Evaluation on Thermal Protection Performance of TiO2@ATO Coated Aramid Nonwoven
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of TiO2@ATO and Coated Nonwoven with TiO2@ATO/Silicone Coating
2.2. Characterization of TiO2@ATO and Coated Nonwoven with TiO2@ATO/Silicone Coating
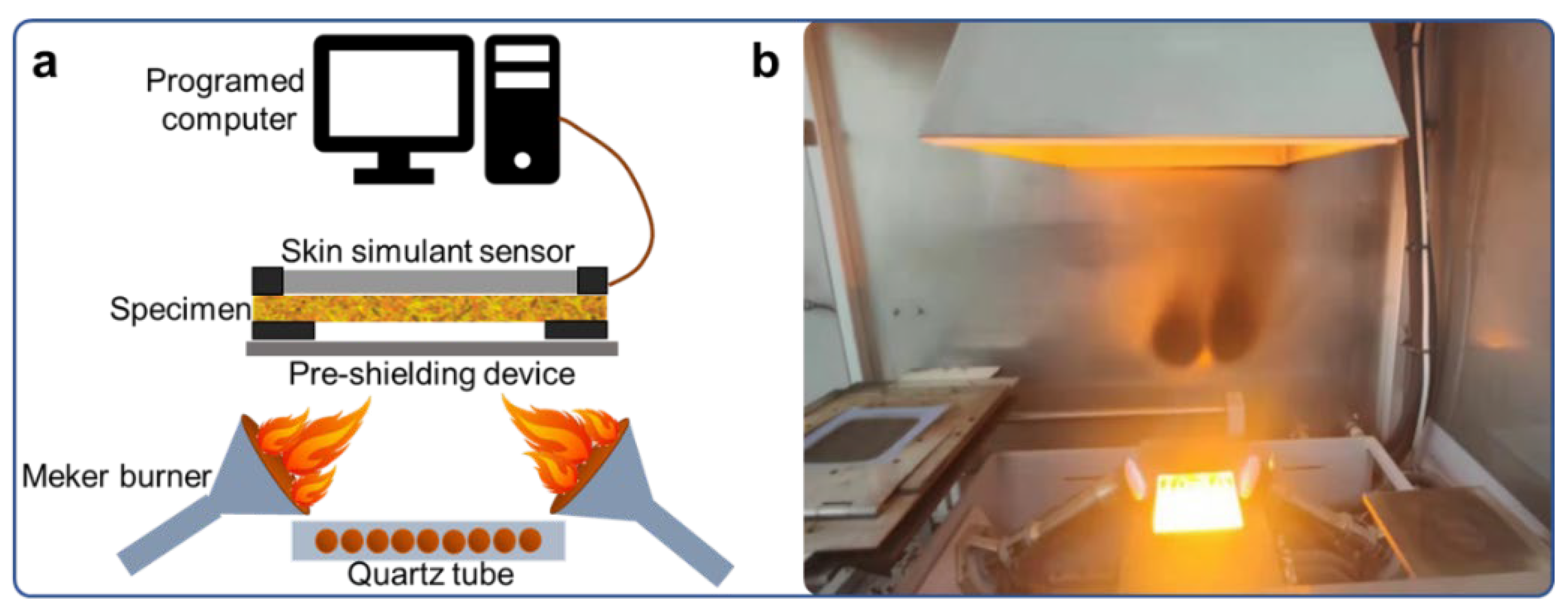
2.3. Evaluation of TPP
3. Results and Discussion
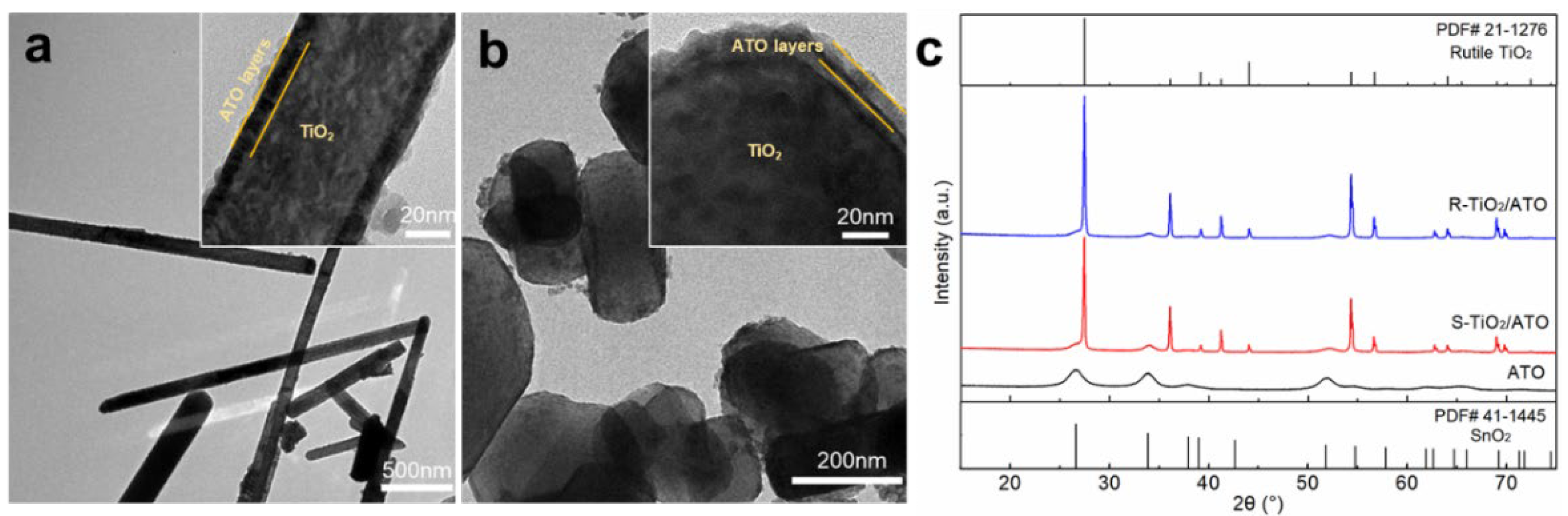
3.1. Micro-Morphology of TiO2@ATO
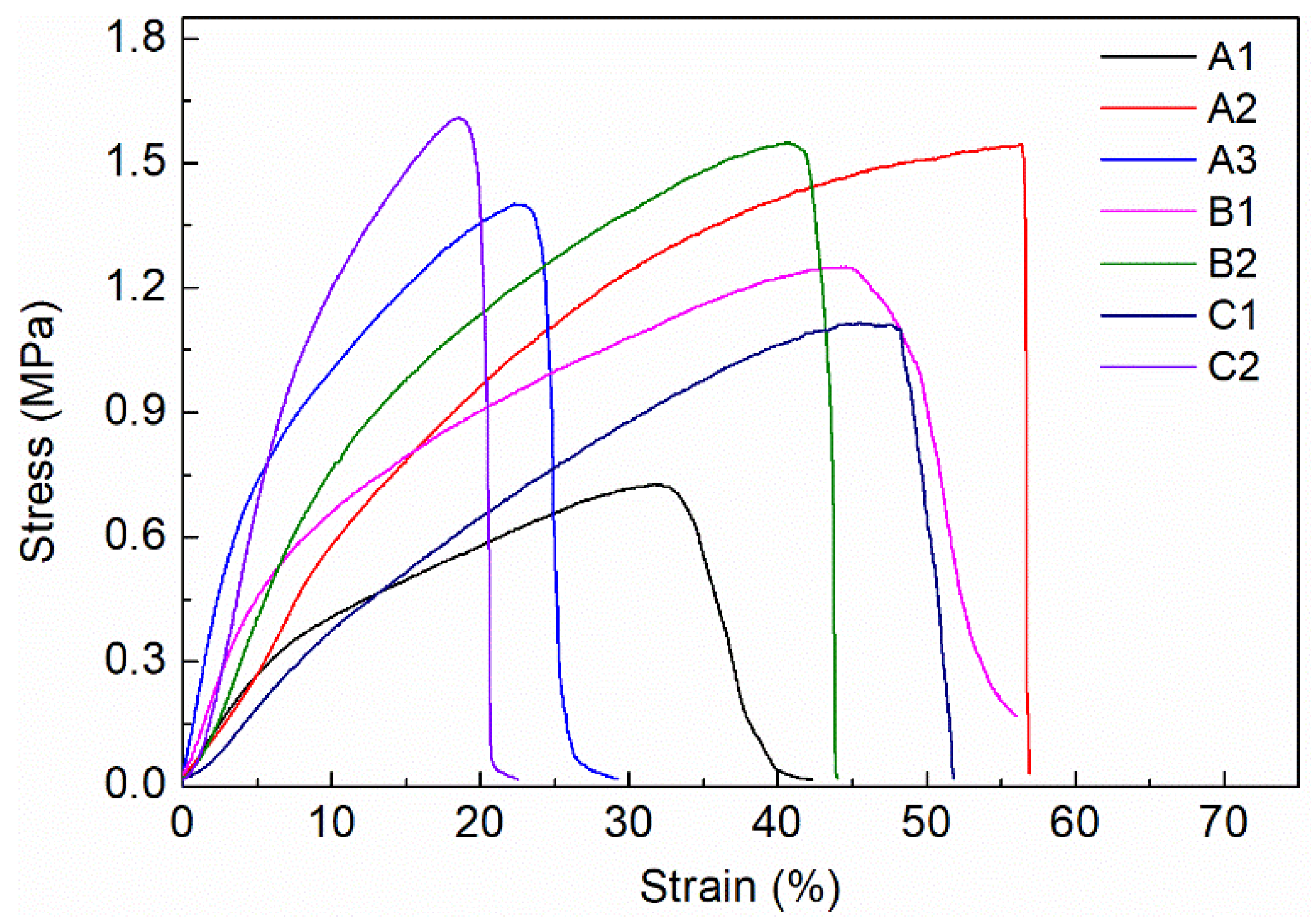
3.2. Mechanical Properties
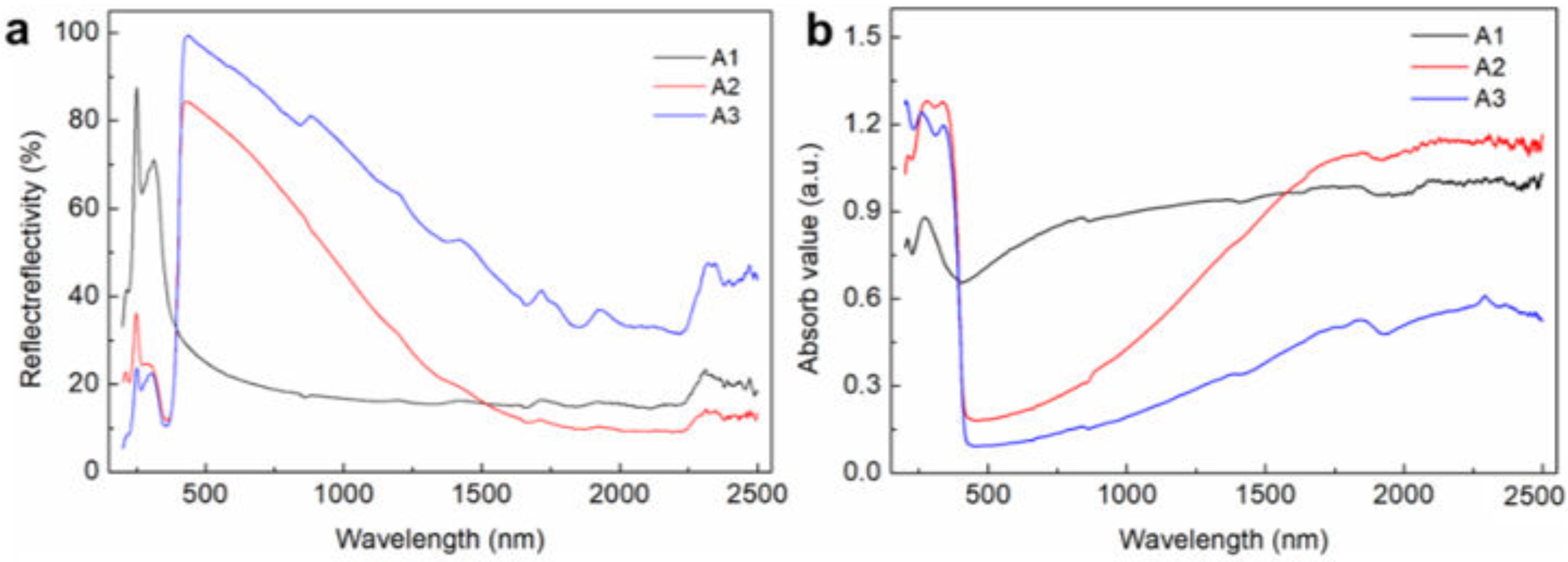
3.3. UV–Vis–NIR Spectrophotometric Analysis
3.4. Thermal Insulation Performances
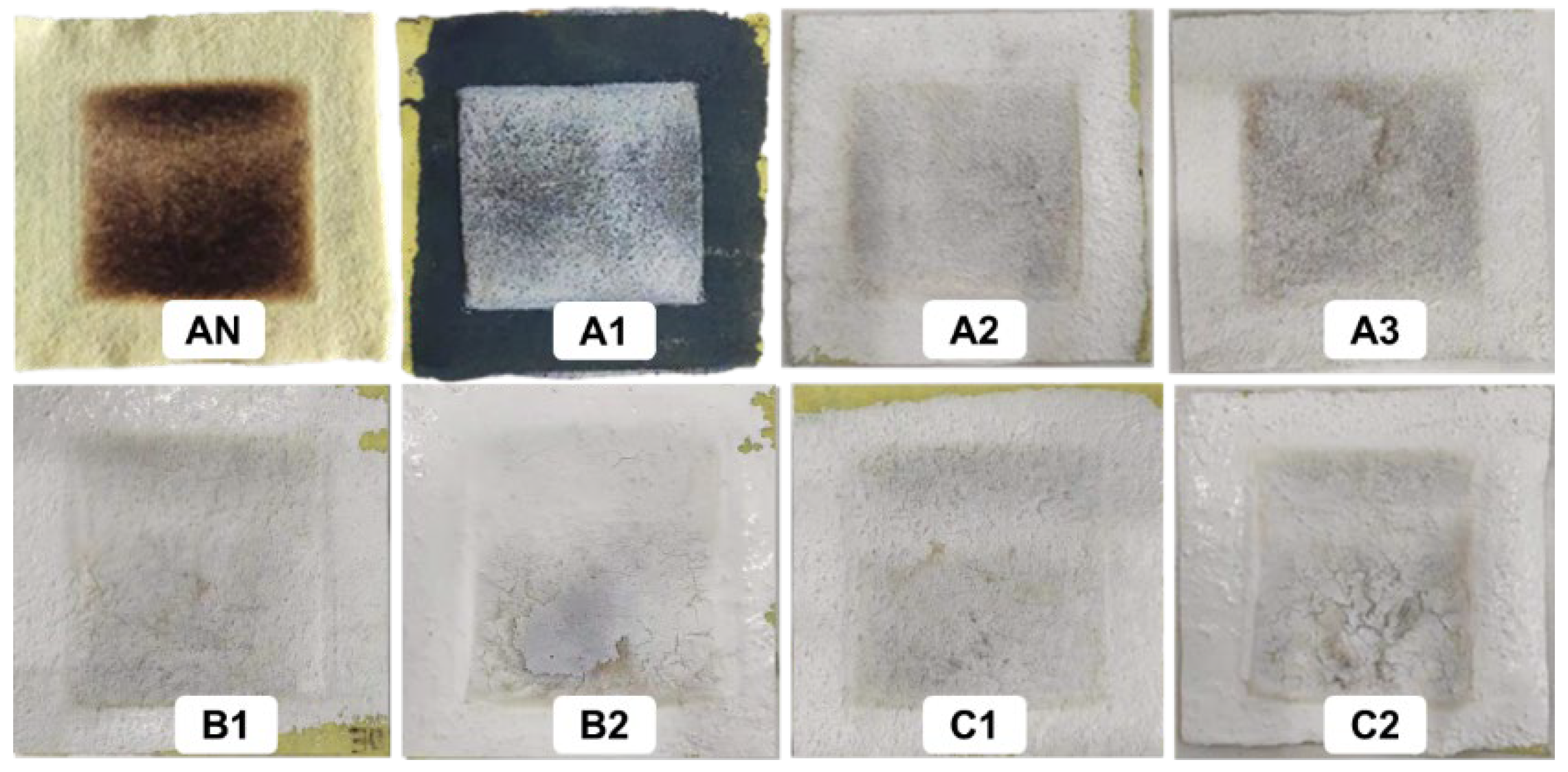
3.5. TPP Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, S.; Chen, L.; Gong, M.; Hu, X.; Zhang, X.; Zhou, Z. Characterization and engineering application of a novel ceramic composite insulation material. Compos. Part B Eng. 2017, 111, 143–147. [Google Scholar] [CrossRef]
- Wang, H.; Chiang, P.C.; Cai, Y.; Li, C.; Wang, X.; Chen, T.L.; Wei, S.; Huang, Q. Application of wall and insulation materials on green building: A review. Sustainability 2018, 10, 3331. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Gan, F.; Dong, J.; Fang, Y.; Zhao, X.; Zhang, Q. Facile preparation of continuous and porous polyimide aerogel fibers for multifunctional applications. ACS Appl. Mater. Interfaces 2021, 13, 10416–10427. [Google Scholar] [CrossRef] [PubMed]
- Scarponi, G.E.; Landucci, G.; Tugnoli, A.; Cozzani, V.; Birk, A.M. Performance assessment of thermal protection coatings of hazardous material tankers in the presence of defects. Process Saf. Environ. Prot. 2017, 105, 393–409. [Google Scholar] [CrossRef]
- Levenspiel, O. The three mechanisms of heat transfer: Conduction, convection, and radiation. In Engineering Flow and Heat Exchange; Springer: Boston, MA, USA, 2014; pp. 179–210. [Google Scholar]
- Iqbal, M. The Solar constant and its spectral distribution. In An Introduction to Solar Radiation; Elsevier: Amsterdam, The Netherland, 2012; pp. 43–57. [Google Scholar]
- Stewart, S.M. Spectral peaks and Wien’s displacement law. J. Thermophys. Heat Transf. 2012, 26, 689–691. [Google Scholar] [CrossRef]
- Xu, R.; Wang, W.; Yu, D. A novel multilayer sandwich fabric-based composite material for infrared stealth and super thermal insulation protection. Compos. Struct. 2019, 212, 58–65. [Google Scholar] [CrossRef]
- Thapliyal, P.C.; Singh, K. Aerogels as promising thermal insulating materials: An overview. J. Mater. 2014. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Chen, X.; Xiong, X.; Wu, X.; Xie, Z.; Liu, Z. Synthesis of hollow TiO2@ SiO2 spheres via a recycling template method for solar heat protection coating. Ceram. Int. 2021, 47, 2678–2685. [Google Scholar] [CrossRef]
- Bhuiyan, M.A.; Wang, L.; Shaid, A.; Jahan, I.; Shanks, R.A. Silica aerogel-integrated nonwoven protective fabrics for chemical and thermal protection and thermophysiological wear comfort. J. Mater. Sci. 2020, 55, 2405–2418. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, X.; Xu, X.; Liu, L.; Yu, B.; Maluk, C.; Huang, G.; Wang, H.; Song, P. Bioinspired, highly adhesive, nanostructured polymeric coatings for superhydrophobic fire-extinguishing thermal insulation foam. ACS Nano 2021, 15, 11667–11680. [Google Scholar] [CrossRef]
- Bühler, M.; Popa, A.M.; Scherer, L.J.; Lehmeier, F.K.S.; Rossi, R.M. Heat protection by different phase change materials. Appl. Therm. Eng. 2013, 54, 359–364. [Google Scholar] [CrossRef]
- Zhou, B.; Dong, Y.; Chi, Q.; Zhang, Y.; Chang, L.; Gong, M.; Huang, J.; Pan, Y.; Wang, X. Fe-based amorphous soft magnetic composites with SiO2 insulation coatings: A study on coatings thickness, microstructure and magnetic properties. Ceram. Int. 2020, 46, 13449–13459. [Google Scholar] [CrossRef]
- Huang, H.; Ng, M.; Wu, Y.; Kong, L. Solvothermal synthesis of Sb: SnO2 nanoparticles and IR shielding coating for smart window. Mater. Des. 2015, 88, 384–389. [Google Scholar] [CrossRef]
- Jia, S.K.; Yong, Z.O.U.; Xu, J.Y.; Jing, W.A.N.G.; Lei, Y.U. Effect of TiO2 content on properties of Al2O3 thermal barrier coatings by plasma spraying. Trans. Nonferrous Met. Soc. China 2015, 25, 175–183. [Google Scholar] [CrossRef]
- Wang, M.; Bu, J.; Xu, Y.; Liu, Y.; Yang, Y. Sb-doped SnO2 (ATO) hollow submicron spheres for solar heat insulation coating. Ceram. Int. 2021, 47, 547–555. [Google Scholar] [CrossRef]
- Hu, Y.; Zhong, H.; Wang, Y.; Lu, L.; Yang, H. TiO2/antimony-doped tin oxide: Highly water-dispersed nano composites with excellent IR insulation and super-hydrophilic property. Sol. Energy Mater. Sol. Cells 2018, 174, 499–508. [Google Scholar] [CrossRef]
- Chin, S.S.; Chiang, K.; Fane, A.G. The stability of polymeric membranes in a TiO2 photocatalysis process. J. Membr. Sci. 2006, 275, 202–211. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Ge, M.; Gao, Q. One-dimensional light-colored conductive antimony-doped tin oxide@ TiO2 whiskers: Synthesis and applications. J. Mater. Sci. Mater. Electron. 2018, 29, 619–627. [Google Scholar] [CrossRef]
- ASTM F2703-08; Standard Test Method for Unsteady-State Heat Transfer Evaluation of Flame Resistant Materials for Clothing with Burn Injury Prediction. American Society for Testing and Materials: West Conshohocken, PA, USA, 2013.
- Shanthi, E.; Banerjee, A.; Chopra, K.L. Dopant effects in sprayed tin oxide films. Thin Solid Film. 1982, 88, 93–100. [Google Scholar] [CrossRef]
- Bird, R.E.; Hulstrom, R.L.; Lewis, L.J. Terrestrial solar spectral data sets. Sol. Energy 1983, 30, 563–573. [Google Scholar] [CrossRef]
- Chandrasekar, M.; Suresh, S. A review on the mechanisms of heat transport in nanofluids. Heat Transf. Eng. 2009, 30, 1136–1150. [Google Scholar] [CrossRef]


| Fabric Code | Filler Type | Filler Content | Times of Blade Coating |
|---|---|---|---|
| A1 | ATO | 40 wt% | Twice |
| A2 | S-TiO2@ATO | 40 wt% | Twice |
| A3 | R-TiO2@ATO | 40 wt% | Twice |
| B1 | S-TiO2@ATO | 40 wt% | Once |
| B2 | S-TiO2@ATO | 40 wt% | Three times |
| C1 | S-TiO2@ATO | 30 wt% | Twice |
| C2 | S-TiO2@ATO | 50 wt% | Twice |
| Fabric Code | tn (s) | TPP Value (kW·s/m2) |
|---|---|---|
| AN | 5.31 | 432.23 |
| A1 | 11.61 | 945.054 |
| A2 | 13.51 | 1099.714 |
| A3 | 13.31 | 1083.434 |
| B1 | 8.41 | 684.574 |
| B2 | 17.91 | 1457.874 |
| C1 | 9.91 | 806.674 |
| C2 | 14.21 | 1156.694 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Sun, Q.; Xue, C.; Long, X.; Zhang, W. Evaluation on Thermal Protection Performance of TiO2@ATO Coated Aramid Nonwoven. Coatings 2022, 12, 657. https://doi.org/10.3390/coatings12050657
Liu W, Sun Q, Xue C, Long X, Zhang W. Evaluation on Thermal Protection Performance of TiO2@ATO Coated Aramid Nonwoven. Coatings. 2022; 12(5):657. https://doi.org/10.3390/coatings12050657
Chicago/Turabian StyleLiu, Wanwan, Qilong Sun, Chao Xue, Xiaoyun Long, and Wei Zhang. 2022. "Evaluation on Thermal Protection Performance of TiO2@ATO Coated Aramid Nonwoven" Coatings 12, no. 5: 657. https://doi.org/10.3390/coatings12050657
APA StyleLiu, W., Sun, Q., Xue, C., Long, X., & Zhang, W. (2022). Evaluation on Thermal Protection Performance of TiO2@ATO Coated Aramid Nonwoven. Coatings, 12(5), 657. https://doi.org/10.3390/coatings12050657






