Application and Monitoring of Oxidative Alginate–Biocide Hydrogels for Two Case Studies in “The Sassi and the Park of the Rupestrian Churches of Matera”
Abstract
:1. Introduction
2. Materials and Methods
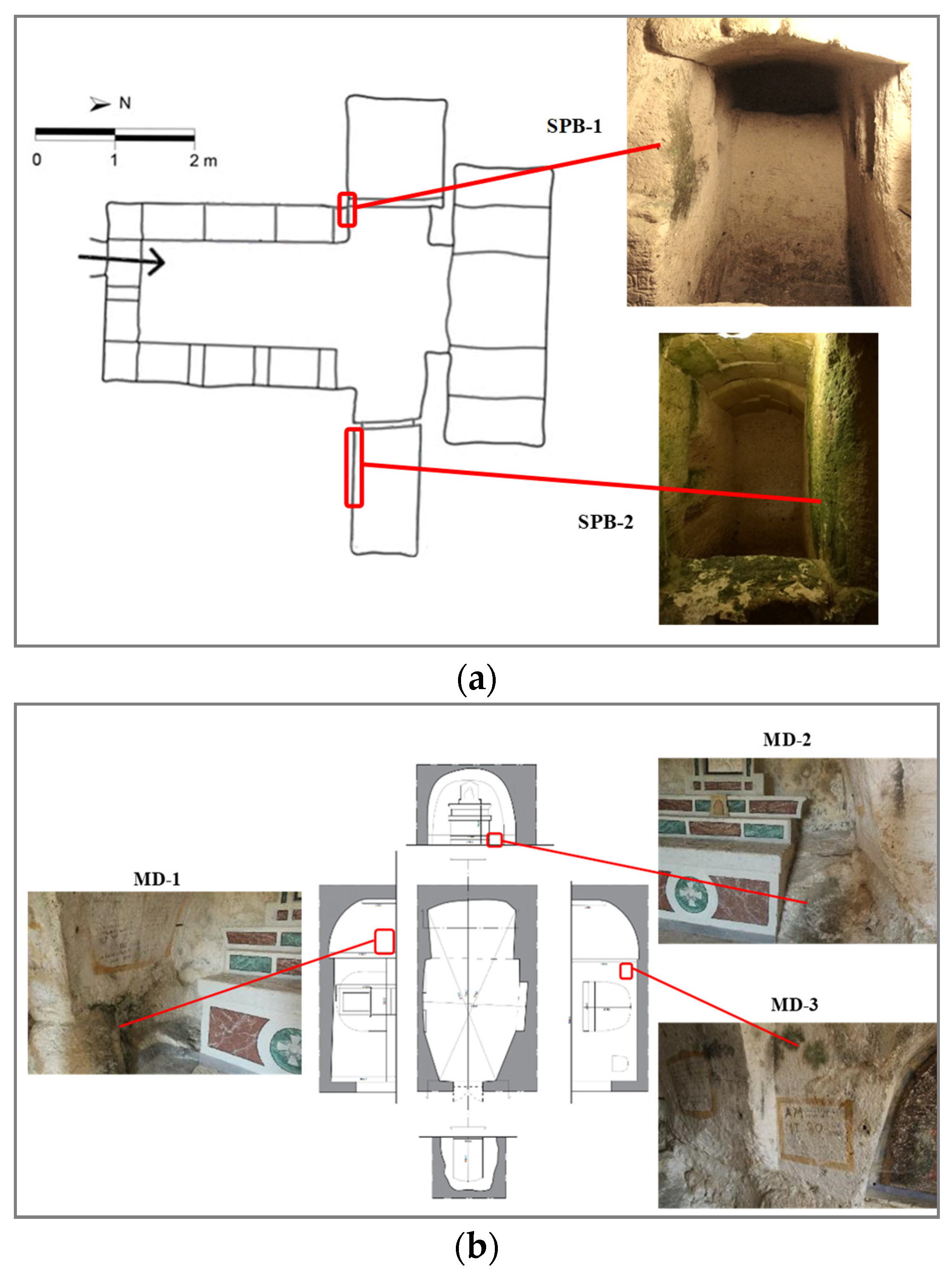
2.1. Areas of Intervention
2.1.1. Site 1: San Pietro Barisano Church
2.1.2. Site 2: Madonna dei Derelitti Church
2.2. Environmental Parameters
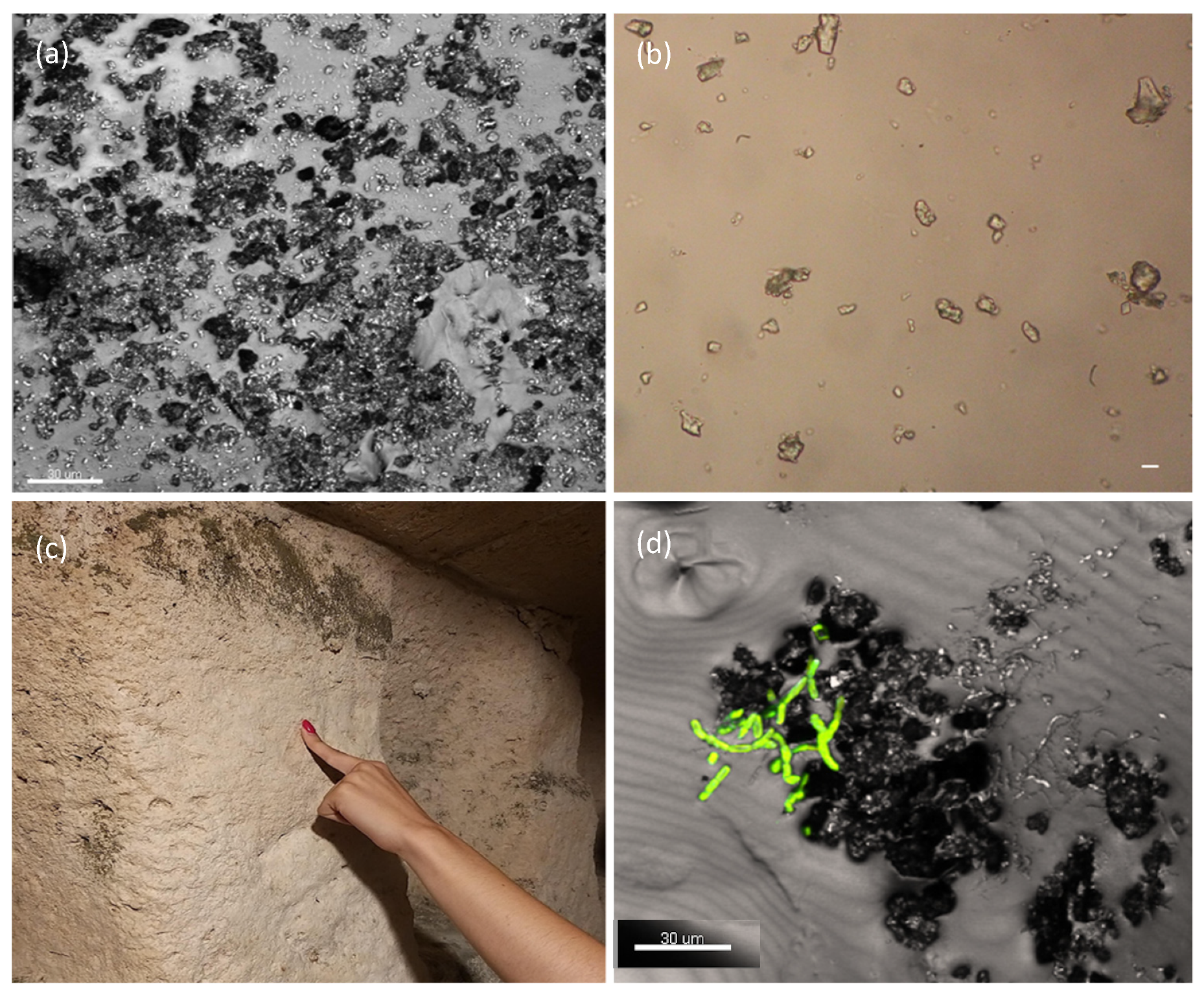
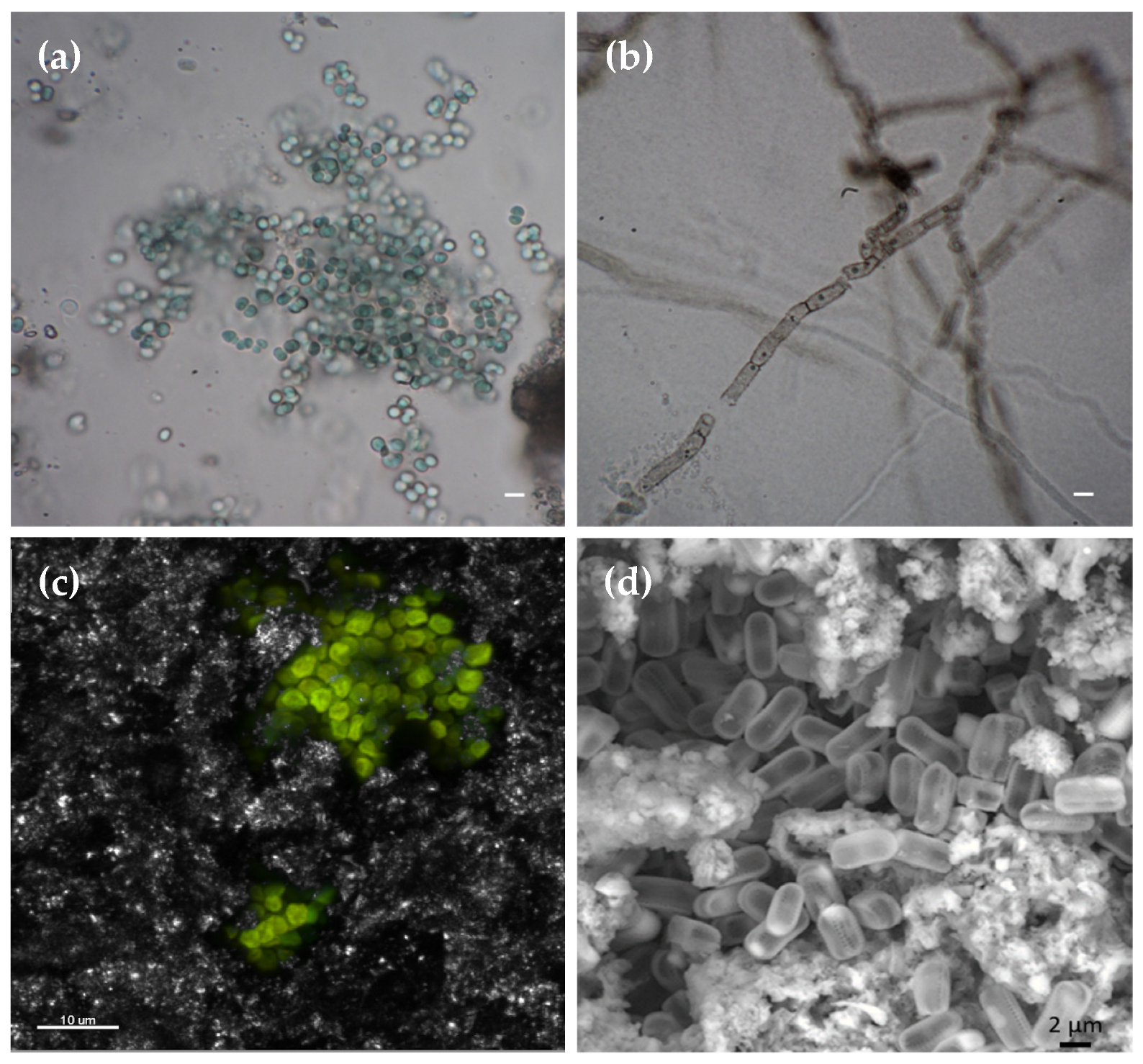
2.3. Sampling of Biofilms and Their Characterization
2.4. Macroscopic and Microscopic Observations
2.5. Colorimetric Measurements
2.6. Preparation and Application of the Alginate–Biocide Hydrogels
2.7. Protective Coatings
2.8. Peeling Test
3. Results and Discussion
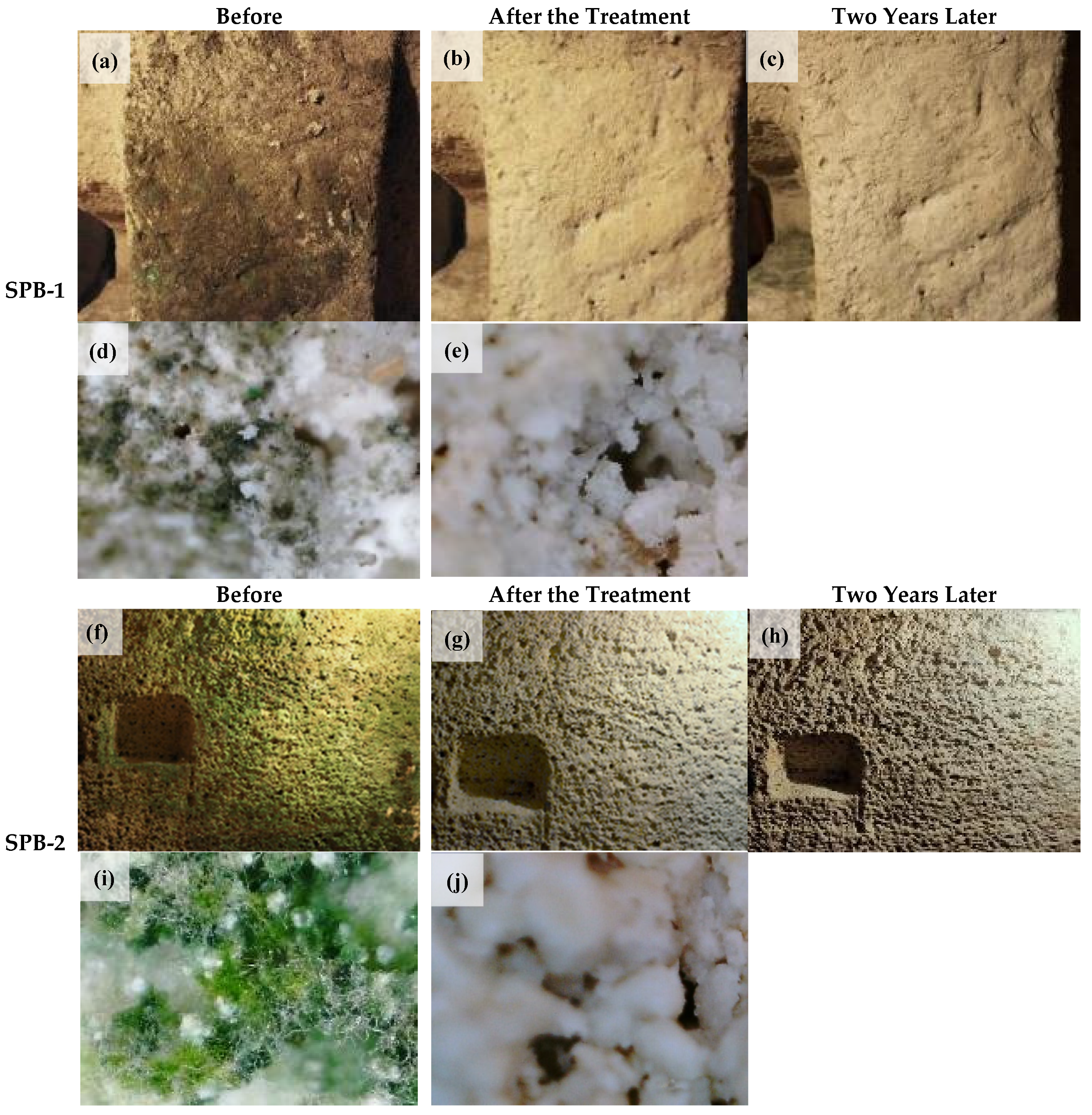
3.1. Site 1: The Putridarium of San Pietro Barisano Church
3.2. Site 2: Madonna dei Derelitti Church
4. Conclusions
Supplementary Materials
 ), BH_1 (
), BH_1 (  ) and BH_2 (
) and BH_2 (  ); Figure S2: SEM images of reference stone materials fragments collected inside the Church of the “Madonna dei Derelitti” and EDS analysis of two selected areas; Figure S3: SEM images of colonized stone materials fragments collected inside the Church of the “Madonna dei Derelitti” and EDS analysis of two selected areas; Figure S4: SEM images of stone materials fragments collected after cleaning treatment inside the Church of the “Madonna dei Derelitti” and EDS analysis of two selected areas; Figure S5: Images of samples collected at different areas of the Church of “Madonna dei Derelitti”, after the cleaning procedures obtained after observations at (a) the light microscope (bar = 5 µm, 40×) and (b) CLSM. It is evident the absence of living microorganisms; mineral material and cellular debris are visible in (a) among which empty frustules of diatoms (arrow); Table S1: Summary of the investigated properties of pure alginate hydrogel (HY) and biocidal gels containing calcium hypochlorite (BH_1) and sodium dichloroisocyanurate (BH_2). References [40,41,42,43] are cited in the supplementary materials.
); Figure S2: SEM images of reference stone materials fragments collected inside the Church of the “Madonna dei Derelitti” and EDS analysis of two selected areas; Figure S3: SEM images of colonized stone materials fragments collected inside the Church of the “Madonna dei Derelitti” and EDS analysis of two selected areas; Figure S4: SEM images of stone materials fragments collected after cleaning treatment inside the Church of the “Madonna dei Derelitti” and EDS analysis of two selected areas; Figure S5: Images of samples collected at different areas of the Church of “Madonna dei Derelitti”, after the cleaning procedures obtained after observations at (a) the light microscope (bar = 5 µm, 40×) and (b) CLSM. It is evident the absence of living microorganisms; mineral material and cellular debris are visible in (a) among which empty frustules of diatoms (arrow); Table S1: Summary of the investigated properties of pure alginate hydrogel (HY) and biocidal gels containing calcium hypochlorite (BH_1) and sodium dichloroisocyanurate (BH_2). References [40,41,42,43] are cited in the supplementary materials.Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guillitte, O. Bioreceptivity: A new concept for building ecology studies. Sci. Total Environ. 1995, 167, 215–220. [Google Scholar] [CrossRef]
- Miller, A.Z.; Sanmartín, P.; Pereira-Pardo, L.; Dionísio, A.; Saiz-Jimenez, C.; Macedo, M.F.; Prieto, B. Bioreceptivity of building stones: A review. Sci. Total Environ. 2012, 426, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín, P.; Miller, A.Z.; Prieto, B.; Viles, H.A. Revisiting and reanalysing the concept of bioreceptivity 25 years on. Sci. Total Environ. 2021, 770, 145314. [Google Scholar] [CrossRef] [PubMed]
- Scheerer, S.; Ortega-Morales, O.; Gaylarde, C. Microbial Deterioration of Stone Monuments-An Updated Overview. Adv. Appl. Microbiol. 2009, 66, 97–139. [Google Scholar] [CrossRef] [PubMed]
- Warscheid, T.; Braams, J. Biodeterioration of stone: A review. Int. Biodeterior. Biodegrad. 2000, 46, 343–368. [Google Scholar] [CrossRef]
- Tiano, P. Biodeterioration of Stone Monuments a Worldwide Issue. Open Conf. Proc. J. 2016, 7, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Sterflinger, K.; Piñar, G. Microbial deterioration of cultural heritage and works of art-tilting at windmills? Appl. Microbiol. Biotechnol. 2013, 97, 9637–9646. [Google Scholar] [CrossRef] [Green Version]
- Macedo, M.F.; Miller, A.Z.; Dionísio, A.; Saiz-Jimenez, C. Biodiversity of cyanobacteria and green algae on monuments in the Mediterranean Basin: An overview. Microbiology 2009, 155, 3476–3490. [Google Scholar] [CrossRef] [Green Version]
- Alexander, S.A.; Schiesser, C.H. Heteroorganic molecules and bacterial biofilms: Controlling biodeterioration of cultural heritage. Arkivoc 2016, 2017, 180–222. [Google Scholar] [CrossRef] [Green Version]
- Kakakhel, M.A.; Wu, F.; Gu, J.D.; Feng, H.; Shah, K.; Wang, W. Controlling biodeterioration of cultural heritage objects with biocides: A review. Int. Biodeterior. Biodegrad. 2019, 143, 104721. [Google Scholar] [CrossRef]
- Albertano, P. Cyanobacterial biofilms in monuments and caves. In Ecology of Cyanobacteria II: Their Diversity in Space and Time; Witton, B.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 317–343. [Google Scholar] [CrossRef]
- Rossi, F.; Micheletti, E.; Bruno, L.; Adhikary, S.P.; Albertano, P.; de Philippis, R. Characteristics and role of the exocellular polysaccharides produced by five cyanobacteria isolated from phototrophic biofilms growing on stone monuments. Biofouling 2012, 28, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Pozo-Antonio, J.S.; Rivas, T.; López, A.J.; Fiorucci, M.P.; Ramil, A. Effectiveness of granite cleaning procedures in cultural heritage: A review. Sci. Total Environ. 2016, 571, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Ranalli, G.; Zanardini, E. Biocleaning on Cultural Heritage: New frontiers of microbial biotechnologies. J. Appl. Microbiol. 2021, 131, 583–603. [Google Scholar] [CrossRef] [PubMed]
- Cappitelli, F.; Cattò, C.; Villa, F. The control of cultural heritage microbial deterioration. Microorganisms 2020, 8, 1542. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, P.; Chelazzi, D.; Giorgi, R.; Poggi, G. Colloid and materials science for the conservation of cultural heritage: Cleaning, consolidation, and deacidification. Langmuir 2013, 29, 5110–5122. [Google Scholar] [CrossRef]
- Baglioni, P.; Berti, D.; Bonini, M.; Carretti, E.; Dei, L.; Fratini, E.; Giorgi, R. Micelle, microemulsions, and gels for the conservation of cultural heritage. Adv. Colloid Interface Sci. 2014, 205, 361–371. [Google Scholar] [CrossRef]
- Boccalon, E.; Nocchetti, M.; Pica, M.; Romani, A.; Sterflinger, K. Hydrogels: A ‘Stepping Stone’ Towards New Cleaning Strategies for Biodeteriorated Surfaces. J. Cult. Herit. 2021, 47, 1–11. [Google Scholar] [CrossRef]
- Gabriele, F.; Tortora, M.; Bruno, L.; Casieri, C.; Chiarini, M.; Germani, R.; Spreti, N. Alginate-biocide hydrogel for the removal of biofilms from calcareous stone artworks. J. Cult. Herit. 2021, 49, 106–114. [Google Scholar] [CrossRef]
- Gabriele, F.; Vetrano, A.; Bruno, L.; Casieri, C.; Germani, R.; Rugnini, L.; Spreti, N. New oxidative alginate-biocide hydrogels against stone biodeterioration. Int. Biodeter. Biodegr. 2021, 163, 105281. [Google Scholar] [CrossRef]
- Tortora, M.; Chiarini, M.; Spreti, N.; Casieri, C. 1H-NMR-relaxation and colorimetry for evaluating nanopolymeric dispersions as stone protective coatings. J. Cult. Herit. 2020, 44, 204–210. [Google Scholar] [CrossRef]
- Rugnini, L.; Migliore, G.; Tasso, F.; Ellwood, N.T.W.; Sprocati, A.R.; Bruno, L. Biocidal activity of phyto-derivative products used on phototrophic biofilms growing on stone surfaces of the domus Aurea in Rome (Italy). Appl. Sci. 2020, 10, 6584. [Google Scholar] [CrossRef]
- UNI EN 15886; Conservation of Cultural Property-Test Methods-Colour Measurement of Surfaces. Official Italian Version of EN 15886:2010. UNI Ente Italiano di Normazione: Milan, Italy, 2010.
- Drdácký, M.; Slížková, Z. In situ peeling tests for assessing the cohesion and consolidation characteristics of historic plaster and render surfaces. Stud. Conserv. 2015, 60, 121–130. [Google Scholar] [CrossRef]
- Urzì, C.; De Leo, F. Sampling with adhesive tape strips: An easy and rapid method to monitor microbial colonization on monument surfaces. J. Microbiol. Methods 2001, 44, 1–11. [Google Scholar] [CrossRef]
- Bruno, L.; Bellezza, S.; Urzì, C.; De Leo, F. A study for monitoring and conservation in the Roman Catacombs of St. Callistus and Domitilla, Rome (Italy). In The Conservation of Subterranean Cultural Heritage; Saiz-Jimenez, C., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 37–44. [Google Scholar] [CrossRef]
- Vigliano, G. Graffiti and Antigraffiti Project [WWW Document]. Available online: http://www.oocities.org/emuseoros/Docs/antigraffiti.htm (accessed on 1 October 2009).
- Roldán, M.; Hernández-Mariné, M. Exploring the secrets of the three-dimensional architecture of phototrophic biofilms in caves. Int. J. Speleol. 2009, 38, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Czerwik-Marcinkowska, J.; Wojciechowska, A.; Massalski, A. Biodiversity of limestone caves: Aggregations of aerophytic algae and cyanobacteria in relation to site factors. Pol. J. Ecol. 2015, 63, 481–499. [Google Scholar] [CrossRef]
- Nikolić, N.; Zarubica, N.; Gavrilović, B.; Predojević, D.; Trbojević, I.; Simić, G.S.; Popović, S. Lampenflora and the entrance biofilm in two show caves: Comparison of microbial community, environmental, and biofilm parameters. J. Cave Karst Stud. 2020, 82, 69–81. [Google Scholar] [CrossRef]
- Nugari, M.P.; Pietrini, A.M.; Caneva, G.; Imperi, F.; Visca, P. Biodeterioration of mural paintings in a rocky habitat: The Crypt of the Original Sin (Matera, Italy). Int. Biodeterior. Biodegrad. 2009, 63, 705–711. [Google Scholar] [CrossRef]
- Caneva, G.; Bartoli, F.; Fontani, M.; Mazzeschi, D.; Visca, P. Changes in biodeterioration patterns of mural paintings: Multi-temporal mapping for a preventive conservation strategy in the Crypt of the Original Sin (Matera, Italy). J. Cult. Herit. 2019, 40, 59–68. [Google Scholar] [CrossRef]
- Mang, S.M.; Scrano, L.; Camele, I. Preliminary studies on fungal contamination of two rupestrian churches from matera (Southern Italy). Sustainability 2020, 12, 6988. [Google Scholar] [CrossRef]
- Pozo-Antonio, J.S.; Montojo, C.; López de Silanes, M.E.; de Rosario, I.; Rivas, T. In situ evaluation by colour spectrophotometry of cleaning and protective treatments in granitic Cultural Heritage. Int. Biodeterior. Biodegrad. 2017, 123, 251–261. [Google Scholar] [CrossRef]
- Favero-Longo, S.E.; Brigadeci, F.; Segimiro, A.; Voyron, S.; Cardinali, M.; Girlanda, M.; Piervittori, R. Biocide efficacy and consolidant effect on the mycoflora of historical stuccos in indoor environment. J. Cult. Herit. 2018, 34, 33–42. [Google Scholar] [CrossRef]
- Favero-Longo, S.E.; Matteucci, E.; Pinna, D.; Ruggiero, M.G.; Riminesi, C. Efficacy of the environmentally sustainable microwave heating compared to biocide applications in the devitalization of phototrophic communities colonizing rock engravings of Valle Camonica, UNESCO world heritage site, Italy. Int. Biodeterior. Biodegrad. 2021, 165, 105327. [Google Scholar] [CrossRef]
- Urzì, C.; De Leo, F.; Lucia Krakova, L.; Domenico Pangallo, D.; Bruno, L. Effects of biocide treatments on the biofilm community in Domitilla’s catacombs in Rome. Sci. Total Environ. 2016, 572, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Pfendler, S.; Fabien Borderie, F.; Faisl Bousta, F.; Laurence Alaoui-Sosse, L.; Badr Alaoui-Sosse, B.; Aleya, L. Comparison of biocides, allelopathic substances and UV-C as treatments for biofilm proliferation on heritage monuments. J. Cult. Herit. 2018, 33, 117–124. [Google Scholar] [CrossRef]
- Mascalchi, M.; Orsini, C.; Pinna, D.; Salvadori, B.; Siano, S.; Riminesi, C. Assessment of different methods for the removal of biofilms and lichens on gravestones of the English Cemetery in Florence. Int. Biodeterior. Biodegrad. 2020, 154, 105041. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Rupérez, P. FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocoll. 2011, 25, 1514–1520. [Google Scholar] [CrossRef]
- Sakugawa, K.; Ikeda, A.; Takemura, A.; Ono, H. Simplified method for estimation of composition of alginates by FTIR. J. Appl. Polym. Sci. 2004, 93, 1372–1377. [Google Scholar] [CrossRef]
- Liu, Q.; Hedberg, E.L.; Liu, Z.; Bahulekar, R.; Meszlenyi, R.K.; Mikos, A.G. Preparation of macroporous poly(2-hydroxyethyl methacrylate) hydrogels by enhanced phase separation. Biomaterials 2000, 21, 2163–2169. [Google Scholar] [CrossRef]
- Bonelli, N.; Poggi, G.; Chelazzi, D.; Giorgi, R.; Baglioni P. Poly(vinyl alcohol)/poly(vinyl pyrrolidone) hydrogels for the cleaning of art. J. Colloid Interface Sci. 2019, 536, 339–348. [Google Scholar] [CrossRef]




| REF-SPB | L* | a* | b* | |||
| 72 ± 5 | 4.8 ± 0.9 | 13 ± 2 | ||||
| ΔL* | Δa* | Δb* | ΔE* | |||
| SPB-1 | COLONIZED | - | −28.0 | −4.8 | 3.3 | 29 |
| CLEANED | NT | −4.6 | 0.1 | 2.2 | 5 | |
| AC012 | −1.9 | 1.9 | 2.4 | 4 | ||
| P2 | −4.7 | −0.5 | 1.7 | 5 | ||
| CLEANED 2y | NT | −4.6 | 1.7 | 2.0 | 5 | |
| AC012 | −3.9 | 0.9 | 0.9 | 4 | ||
| P2 | −3.1 | 0.1 | 0.7 | 3 | ||
| SPB-2 | COLONIZED | - | −28.4 | −3.4 | 0.8 | 29 |
| CLEANED | NT | 3.6 | 0.5 | 2.8 | 5 | |
| AC012 | −3.9 | 0.4 | 1.5 | 4 | ||
| P2 | 0.9 | 0.1 | 2.0 | 2 | ||
| CLEANED 2y | NT | 0.9 | 0.3 | 0.3 | 1 | |
| AC012 | 2.4 | 0.4 | 0.6 | 3 | ||
| P2 | 0.3 | 0.1 | 0.5 | 1 | ||
| Element | REF | COLONIZED | CLEANED |
|---|---|---|---|
| O | 48 ± 3 | 35 ± 9 | 47 ± 2 |
| Ca | 35 ± 3 | 11 ± 3 | 37 ± 5 |
| C | 13 ± 3 | 33 ± 7 | 13 ± 3 |
| Cl | 1.0 ± 0.3 | 2 ± 1 | 0.8 ± 0.3 |
| S | 0.8 ± 0.2 | 0.5 ± 0.4 | 0.6 ± 0.2 |
| Si | 0.8 ± 0.3 | 16 ± 7 | 0.8 ± 0.5 |
| Na | 0.6 ± 0.3 | 0.3 ± 0.1 | 0.5 ± 0.1 |
| REF-MD-1/2 | L* | a* | b* | ||
| 67 ± 4 | 5 ± 1 | 12.0 ± 0.9 | |||
| ΔL* | Δa* | Δb* | ΔE* | ||
| MD-1 | COLONIZED | −36.1 | −3.8 | −6.4 | 37 |
| CLEANED | −0.6 | −0.5 | 2.2 | 2 | |
| AC012 TREATED | 1.0 | −1.7 | 0.8 | 2 | |
| AC012 TREATED 2y | 2.7 | −1.2 | 0.1 | 3 | |
| MD-2 | COLONIZED | −29.2 | −4.2 | −4.7 | 30 |
| CLEANED | 3.8 | −1.2 | −2.9 | 5 | |
| P2 TREATED | 2.5 | −1.7 | −1.6 | 3 | |
| P2 TREATED 2y | 2.4 | −1.2 | −1.3 | 3 | |
| REF-MD-3 | L* | a* | b* | ||
| 76 ± 2 | 3.4 ± 0.61 | 8.6 ± 0.9 | |||
| ΔL* | Δa* | Δb* | ΔE* | ||
| MD-3 | COLONIZED | −13.7 | −6.5 | 0.8 | 15 |
| CLEANED | −2.7 | −2.3 | −1.0 | 4 | |
| P2 TREATED | 0.0 | −1.2 | −1.1 | 1 | |
| P2 TREATED 2y | −3.4 | 0.1 | 2.1 | 4 | |
| Weight of Detached Material, mg/cm2 | ||
|---|---|---|
| MD-1 | CLEANED | 2.5 ± 0.9 |
| AC012 TREATED | 0.4 ± 0.1 | |
| MD-2 | CLEANED | 1.6 ± 0.9 |
| P2 TREATED | 0.2 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabriele, F.; Bruno, L.; Casieri, C.; Ranaldi, R.; Rugnini, L.; Spreti, N. Application and Monitoring of Oxidative Alginate–Biocide Hydrogels for Two Case Studies in “The Sassi and the Park of the Rupestrian Churches of Matera”. Coatings 2022, 12, 462. https://doi.org/10.3390/coatings12040462
Gabriele F, Bruno L, Casieri C, Ranaldi R, Rugnini L, Spreti N. Application and Monitoring of Oxidative Alginate–Biocide Hydrogels for Two Case Studies in “The Sassi and the Park of the Rupestrian Churches of Matera”. Coatings. 2022; 12(4):462. https://doi.org/10.3390/coatings12040462
Chicago/Turabian StyleGabriele, Francesco, Laura Bruno, Cinzia Casieri, Roberta Ranaldi, Lorenza Rugnini, and Nicoletta Spreti. 2022. "Application and Monitoring of Oxidative Alginate–Biocide Hydrogels for Two Case Studies in “The Sassi and the Park of the Rupestrian Churches of Matera”" Coatings 12, no. 4: 462. https://doi.org/10.3390/coatings12040462







