Structural and Mechanical Properties of Fluorine-Containing TaCxNy Thin Films Deposited by Reactive Magnetron Sputtering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Deposition
2.2. Thin Film Characterization
3. Results and discussion
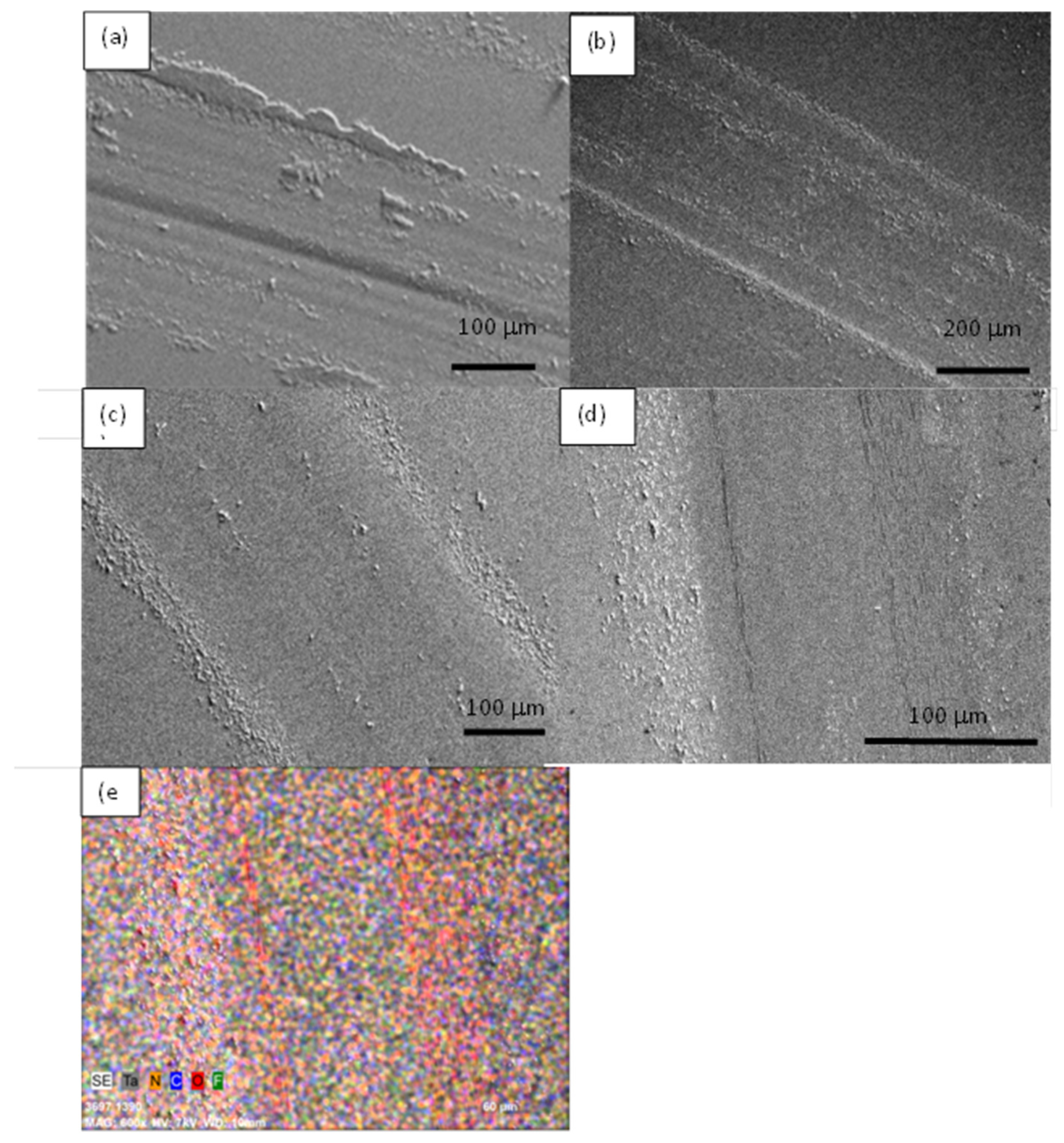
3.1. Structural Analysis
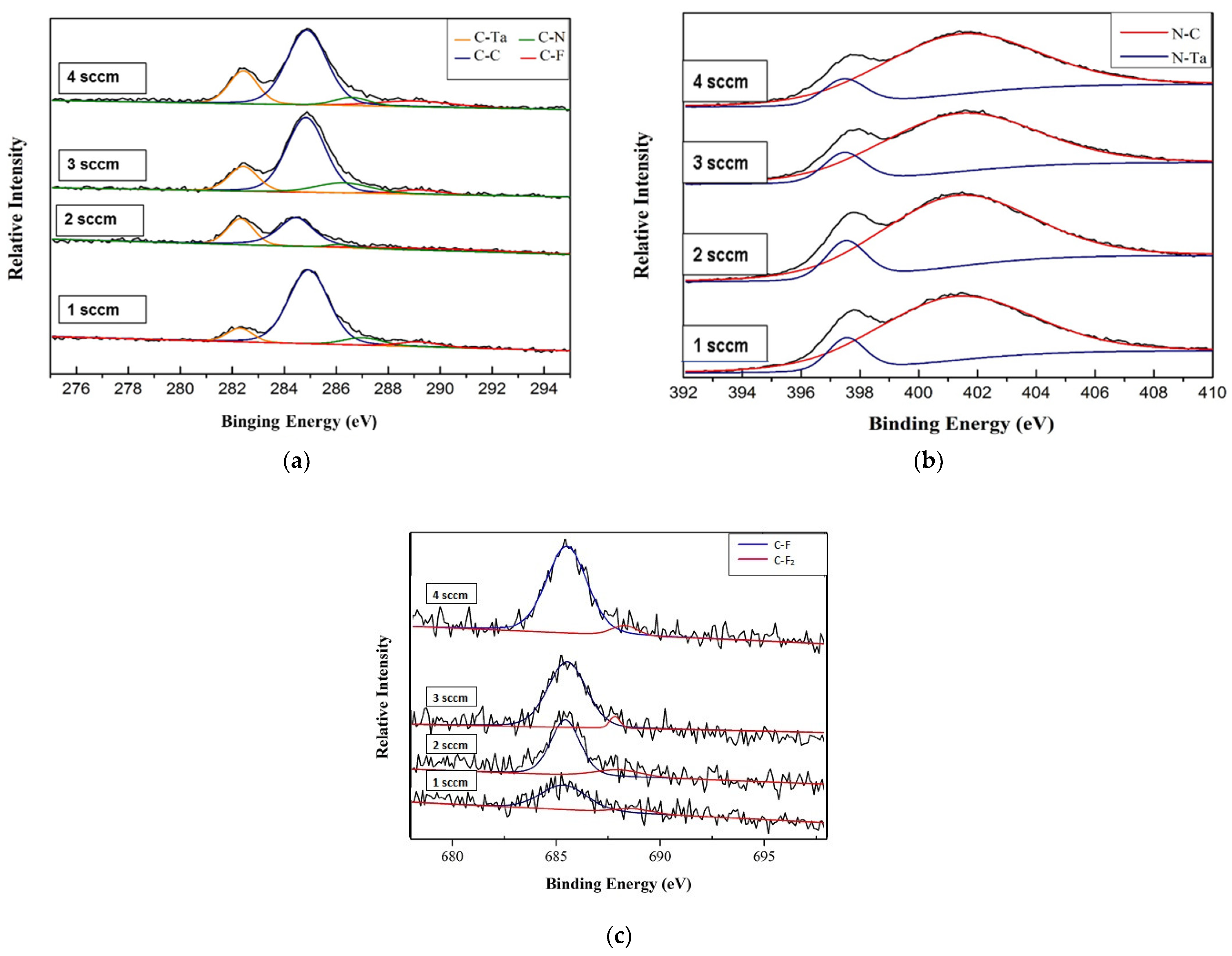
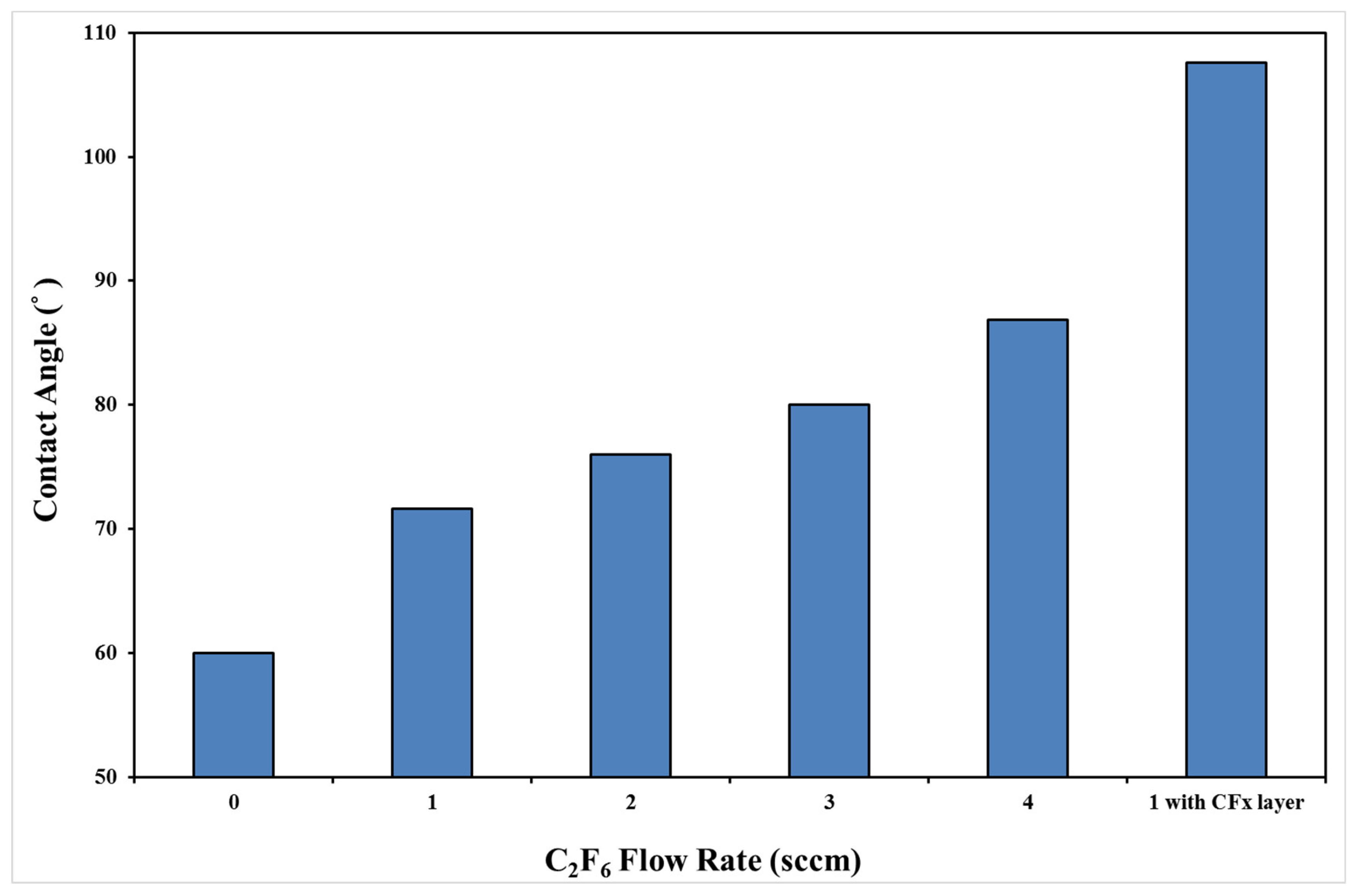
3.2. Chemical Composition and Bond Analysis
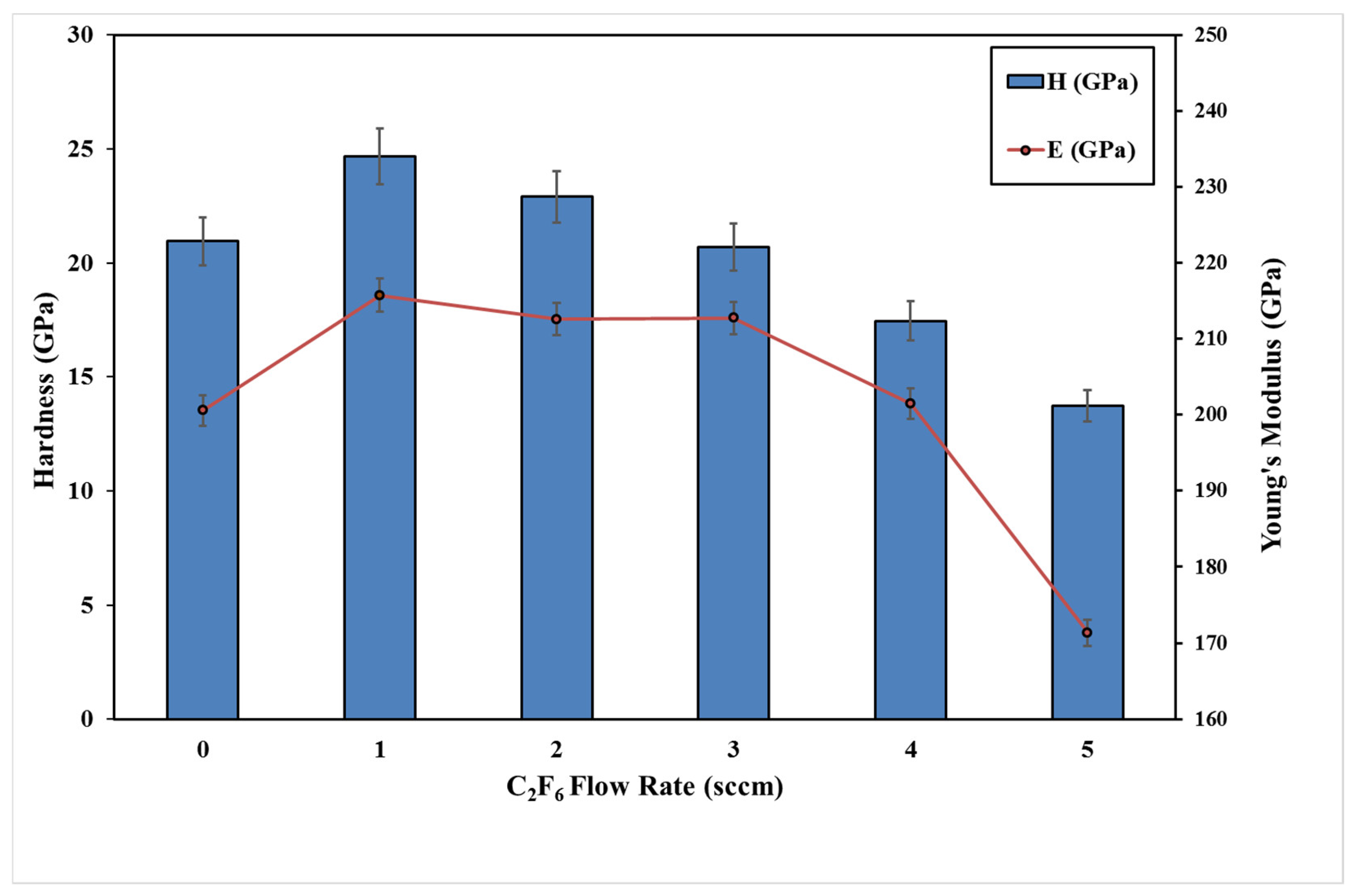
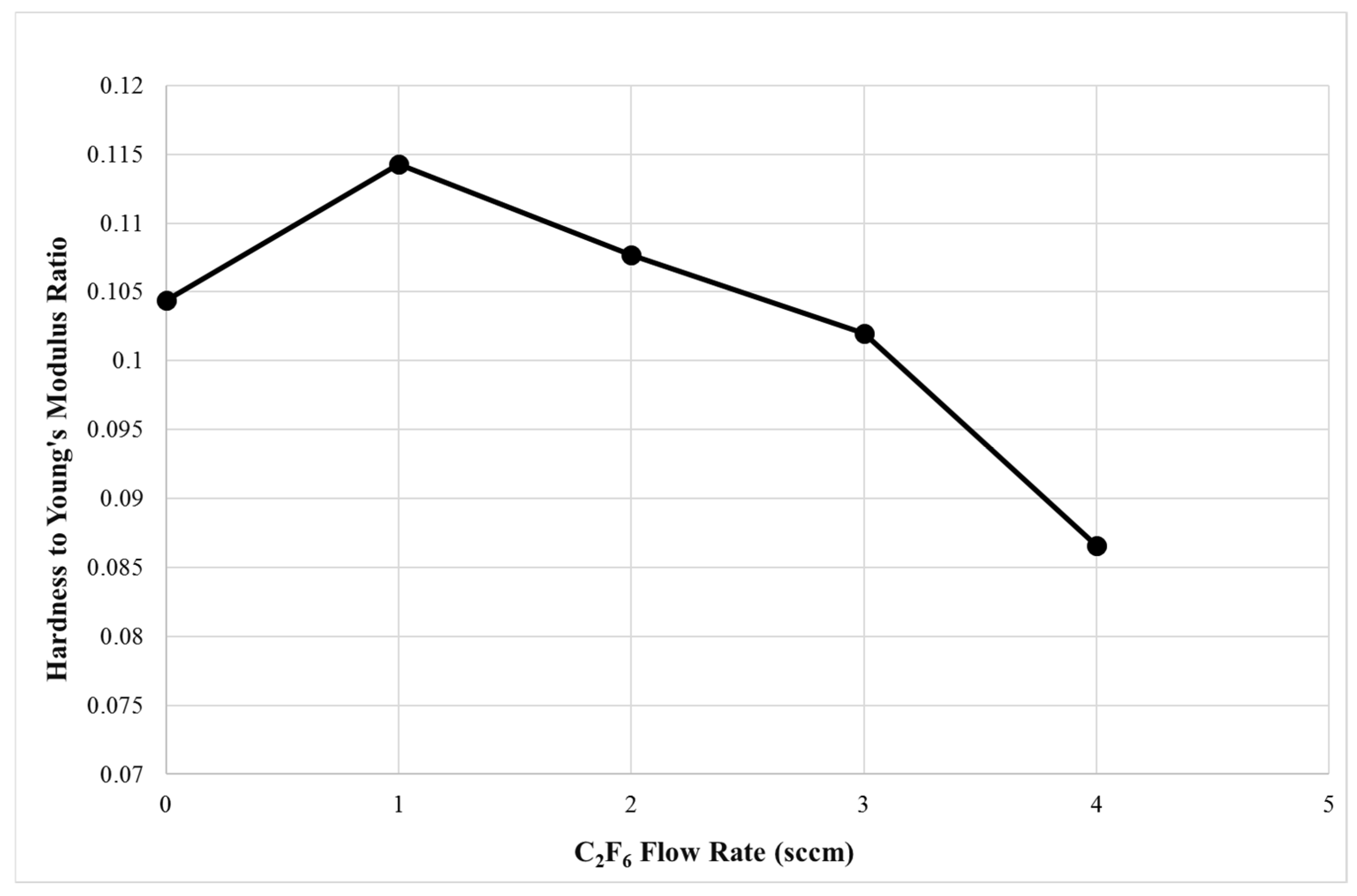
3.3. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manivasagam, V.K.; Popat, K.C. In Vitro Investigation of Hemocompatibility of Hydrothermally Treated Titanium and Titanium Alloy Surfaces. ACS Omega 2020, 5, 8108–8120. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Wang, Y.; Li, J.; Zhao, H. Effect of Nitrogen Flow Ratio on Degradation Behaviors and Failure of Magnetron Sputter Deposited Tantalum Nitride. Coatings 2021, 11, 1133. [Google Scholar] [CrossRef]
- Zaman, A.; Meletis, E.I. Microstructure and Mechanical Properties of TaN Thin Films Prepared by Reactive Magnetron Sputtering. Coatings 2017, 7, 209. [Google Scholar] [CrossRef] [Green Version]
- Radhakrishnan, K.; Ing, N.G.; Gopalakrishnan, R. Reactive sputter deposition and characterization of tantalum nitride thin films. Mater. Sci. Eng. 1999, 57, 224–227. [Google Scholar] [CrossRef]
- Dai, W.; Shi, Y. Effect of Bias Voltage on Microstructure and Properties of Tantalum Nitride Coatings Deposited by RF Magnetron Sputtering. Coatings 2021, 11, 911. [Google Scholar] [CrossRef]
- Stavrev, M.; Fischer, D.; Wenzel, C.; Drescher, K.; Mattern, M. Crystallographic and morphological characterization of reactively sputtered Ta, TaN, TaNO thin films. Thin Solid Film. 1997, 307, 79–88. [Google Scholar] [CrossRef]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science, 2nd ed.; Elsevier: Cambridge, MA, USA, 2004. [Google Scholar]
- Balla, V.K.; Bodhak, S.; Bose, S.; Bandyopadhyay, A. Porous tantalum structures for bone implants: Fabrication, mechanical and in vitro biological properties. Acta Biomater. 2010, 6, 3349–3359. [Google Scholar] [CrossRef] [Green Version]
- Zitter, H.; Plenk, H., Jr. The electrochemical behavior of metallic implant materials as an indicator of their biocompatibility. J. Biomed. Mater. Res. 1987, 21, 881–896. [Google Scholar] [CrossRef]
- Huang, H.L.; Chang, Y.Y.; Lai, M.C.; Lin, C.R.; Lai, C.H.; Shieh, T.M. Antibacterial TaN-Ag coatings on titanium dental implants. Surf. Coat. Technol. 2010, 205, 1636–1641. [Google Scholar] [CrossRef]
- Leng, Y.X.; Sun, H.; Yang, P.; Chen, J.Y.; Wang, J.; Wan, G.J.; Huang, N.; Tian, X.B.; Wang, L.P.; Chu, P.K. Biomedical properties of tantalum nitride films synthesized by reactive magnetron sputtering. Thin Solid Film. 2001, 398–399, 471–475. [Google Scholar] [CrossRef]
- Piallat, F.; Beugin, V.; Gassilloud, R.; Michallon, P.; Dussault, L.; Pelissier, B.; Asikainen, T.; Maes, J.W.; Martin, F.; Morin, P.; et al. Evaluation of plasma parameters on PEALD deposited TaCN. Microelectron. Eng. 2013, 107, 156–160. [Google Scholar] [CrossRef]
- Bendavid, A.; Martin, P.J.; Randeniya, L.; Amin, M.S.; Rohanizadeh, R. The properties of fluorine-containing diamond-like carbon films prepared by pulsed DC plasma-activated chemical vapour deposition. Diam. Relat. Mater. 2010, 19, 1466–1471. [Google Scholar] [CrossRef]
- Schmidt, S.; Goyenola, C.; Gueorguiev, G.K.; Jensen, J.; Greczynski, G.; Ivanov, I.G.; Czigány, Z.; Hultman, L. Reactive high power impulse magnetron sputtering of CFx thin films in mixed Ar/CF4 and Ar/C4F8 discharges. Thin Solid Film. 2013, 542, 21–30. [Google Scholar] [CrossRef]
- Schmidt, D.S.; Greczynski, G.; Goyenola, C.; Gueorguiev, G.K.; Czigany, Z.; Jensen, J.; Ivanov, I.G.; Hultman, L. CFx thin solid films deposited by high power impulse magnetron sputtering: Synthesis and characterization. Surf. Coat. Technol. 2011, 206, 646–653. [Google Scholar] [CrossRef] [Green Version]
- Nobili, E.L.; Guglielmini, A. Thermal stability and mechanical properties of fluorinated diamond-like carbon coatings. Surf. Coat. Technol. 2013, 219, 144–150. [Google Scholar]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Chen, J.; Bull, S.J. On the factors affecting the critical indenter penetration for measurement of coating hardness. Vacuum 2009, 83, 911–920. [Google Scholar] [CrossRef]
- Yao, Z.Q.; Yang, P.; Huang, N.; Sun, H.; Wang, J. Structural, mechanical and hydrophobic properties of fluorine-doped diamond-like carbon films synthesized by plasma immersion ion implantation and deposition (PIII–D). Appl. Surf. Sci. 2004, 230, 172–178. [Google Scholar] [CrossRef]
- Arshi, N.; Lu, J.; Joo, Y.K.; Yoon, J.H.; Koo, B.H. Effects of nitrogen composition on the resistivity of reactively sputtered TaN thin films. Surf. Interface Anal. 2015, 47, 154–160. [Google Scholar] [CrossRef]
- Li, L.; Niu, E.; Lv, G.; Zhang, X.; Chen, H.; Fan, S.; Liu, C.; Yang, S.Z. Synthesis and electrochemical characteristics of Ta–N thin films fabricated by cathodic arc deposition. Appl. Surf. Sci. 2007, 253, 6811–6816. [Google Scholar] [CrossRef]
- Wang, X.; Harris, H.R.; Bouldin, K.; Temkin, H.; Gangopadhyay, S. Structural properties of fluorinated amorphous carbon films. J. Appl. Phys. 2000, 87, 621–623. [Google Scholar] [CrossRef] [Green Version]
- Leyland, A.; Matthew, A. On the sigficance of H/E ratio in wear control: A nanocomposite coating approach to optimized tribological behavior. Wear 2000, 246, 1–11. [Google Scholar] [CrossRef]
- Hong, S.; Li, J.; Zhao, P.; Xu, Y.; Li, W. Evolution in Wear and High-Temperature Oxidation Resistance of Laser-Clad AlxMoNbTa Refractory High-Entropy Alloys Coatings with Al Addition Content. Coatings 2022, 12, 121. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, F.; Qiang, L.; Gao, K.; Zhang, B.; Zhang, J. Recent advances in the mechanical and tribological properties of fluorine-containing DLC films. RSC Adv. 2015, 5, 9635–9649. [Google Scholar]
- Yamada, N.; Kato, Y.; Kanda, K.; Haruyama, Y.; Matsui, S. Surface modification of diamond-like carbon by synchrotron radiation exposure under the perfluorohexane gas atmosphere. Jpn. J. Appl. Phys. Part 1 2006, 45, 6400. [Google Scholar] [CrossRef]
- Nakamura, T.; Ohana, T.; Suzuki, M.; Ishihara, M.; Tanaka, A.; Koga, Y. Surface modification of diamond-like carbon films with perfluorooctyl functionalities and their surface properties. Surf. Sci. 2005, 580, 101–106. [Google Scholar] [CrossRef]
- Zhang, L.; Zong, X.; Guo, F.; He, B.; Yuan, X. Effect of fluorine incorporation on DLC films deposited by pulsed cathodic arc deposition on nitrile butadiene rubber and polyurethane rubber substrates. Coatings 2020, 10, 878. [Google Scholar] [CrossRef]






| Process Parameters | |
|---|---|
| Power of Ta target (W) | (DC) 190 |
| RF Bias (W) | (RF) 50 |
| Target–substrate distance (cm) | 10 |
| Background pressure (torr) | 4 × 10−6 |
| Flow Rate of Ar (sccm) | 35 |
| Flow Rate of N2 (sccm) | 4.5 |
| Flow Rate of C2F6 (sccm) | 0/1/2/3/4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, J.-H.; Li, C.; Wu, W.; Liu, S.-L. Structural and Mechanical Properties of Fluorine-Containing TaCxNy Thin Films Deposited by Reactive Magnetron Sputtering. Coatings 2022, 12, 508. https://doi.org/10.3390/coatings12040508
Hsieh J-H, Li C, Wu W, Liu S-L. Structural and Mechanical Properties of Fluorine-Containing TaCxNy Thin Films Deposited by Reactive Magnetron Sputtering. Coatings. 2022; 12(4):508. https://doi.org/10.3390/coatings12040508
Chicago/Turabian StyleHsieh, Jang-Hsing, Chuan Li, Weite Wu, and Shan-Lun Liu. 2022. "Structural and Mechanical Properties of Fluorine-Containing TaCxNy Thin Films Deposited by Reactive Magnetron Sputtering" Coatings 12, no. 4: 508. https://doi.org/10.3390/coatings12040508
APA StyleHsieh, J.-H., Li, C., Wu, W., & Liu, S.-L. (2022). Structural and Mechanical Properties of Fluorine-Containing TaCxNy Thin Films Deposited by Reactive Magnetron Sputtering. Coatings, 12(4), 508. https://doi.org/10.3390/coatings12040508








