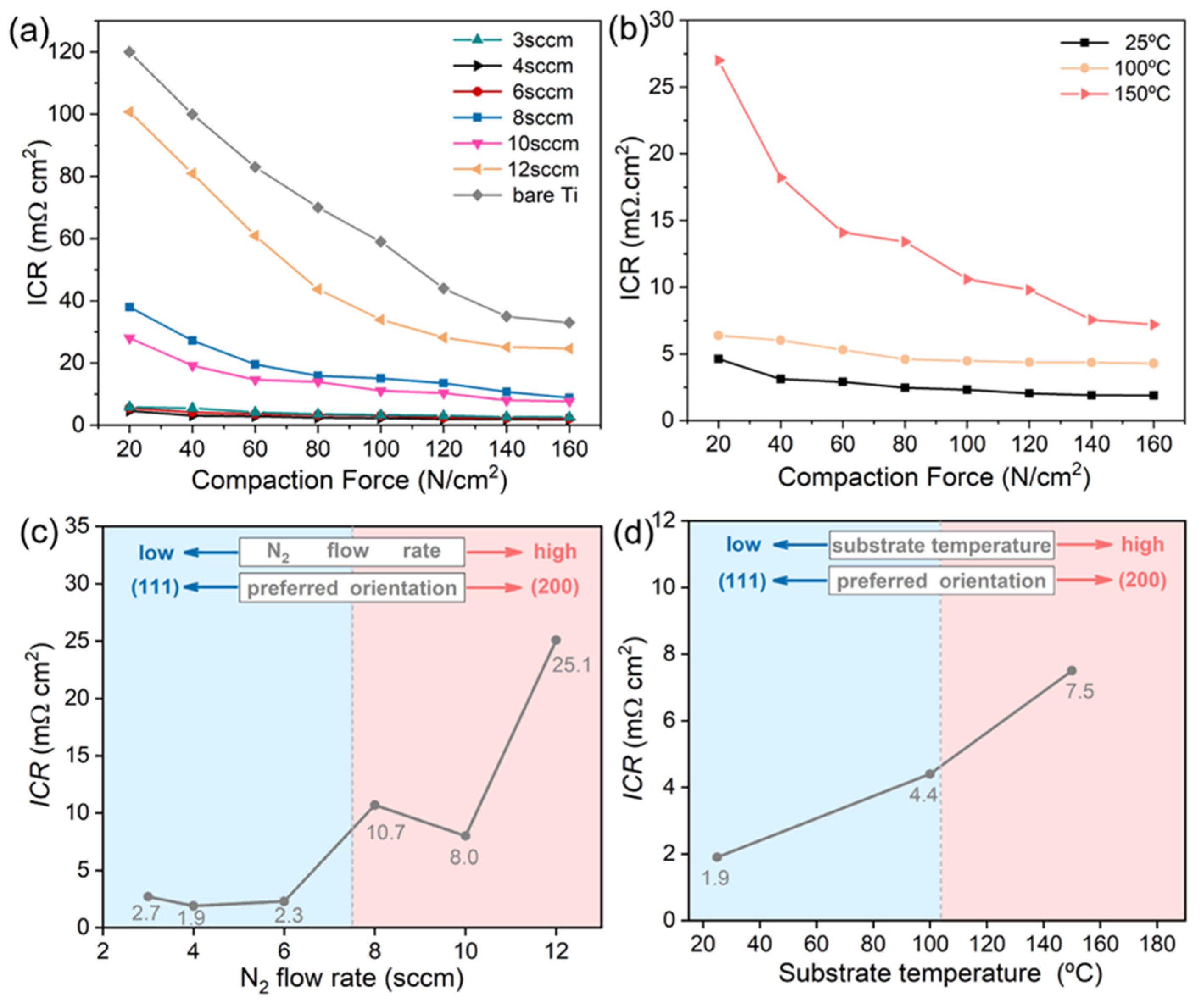
3.2. ICR Test
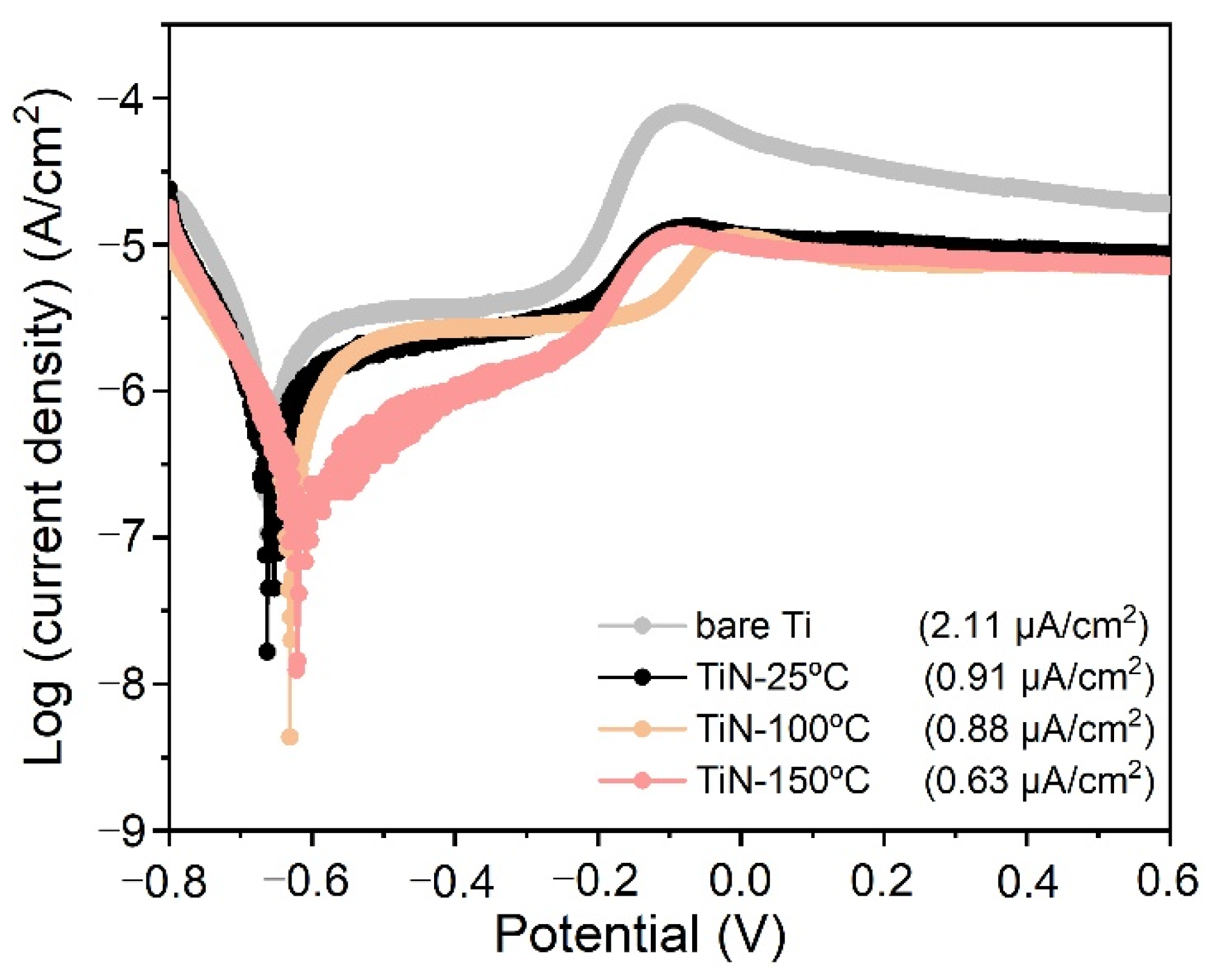
3.3. Electrochemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chandan, A.; Hattenberger, M.; El-kharouf, A.; Du, S.; Dhir, A.; Self, V.; Pollet, B.G.; Ingram, A.; Bujalski, W. High temperature (HT) polymer electrolyte membrane fuel cells (PEMFC)—A review. J. Power Sour. 2013, 231, 264–278. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Z.; Chen, J.; Li, C.; Zhang, Z.; Yan, X.; Liu, X.; Yang, S. Recent development and progress of structural energy devices. Chin. Chem. Lett. 2021; in press. [Google Scholar] [CrossRef]
- Xiong, K.; Wu, W.; Wang, S.; Zhang, L. Modeling, design, materials and fabrication of bipolar plates for proton exchange membrane fuel cell: A review. Appl. Energy 2021, 301, 117443. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, C.; Ling, C.-Y.; Han, M.; Yong, R.-Y.; Sun, D.; Chen, J. Review on current research of materials, fabrication and application for bipolar plate in proton exchange membrane fuel cell. Int. J. Hydrog. Energy 2020, 45, 29832–29847. [Google Scholar] [CrossRef]
- Singh, R.S.; Gautam, A.; Rai, V. Graphene-based bipolar plates for polymer electrolyte membrane fuel cells. Front. Mater. Sci. 2019, 13, 217–241. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Rehman, A.-U.; Siddique, S. Prospects and challenges of graphene based fuel cells. J. Energ. Chem. 2019, 39, 217–234. [Google Scholar] [CrossRef] [Green Version]
- Brady, M.P.; Weisbrod, K.; Paulauskas, I.; Buchanan, R.A.; More, K.L.; Wang, H.; Wilson, M.; Garzon, F.; Walker, L.R. Preferential thermal nitridation to form pin-hole free Cr-nitrides to protect proton exchange membrane fuel cell metallic bipolar plates. Scr. Mater. 2004, 50, 1017–1022. [Google Scholar] [CrossRef]
- Taherian, R. A review of composite and metallic bipolar plates in proton exchange membrane fuel cell: Materials, fabrication, and material selection. J. Power Sour. 2014, 265, 370–390. [Google Scholar] [CrossRef]
- He, R.Y.; Jiang, J.; Wang, R.F.; Yue, Y.; Chen, Y.; Pan, T.J. Anti-corrosion and conductivity of titanium diboride coating on metallic bipolar plates. Corros. Sci. 2020, 170, 108646. [Google Scholar] [CrossRef]
- Wu, S.; Yang, W.; Yan, H.; Zuo, X.; Cao, Z.; Li, H.; Shi, M.; Chen, H. A review of modified metal bipolar plates for proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2021, 46, 8672–8701. [Google Scholar] [CrossRef]
- Wang, S.-H.; Peng, J.; Lui, W.-B. Surface modification and development of titanium bipolar plates for PEM fuel cells. J. Power Sour. 2006, 160, 485–489. [Google Scholar] [CrossRef]
- Jin, J.; He, Z.; Zhao, X. Formation of a protective TiN layer by liquid phase plasma electrolytic nitridation on Ti–6Al–4V bipolar plates for PEMFC. Int. J. Hydrog. Energy 2020, 45, 12489–12500. [Google Scholar] [CrossRef]
- Zhang, P.; Hao, C.; Han, Y.; Du, F.; Wang, H.; Wang, X.; Sun, J. Electrochemical behavior and surface conductivity of NbC modified Ti bipolar plate for proton exchange membrane fuel cell. Surf. Coat. Technol. 2020, 397, 126064. [Google Scholar] [CrossRef]
- Yi, P.; Dong, C.; Zhang, T.; Xiao, K.; Ji, Y.; Wu, J.; Li, X. Effect of plasma electrolytic nitriding on the corrosion behavior and interfacial contact resistance of titanium in the cathode environment of proton-exchange membrane fuel cells. J. Power Sour. 2019, 418, 42–49. [Google Scholar] [CrossRef]
- Wang, S.-H.; Peng, J.; Lui, W.-B.; Zhang, J.-S. Performance of the gold-plated titanium bipolar plates for the light weight PEM fuel cells. J. Power Sour. 2006, 162, 486–491. [Google Scholar] [CrossRef]
- Li, T.; Yan, Z.; Liu, Z.; Yan, Y.; Chen, Y. Surface microstructure and performance of TiN monolayer film on titanium bipolar plate for PEMFC. Int. J. Hydrog. Energy 2021, 46, 31382–31390. [Google Scholar] [CrossRef]
- Lin, M.-T.; Wan, C.-H.; Wu, W. Comparison of corrosion behaviors between SS304 and Ti substrate coated with (Ti,Zr)N thin films as Metal bipolar plate for unitized regenerative fuel cell. Thin Solid Films 2013, 544, 162–169. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, P.; Han, Y.; Wang, H.; Wang, X.; Yu, Y.; Sun, J. Investigation on electrochemical behavior and surface conductivity of titanium carbide modified Ti bipolar plate of PEMFC. Int. J. Hydrog. Energy 2020, 45, 10050–10058. [Google Scholar] [CrossRef]
- Xu, J.; Huang, H.J.; Li, Z.; Xu, S.; Tao, H.; Munroe, P.; Xie, Z.-H. Corrosion behavior of a ZrCN coated Ti alloy with potential application as a bipolar plate for proton exchange membrane fuel cell. J. Alloys Compd. 2016, 663, 718–730. [Google Scholar] [CrossRef] [Green Version]
- Gao, P.; Xie, Z.; Wu, X.; Ouyang, C.; Lei, T.; Yang, P.; Liu, C.; Wang, J.; Ouyang, T.; Huang, Q. Development of Ti bipolar plates with carbon/PTFE/TiN composites coating for PEMFCs. Int. J. Hydrog. Energy 2018, 43, 20947–20958. [Google Scholar] [CrossRef]
- Gao, P.; Xie, Z.; Ouyang, C.; Tao, T.; Wu, X.; Huang, Q. Electrochemical characteristics and interfacial contact resistance of Ni-P/TiN/PTFE coatings on Ti bipolar plates. J. Solid State Electr. 2018, 22, 1971–1981. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, Q.; Huang, B. Synthesis and properties of novel N/Ta-co-doped TiO2 coating on titanium in simulated PEMFC environment. J. Alloys Compd. 2021, 879, 160470. [Google Scholar] [CrossRef]
- Chou, W.J.; Yu, G.P.; Huang, J.H. Deposition of TiN thin films on Si 100 by HCD ion plating. Surf. Coat. Technol. 2001, 140, 206–214. [Google Scholar] [CrossRef]
- Nakamori, Y.; Miwa, K.; Ninomiya, A.; Li, H.W.; Ohba, N.; Towata, S.I.; Zuttel, A.; Orimo, S.I. Correlation between thermodynamical stabilities of metal borohydrides and cation electronegativites: First-principles calculations and experiments. Phys. Rev. B 2006, 74, 045126. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.H.Q.; Zhao, L.R.; Immarigeon, J.-P. Preferred orientation and hardness enhancement of TiN/CrN superlattice coatings deposited by reactive magnetron sputtering. Scr. Mater. 2002, 46, 293–297. [Google Scholar] [CrossRef]
- Lu, L.; Luo, F.; Huang, Z.; Zhou, W.; Zhu, D. Influence of the nitrogen flow rate on the infrared emissivity of TiNx films. Infrared Phys Techn. 2018, 88, 144–148. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; He, W.; Ma, C.; Liao, B.; Li, Y. Mechanical modification and damage mechanism evolution of TiN films subjected to cyclic nano-impact by adjusting N/Ti ratios. J. Alloys Compd. 2019, 809, 151816. [Google Scholar] [CrossRef]
- Jeyachandran, Y.L.; Narayandass, S.K.; Mangalaraj, D.; Areva, S.; Mielczarski, J.A. Properties of titanium nitride films prepared by direct current magnetron sputtering. Mater. Sci. Eng. A 2007, 445, 223–236. [Google Scholar] [CrossRef]
- Arshi, N.; Lu, J.; Joo, Y.K.; Lee, C.G.; Yoon, J.H.; Ahmed, F. Influence of nitrogen gas flow rate on the structural, morphological and electrical properties of sputtered TiN films. J. Mater. Sci. Mater. Electron. 2012, 24, 1194–1202. [Google Scholar] [CrossRef]
- Huang, J.-H.; Lau, K.-W.; Yu, G.-P. Effect of nitrogen flow rate on structure and properties of nanocrystalline TiN thin films produced by unbalanced magnetron sputtering. Surf. Coat. Technol. 2005, 191, 17–24. [Google Scholar] [CrossRef]
- Kim, H.T.; Park, J.Y.; Park, C. Effects of nitrogen flow rate on titanium nitride films deposition by DC facing target sputtering method. Korean J. Chem. Eng. 2012, 29, 676–679. [Google Scholar] [CrossRef]
- Montes de Oca Valero, J.A.; Le Petitcorps, Y.; Manaud, J.P.; Chollon, G.; Carrillo Romo, F.J.; López, A.M. Low temperature, fast deposition of metallic titanium nitride films using plasma activated reactive evaporation. J. Vac. Sci. Technol. A 2005, 23, 394–400. [Google Scholar] [CrossRef]
- Huang, H.H.; Hon, M.H. Effect of N2 addition on growth and properties of titanium nitride films obtained by atmospheric pressure chemical vapor deposition. Thin Solid Films 2002, 416, 54–61. [Google Scholar] [CrossRef]
- Lee, W.J.; Yun, E.Y.; Hong, S.W.; Kwon, S.H. Ultrathin effective tin protective films prepared by plasma-enhanced atomic layer deposition for high performance metallic bipolar plates of polymer electrolyte membrane fuel cells. Appl. Surf. Sci. 2020, 519, 146215. [Google Scholar] [CrossRef]
- Jones, M.I.; McColl, I.R.; Grant, D.M. Effect of substrate preparation and deposition conditions on the preferred orientation of TiN coatings deposited by RF reactive sputtering. Surf. Coat. Technol. 2000, 132, 143–151. [Google Scholar] [CrossRef]
- Li, H.W.; Kikuchi, K.; Sato, T.; Nakamori, Y.; Ohba, N.; Aoki, M.; Miwa, K.; Towata, S.; Orimo, S. Synthesis and Hydrogen Storage Properties of a Single-Phase Magnesium Borohydride Mg(BH4)(2). Mater. Trans. 2008, 49, 2224–2228. [Google Scholar] [CrossRef] [Green Version]
- Li, H.W.; Kikuchi, K.; Nakamori, Y.; Ohba, N.; Miwa, K.; Towata, S.; Orimo, S. Dehydriding and rehydriding processes of well-crystallized Mg(BH(4))(2) accompanying with formation of intermediate compounds. Acta Mater. 2008, 56, 1342–1347. [Google Scholar] [CrossRef]
- Lengauer, W. Properties of bulk δ-TiN1-x prepared by nitrogen diffusion into titanium metal. J. Alloys Compd. 1992, 186, 293–307. [Google Scholar] [CrossRef]
- Amin, M.A.; Khaled, K.F.; Fadl-Allah, S.A. Testing validity of the Tafel extrapolation method for monitoring corrosion of cold rolled steel in HCl solutions—Experimental and theoretical studies. Corros. Sci. 2010, 52, 140–151. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Z.; Li, T.; Wang, Q.; Li, H.; Wang, Y.; Wu, C.; Yan, Y.; Chen, Y. Surface Conductivity and Preferred Orientation of TiN Film for Ti Bipolar Plate. Coatings 2022, 12, 454. https://doi.org/10.3390/coatings12040454
Yan Z, Li T, Wang Q, Li H, Wang Y, Wu C, Yan Y, Chen Y. Surface Conductivity and Preferred Orientation of TiN Film for Ti Bipolar Plate. Coatings. 2022; 12(4):454. https://doi.org/10.3390/coatings12040454
Chicago/Turabian StyleYan, Zhi, Tao Li, Qian Wang, Hongjiao Li, Yao Wang, Chaoling Wu, Yigang Yan, and Yungui Chen. 2022. "Surface Conductivity and Preferred Orientation of TiN Film for Ti Bipolar Plate" Coatings 12, no. 4: 454. https://doi.org/10.3390/coatings12040454






