A Multi-Analytical Investigation of Roman Frescoes from Rapoltu Mare (Romania)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
3. Results and Discussion
3.1. XRD/WDXRF Studies
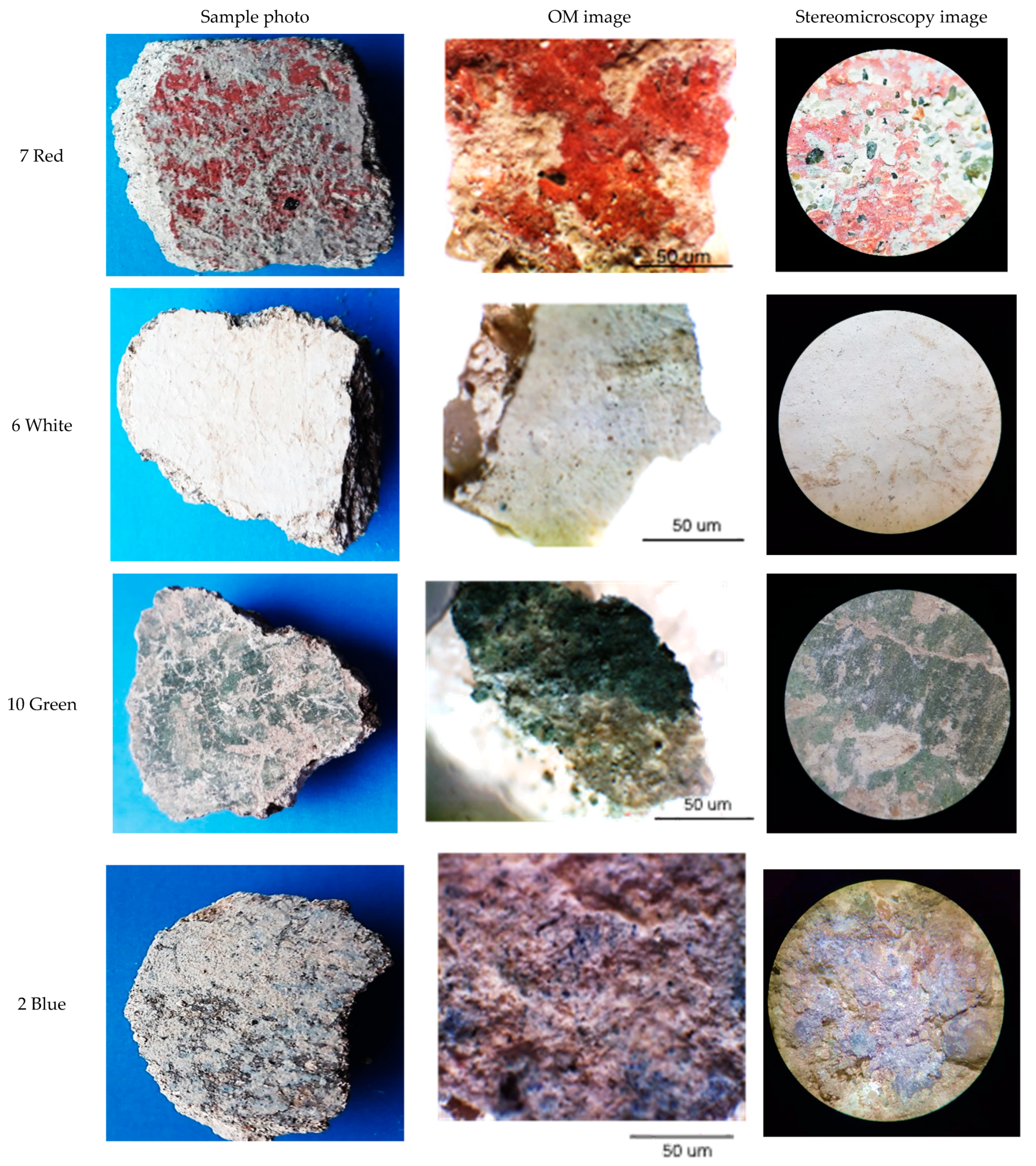
3.2. Microscopic Observations
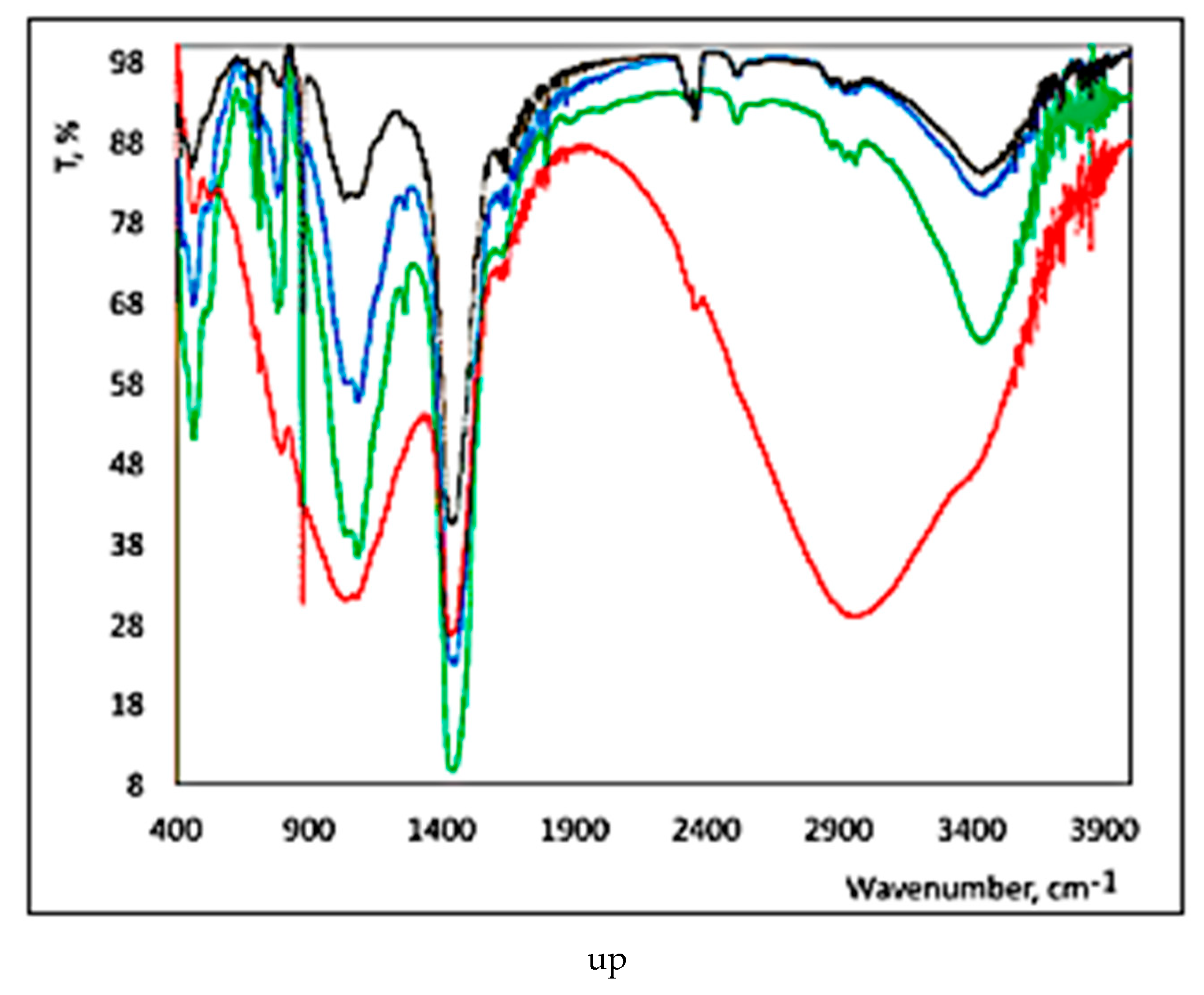
3.3. FTIR Results
3.4. Blue Zone
3.5. Red Zone
3.6. Green Zone
3.7. Diffuse Reflectance UV/Vis Spectroscopy (DR-UV)
3.8. Thermal Gravimetric Analysis
- −
- the endothermic peak at 100 °C is attributed to hygroscopic humidity (also known as physically adsorbed water), while those occurring at about 200–250 °C are due to hydrated inter-layer cations (i.e., bound water) [73].
- −
- weight loss between 120 and 200 °C can be attributed to hydrated salts.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fermo, P.; Piazzalunga, A.; de Vos, M.; Andreoli, M. A multi-analytical approach for the study of the pigments used in the wall paintings from a building complex on the Caelian Hill (Rome). Appl. Phys. A 2013, 25, 2621–2630. [Google Scholar] [CrossRef]
- Westlake, P.; Siozos, P.; Philippidis, A.; Apostolaki, C.; Derham, B.; Terlixi, A.; Perdikatsis, V.; Jones, R.; Anglos, D. Studying pigments on painted plaster in Minoan, Roman and Early Byzantine Crete. A multi-analytical technique approach. Anal. Bioanal. Chem. 2012, 402, 1413–1432. [Google Scholar] [CrossRef] [PubMed]
- Gliozzo, E.; Cavari, F.; Damiani, D.; Memmi, I. Pigments and plasters from the roman settlement of thamusida (Rabat, Morocco). Archaeometry 2012, 54, 278–293. [Google Scholar] [CrossRef]
- Clementi, C.; Ciocan, V.; Vagnini, M.; Doherty, B.; Tabasso, M.L.; Conti, C.; Brunetti, B.G.; Miliani, C. Non-invasive and micro-destructive investigation of the Domus Aurea wall painting decorations. Anal. Bioanal. Chem. 2011, 401, 1815–1826. [Google Scholar] [CrossRef]
- Piovesan, R.; Siddall, R.; Mazzoli, C.; Nodari, L. The Temple of Venus (Pompeii): A study of the pigments and painting techniques. J. Archaeol. Sci. 2011, 38, 2633–2643. [Google Scholar] [CrossRef]
- Weber, J.; Prochaska, W.; Zimmermann, N. Microscopic techniques to study Roman renders and mural paintings from various sites. Mater. Charact. 2009, 60, 586–593. [Google Scholar] [CrossRef]
- Vaquerizo, D.; Carrillo, J.R. The roman ville of El Ruedo (Almedinilla, Córdoba). J. Roman Archaeol. 1995, 8, 12–152. [Google Scholar]
- Plinius, C.S. Naturalis Historia: Enciclopedia Cunoștințelor din Antichitate; Polirom: Iași, Romania, 2004; p. 408. [Google Scholar]
- Vitruvius. Despre arhitectură (Scriptores Graeci et Latini V); Editura Academiei Republicii Populare Române: Bucureşti, Romania, 1964. [Google Scholar]
- Cuní, J.; Cuní, P.; Eisen, B.; Savizky, R.; Bové, J. Characterization of the binding medium used in Roman encaustic paintings on wall and wood. Anal. Methods 2012, 4, 659. [Google Scholar] [CrossRef]
- Amadori, M.L.; Barcelli, S.; Poldi, G.; Ferrucci, F.; Andreotti, A.; Baraldi, P.; Colombini, M.P. Invasive and non-invasive analyses for knowledge and conservation of Roman wall paintings of the Villa of the Papyri in Herculaneum. Microchem. J. 2015, 118, 183–192. [Google Scholar] [CrossRef]
- Tamburini, D.; Łucejko, J.J.; Modugno, F.; Colombini, M.P.; Pallecchi, P.; Giachi, G. Microscopic techniques (LM, SEM) and a multi-analytical approach (EDX, FTIR, GC/MS, Py-GC/MS) to characterise the decoration technique of the wooden ceiling of the House of the Telephus Relief in Herculaneum (Italy). Microchem. J. 2014, 116, 7–14. [Google Scholar] [CrossRef]
- Brecoulaki, H.; Andreotti, A.; Bonaduce, I.; Colombini, M.P.; Lluveras, A. Characterization of organic media in the wall-paintings of the “Palace of Nestor” at Pylos, Greece: Evidence for a secco painting techniques in the Bronze Age. J. Archaeol. Sci. 2012, 39, 2866. [Google Scholar] [CrossRef]
- Pallecchi, P.; Giachi, G.; Colombini, M.P.; Modugno, M.; Ribechini, R. The painting of the Etruscan ‘‘Tomba della Quadriga Infernale’’ (4th century BC), in Sarteano (Siena, Italy): Technical features. J. Archaeol. Sci. 2009, 36, 2635. [Google Scholar] [CrossRef]
- Andreotti, A.; Bonaduce, I.; Colombini, M.P.; Gautier, G.; Modugno, F.; Ribechini, E. Combined GC/MS analytical procedure for the characterization of glycerolipid, waxy, resinous, and proteinaceous materials in a unique paint microsample. Anal. Chem. 2006, 78, 4499. [Google Scholar] [CrossRef] [PubMed]
- Băeştean, G.; Bălos, A.; Barbu, M.G.; Bărbat, I.A.; Barbu, I.L.; Țuțuianu, C.-D.; Marc, A.T.; Barbu, M.-M.; Gonciar, A. Rapoltu Mare, jud. Hunedoara, La Vie, CCA Campania 2019; Mega Print: Cluj-Napoca, Romania, 2020; pp. 286–287. [Google Scholar]
- Barbu, M.G.; Bărbat, I.A. New Archaeological Information Regarding the Exploitation of Andesite in Măgura Uroiului (Hunedoara County), Sargetia (S.N.); Altip: Alba Iulia, Romania, 2017; Volume VIII, pp. 71–121. [Google Scholar]
- Ion, R.-M.; Rizescu, C.E.; Vasile, D.A.; Vasilievici, G.; Atkinson, I.; Rusu, A.; Predoana, L.; Miculescu, F. Layered double hydroxides (LDHs) as new consolidants for cultural heritage masonry. Crystals 2022, 12, 490. [Google Scholar] [CrossRef]
- Bălos, A.; Ţuţuianu, C.D. Rapoltu Mare, com. Rapoltu Mare, jud. Hunedoara, Punt: La Vie, CCA, Campania 1999; Mega Print: Cluj-Napoca, Romania, 2000; p. 80. [Google Scholar]
- Băeştean, G.; Bălos, A.; Barbu, M.G.; Bărbat, I.A.; Gonciar, A.; Brown, A.; Barbu, I.L.; Tutuianu, D.C.; Tutilă, O.C.; Barbu, M.M.; et al. Rapoltu Mare, com. Rapoltu Mare, jud. Hunedoara. Punct: La Vie, CCA, Campania 2016; Mega Print: Cluj-Napoca, Romania, 2017; pp. 109–111. [Google Scholar]
- Ion, R.M.; Iancu, L.; David, M.E.; Grigorescu, R.M.; Trica, B.; Somoghi, R.; Vasile, S.F.; Dulama, I.D.; Gheboianu, A.I.; Tincu, S. Multi-analytic characterization of Corvins’ Castle—Deserted Tower. Construction materials and conservation tests. Heritage 2020, 3, 941–964. [Google Scholar] [CrossRef]
- Ion, R.M.; Tincu, S.; Minca, I.; Dulama, I.D.; Bucurica, I.A.; Ion, M.L.; Gheboianu, A.I. Instrumental analytical techniques applied to Old Gate Tower from Corvins’ Castle. IOP Conf. Ser. Mater. Sci. Eng. 2019, 877, 012050. [Google Scholar] [CrossRef]
- Rietveld, H.M. Rietveld method. Phys. Scr. 2014, 89, 098002. [Google Scholar] [CrossRef]
- Dal Fovo, A.; Mazzinghi, A.; Omarini, S.; Pampaloni, E.; Ruberto, C.; Striova, J.; Fontana, R. Non-invasive mapping methods for pigments analysis of Roman mural paintings. J. Cult. Herit. 2020, 43, 311–318. [Google Scholar] [CrossRef]
- Martinetto, P.; Blanc, N.; Bordet, P.; Champdavoine, S.; Fabre, F.; Guiblain, T.; Hodeau, J.-L.; Lelong, F.; Leynaud, O.; Prat, A.; et al. Non-invasive X-ray investigations of medieval sculptures: New insights on “applied tin-relief brocade” technique. J. Cult. Herit. 2021, 47, 89–99. [Google Scholar] [CrossRef]
- Eastaugh, N.; Walsh, V.; Chaplin, T.; Siddall, R. Pigment Compendium: A Dictionary of Historical Pigments; Butterworth Heinemann: Oxford, UK, 2004. [Google Scholar]
- Buckley, H.; Bevan, J.; Brown, K.; Johnson, L.; Farmer, V. Glauconite and celadonite: Two separate mineral species. Mineral. Mag. 1978, 42, 373–382. [Google Scholar] [CrossRef]
- Bearat, H.; Fuchs, M.; Maggetti, M.; Paunier, D. Roman Wall: Materials, Techniques, Analysis and Conservation. In Proceedings of the International Workshop on Roman Wall Painting, Fribourg, Switzerland, 7–9 March 1996; Institute of Mineralogy and Petrography, Fribourg University: Fribourg, Switzerland, 1997. [Google Scholar]
- Guglielmi, V.; Comite, V.; Andreoli, M.; Demartin, F.; Lombardi, C.A.; Fermo, P. Pigments on Roman wall painting and stucco fragments from the Monte d’Oro Area (Rome): A multi-technique approach. Appl. Sci. 2020, 10, 7121. [Google Scholar] [CrossRef]
- Paternoster, G.; Rinzivillo, R.; Nunziata, F.; Castellucci, E.M.; Lofrumento, C.; Zoppi, A.; Felici, A.C.; Fronterotta, G.; Nicolais, C.; Piacentini, M.; et al. Study on the technique of the Roman age mural paintings by micro-XRF with polycapillary conic collimator and micro-Raman analyses. J. Cult. Herit. 2005, 6, 21–28. [Google Scholar] [CrossRef]
- Aliatis, I.; Bersani, D.; Campani, E.; Casoli, A.; Lottici, P.P.; Mantovan, S.; Marino, I.G. Pigments used in wall paintings in the Vesuvian area. J. Raman Spectrosc. 2009, 41, 1537–1542. [Google Scholar] [CrossRef]
- Baraldi, P.; Bonazzi, A.; Giodani, N.; Paccagnella, F.; Zannini, P. Analytical characterization of Roman plaster of the “Domuns Farini” in Modena. Archeometry 2006, 48, 481–499. [Google Scholar] [CrossRef]
- Siddal, R. Mineral pigments in archaeology. Their analysis and the range of available materials. Minerals 2018, 8, 201. [Google Scholar] [CrossRef] [Green Version]
- Ion, R.-M.; Iancu, L.; Vasilievici, G.; Grigore, M.E.; Andrei, R.E.; Radu, G.-I.; Grigorescu, R.M.; Teodorescu, S.; Bucurica, I.A.; Ion, M.-L.; et al. Ion-substituted carbonated hydroxyapatite coatings for model stone samples. Coatings 2019, 9, 231. [Google Scholar] [CrossRef] [Green Version]
- Odom, I.E. GLAUCONITE and CELADONITE MINERALS. In Micas; Bailey, S.W., Ed.; De Gruyter: Berlin, Germany, 2018; pp. 545–572. [Google Scholar]
- Čermáková, Z.; Hradilová, J.; Jehlička, J.; Osterrothová, K.; Massanek, A.; Bezdička, P.; Hradil, D. Identification of vivianite in painted works of art and its significance for provenance and authorship studies. Archaeometry 2014, 56, 148–167. [Google Scholar] [CrossRef]
- Schiegl, S.; Weiner, K.L. Discovery of copper chloride cancer in ancient Egyptian polychromic wall paintings and faience: A developing archaeological disaster. Naturwissenschaften 1989, 400, 393–400. [Google Scholar] [CrossRef]
- Moussa, A.; Ali, M.F. Color alteration of ancient egyptian blue faience. Int. J. Archit. Herit. 2013, 7, 261–274. [Google Scholar] [CrossRef]
- Béarat, H. Chemical and mineralogical analyses of Gallo-Roman wall painting from Dietikon, Switzerland. Archaeometry 1996, 38, 81–95. [Google Scholar] [CrossRef]
- Brindley, G.W.; Brown, G. Crystal Structures of Clay Minerals and Their X-ray Identification; Mineralogical Society: London, UK, 1980. [Google Scholar]
- Stacey, R.J. The composition of some Roman medicines: Evidence for Pliny’s Punic wax? Anal. Bioanal. Chem. 2011, 401, 1749–1759. [Google Scholar] [CrossRef] [PubMed]
- Stacey, R.J.; Dyer, J.; Mussell, C.; Lluveras-Tenorio, A.; Colombini, M.P.; Duce, C.; La Nasa, J.; Cantisani, E.; Prati, S.; Sciutto, G. Ancient encaustic: An experimental exploration of technology, ageing behaviour and approaches to analytical investigation. Microchem. J. 2018, 138, 472–487. [Google Scholar] [CrossRef]
- Hossain, M.E.; Rahman, M.S.; Ketata, C.; Mann, H.; Islam, M.R. SEM-based structural and chemical analysis of paraffin wax and beeswax for petroleum applications. J. Charact. Dev. Nov. Mater. 2009, 1, 21–38. [Google Scholar]
- Mahmoud, H.H.M. Investigations by Raman microscopy, ESEM and FTIR-ATR of wall paintings from Qasr el-Ghuieta temple. Kharga Oasis, Egypt. Herit. Sci. 2014, 2, 18. [Google Scholar] [CrossRef] [Green Version]
- Mazzocchin, G.A.; Agnoli, F.; Mazzocchim, S.; Colpo, I. Analysis of pigments from Roman wall paintings found in Vicenza. Talanta 2003, 61, 565–572. [Google Scholar] [CrossRef]
- Franquelo, M.L.; Duran, A.; Herrera, L.K.; de Haro, M.C.J.; Perez-Rodriguez, L. Comparison between micro-Raman and micro-FTIR spectroscopy techniques for the characterization of pigments from Southern Spain Cultural Heritage. J. Mol. Struct. 1999, 924–926, 404–412. [Google Scholar] [CrossRef]
- Pagez-Camagra, S.; Collinart, S.; Coupry, C. Fabrication process of archeological Egyptian blue and green pigments enlightened by Raman microscopy and scaning electron microscopy. J. Raman Spectrosc. 1999, 30, 313–317. [Google Scholar] [CrossRef]
- Burgio, L.; Clark, R.J.H. Library of FT-Raman spectra of pigments, minerals, pigment media and varnishes, and supplement to existing library of Raman spectra of pigments with visible excitation. Spectrochim. Acta A 2001, 57, 1491–1521. [Google Scholar] [CrossRef]
- di Stefano, L.M.; Fuchs, R. Characterisation of the pigments in a Ptolemaic Egyptian Book of the Dead Papyrus. Archaeol. Anthropol. Sci. 2011, 3, 229–334. [Google Scholar] [CrossRef]
- Riontino, C.; Sabbioni, C.; Ghedini, N.; Zappiaa, G.; Gobbi, G.; Favoni, O. Evaluation of atmospheric deposition on historic buildings by combined thermal analysis and combustion techniques. Thermochim. Acta 1998, 321, 215–222. [Google Scholar] [CrossRef]
- Mateos, L.D.; Cosano, D.; Mora, M.; Muñiz, I.; Carmona, R.; Jiménez-Sanchidrián, C.; Ruiz, J.R. Raman microspectroscopic analysis of decorative pigments from the Roman villa of El Ruedo (Almedinilla, Spain). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 151, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Vandenabeele, P.; Edwards, H.G.M.; Moens, L. A decade of Raman spectroscopy in art and archaeology. Chem. Rev. 2007, 107, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Clark, R.J. Raman spectroscopy in archaeological science. J. Archaeol. Sci. 2004, 31, 1137–1160. [Google Scholar] [CrossRef]
- Gauldie, R.W.; Sharma, S.K.; Volk, E. Micro-Raman spectral study of vaterite and aragonite otoliths of the coho salmon, Oncorhynchus kisutch. Comp. Biochem. Physiol. A 1997, 118, 753–757. [Google Scholar] [CrossRef]
- BS ISO 9277: 2010; Test Method Determining the Specific Surface Area of a Variety of Materials by the BET Nitrogen Adsorption Technique. International Organization for Standardization: Geneva, Switzerland, 2010.
- BS ISO 9277:2010; Brunauer-Emmett-Teller (BET) Surface Area Determination. International Organization for Standardization: Geneva, Switzerland, 2010.
- Colomban, P.; Sagon, G.; Faurel, X. Differentation of antique ceramics from the Raman spectra of their coloured glazes and paintings. J. Raman Spectrosc. 2001, 32, 351–360. [Google Scholar] [CrossRef]
- Ospitali, F.; Bersani, D.; Lonardo GDi Lottici, P.P. ’Green earths’: Vibrational and elemental characterization of glauconites, celadonites and historical pigments. J. Raman Spectrosc. 2008, 39, 1066. [Google Scholar] [CrossRef]
- Boschetti, C.; Corradi, A.; Baraldi, P. Raman characterization of painted mortar in Republican roman mosaics. J. Raman Spectrosc. 2008, 39, 1085–1090. [Google Scholar] [CrossRef]
- Colomban, P. The destructive/non-destructive identification of enamelled pottery and glass artifacts and associated pigments—A brief overview. Arts 2013, 2, 77–110. [Google Scholar] [CrossRef] [Green Version]
- Jaksch, H.; Seipel, W.; Weiner, K.L.; El Goresy, A. Egyptian blue-cuprorovaite. A window to ancient Egyptian technology. Die Nat. 1983, 70, 525–535. [Google Scholar] [CrossRef]
- Bouherour, S.; Berke, H.; Wiedemann, H.G. Ancient man-made copper silicate pigments studied by Raman microscopy. Chim. Int. J. Chem. 2001, 55, 942–951. [Google Scholar]
- Canevaliet, C. A multi-analytical approach for the characterization of powders from the Pompeii archaeological site. Anal. Bioanal. Chem. 2011, 401, 1801–1814. [Google Scholar] [CrossRef] [PubMed]
- Marucci, G.; Beeby, A.; Parker, A.W.; Nicholson, C.E. Raman spectroscopic library of medieval pigments collected with five different wavelengths for investigation of illuminated manuscripts. Anal. Methods 2018, 10, 1219–1236. [Google Scholar] [CrossRef] [Green Version]
- Cersoyet, S. Identifying and quantifying amorphous and crystalline content in complex powdered samples: Application to archaeological carbon blacks. J. Appl. Cryst. 2016, 49, 585–593. [Google Scholar] [CrossRef]
- Sotiropoulou, S.; Daniilia, S.; Miliani, C.; Rosi, F.; Cartechini, L.; Papanikola Bakirtzis, D. Microanalytical investigation of degradation issues in Byzantine wall paintings. Appl. Phys. A 2008, 92, 143–150. [Google Scholar] [CrossRef]
- Hradil, D.; Grygar, T.; Hradilová, J.; Bezdička, P. Clay and iron oxide pigments in the history of painting. Appl. Clay Sci. 2003, 22, 223–236. [Google Scholar] [CrossRef]
- Sanjurjo Sánchez, J.; Alves, C.A.S.; Vidal Romaní, J.R.; Fernández Mosquera, D. Origin of gypsum-rich coatings on historic buildings. Water Air Soil Pollut. 2009, 204, 53–68. [Google Scholar] [CrossRef]
- Owen, T. Fundamentals of Modern UV-Visible Spectroscopy: A Primer: Hewlett Packard; FAO: Rome, Italy, 1996. [Google Scholar]
- Hunt, G.R.; Salisbury, J.W.; Lenhoff, C.J. Visible and near-infrared spectra of minerals and rocks: III. Oxides and hydroxides. Modern Geol. 1971, 2, 195–205. [Google Scholar]
- Scheinost, A.C.; Chavernas, A.; Barrón, V.; Torrent, J. Use and limitations of second-derivative diffuse reflectance spectroscopy in the visible to near-infrared range to identify and quantify Fe oxide minerals in soils. Clays Clay Miner. 1998, 46, 528–530. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Ruiz-Agudo, E.; Burgos-Cara, A.; Elert, K.; Hansen, E.H. Crystallization and colloidal stabilization of Ca(OH)2 in the presence of nopal juice (Opuntia ficus indica): Implications in architectural heritage conservation. Langmuir 2017, 33, 10936–10950. [Google Scholar] [CrossRef]
- Sanjurjo Sánchez, J.; Vidal Romaní, J.R.; Fernández Mosquera, D.; Alves, C.A. Study of origin and composition of coatings in a monument built with granitic rocks, by SEM, XRD, XRF and DTA-TGA. X-ray Spectrom. 2008, 37, 346–354. [Google Scholar] [CrossRef]
- Moropoulou, A.; Bakolas, A.; Bisbikou, K. Characterization of ancient, byzantine and later historic mortars by thermal and X-ray difraction techniques. Thermochim. Acta 1995, 269–270, 779–795. [Google Scholar]
- Ghedini, N.; Sabbioni, C.; Pantani, M. Thermal analysis in cultural heritage safeguard: An application. Thermochim. Acta 2003, 406, 105–113. [Google Scholar] [CrossRef]








| Oxide | Sample | |||
|---|---|---|---|---|
| - | Blue | White | Red | Green |
| - | Oxide Mass (%) | |||
| CO2 | 19.90 ± 1.514 | - | 24.30 ± 1.849 | 23.78 ± 1.809 |
| Na2O | 0.84 ± 0.063 | 1.21 ± 0.05 | 0.77 ±0.058 | 0.51 ± 0.038 |
| K2O | 1.26 ± 0.096 | 1.53 ± 0.013 | 0.91 ± 0.009 | 0.86 ± 0.065 |
| MgO | 0.45 ± 0.034 | 0.55 ± 0.03 | 0.37 ± 0.028 | 0.51 ± 0.038 |
| Al2O3 | 5.65 ± 0.043 | 7.10 ± 0.032 | 4.92 ± 0.037 | 3.23 ± 0.024 |
| SiO2 | 44.13 ± 0.036 | 53.70 ± 0.079 | 36.37 ± 0.076 | 27.11 ± 0.063 |
| P2O5 | 0.15 ± 0.011 | 0.15 ± 0.009 | 0.09 ± 0.007 | 0.18 ± 0.013 |
| SO3 | 0.08 ± 0.006 | 0.086 ± 0.012 | 0.10 ± 0.007 | 0.19 ± 0.014 |
| CaO | 23.99 ± 0.082 | 31.35 ± 0.037 | 30.13 ± 0.029 | 39.96 ± 0.041 |
| TiO2 | 0.41 ± 0.031 | 0.40 ± 0.009 | 0.22 ± 0.017 | 0.42 ± 0.031 |
| MnO | 0.05 ± 0.003 | 0.08 ± 0.029 | - | 0.08 ± 0.008 |
| Fe2O3 | 1.83 ± 0.139 | 3.74 ± 0.144 | 1.68 ± 0.011 | 2.56 ± 0.192 |
| SrO | 0.02 ± 0.001 | 0.03 ± 0.006 | 0.02 ± 0.002 | 0.5 ± 0.003 |
| Cl | 0.05 ± 0.002 | - | 0.03 ± 0.01 | 0.03 ± 0.01 |
| CuO | 1.19 ± 0.030 | - | 0.09 ± 0.003 | 0.40 ± 0.075 |
| As2O3 | - | - | - | 0.04 ± 0.006 |
| Sample | Element Mass (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| - | C | O | Na | Mg | Al | Si | K | Ca | Fe | Mo |
| White | 11.42 ± 0.1 | 50.29 ± 0.3 | 0.18 ± 0.03 | 0.12 ± 0.01 | 1.79 ± 0.03 | 9.24 ± 0.05 | 1.14 ± 0.03 | 25.27 ± 0.11 | 0.56 ± 0.06 | - |
| Green | 20.17 ± 0.13 | 51.77 ± 0.29 | 0.03 ± 0.01 | 01.4 ± 0.01 | 0.48 ± 0.01 | 0.75 ± 0.02 | 0.11 ± 0.01 | 25.85 ± 0.09 | 0.42 ± 0.02 | 0.27 ± 0.03 |
| Sample | Specific Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| Blue | 4.727 | 0.0197 | 18.29 |
| White | 3.629 | 0.0104 | 8.859 |
| Red | 2.201 | 0.0039 | 7.21 |
| Green | 4.653 | 0.0127 | 10.93 |
| Sample | Absorption Maxima (nm) |
|---|---|
| Blue | 280; 426; 586; 600; 668; 826 |
| Green | 315; 390; 525; 565; 666; 800 |
| Red | 280; 432; 512; 767; 1086 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ion, R.-M.; Barbu, M.G.; Gonciar, A.; Vasilievici, G.; Gheboianu, A.I.; Slamnoiu-Teodorescu, S.; David, M.E.; Iancu, L.; Grigorescu, R.M. A Multi-Analytical Investigation of Roman Frescoes from Rapoltu Mare (Romania). Coatings 2022, 12, 530. https://doi.org/10.3390/coatings12040530
Ion R-M, Barbu MG, Gonciar A, Vasilievici G, Gheboianu AI, Slamnoiu-Teodorescu S, David ME, Iancu L, Grigorescu RM. A Multi-Analytical Investigation of Roman Frescoes from Rapoltu Mare (Romania). Coatings. 2022; 12(4):530. https://doi.org/10.3390/coatings12040530
Chicago/Turabian StyleIon, Rodica-Mariana, Marius Gheorghe Barbu, Andrei Gonciar, Gabriel Vasilievici, Anca Irina Gheboianu, Sofia Slamnoiu-Teodorescu, Madalina Elena David, Lorena Iancu, and Ramona Marina Grigorescu. 2022. "A Multi-Analytical Investigation of Roman Frescoes from Rapoltu Mare (Romania)" Coatings 12, no. 4: 530. https://doi.org/10.3390/coatings12040530
APA StyleIon, R.-M., Barbu, M. G., Gonciar, A., Vasilievici, G., Gheboianu, A. I., Slamnoiu-Teodorescu, S., David, M. E., Iancu, L., & Grigorescu, R. M. (2022). A Multi-Analytical Investigation of Roman Frescoes from Rapoltu Mare (Romania). Coatings, 12(4), 530. https://doi.org/10.3390/coatings12040530








