Recent Advances on Hydrogen Evolution and Oxygen Evolution Catalysts for Direct Seawater Splitting
Abstract
:1. Introduction
2. HER Catalysts
2.1. Precious Metal
2.2. Transition Metal Sulfide
2.3. Transition Metal Selenide
2.4. Transition Metal Phosphide
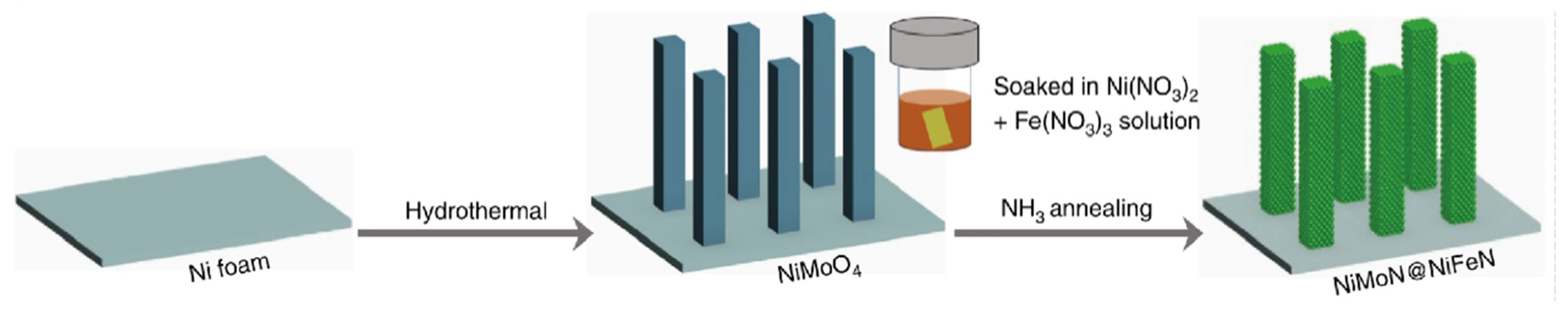
2.5. Transition Metal Carbide and Nitride
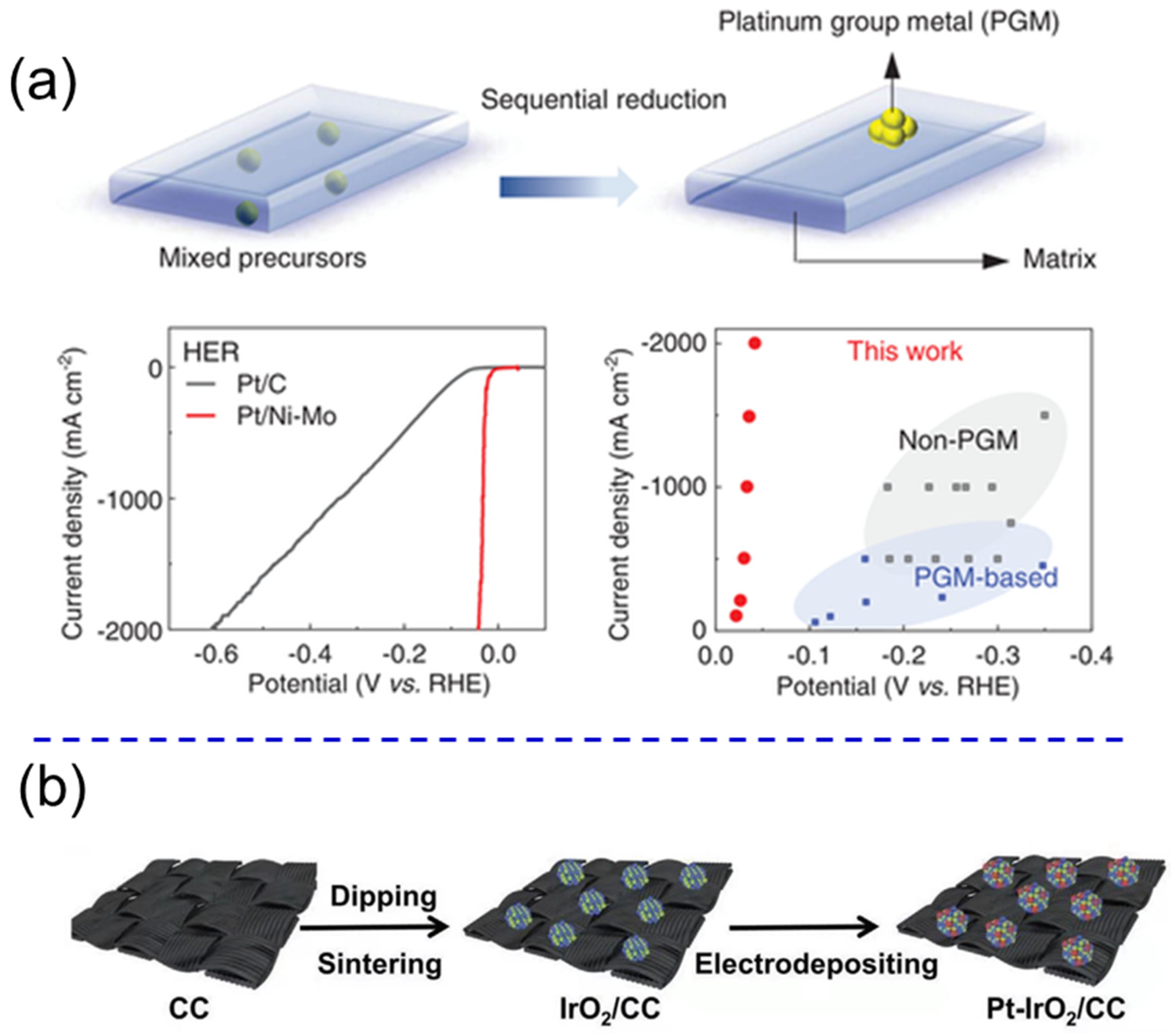
2.6. Transition Metal Alloys
3. OER Catalysts
3.1. Precious Metal
3.2. Transition Metal Oxide/Hydroxides
3.3. Transition Metal Phosphate
3.4. Transition Metal Chalcogenides
3.5. Transition Metal Phosphide
3.6. Transition Metal Nitrides
3.7. Micromolecule Additives to Form the Shielding Layer
3.8. Passivation Layer Covering
4. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, J.; Ding, J.; Wei, D.; Xie, X.; Li, B.; Lu, S.; Zhang, J.; Liu, Y.; Cai, Q.; Zang, S. Coupling of Ru and O-vacancy on 2D Mo-based electrocatalyst via a solid-phase interface reaction strategy for hydrogen evolution reaction. Adv. Energy Mater. 2021, 11, 2100141. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, Z.; Qiu, J. Energy-saving hydrogen production by seawater electrolysis coupling sulfion degradation. Adv. Mater. 2022, 34, 2109321. [Google Scholar] [CrossRef]
- Oh, B.S.; Oh, S.G.; Hwang, Y.Y.; Yu, H.-W.; Kang, J.-W.; Kim, I.S. Formation of hazardous inorganic by-products during electrolysis of seawater as a disinfection process for desalination. Sci. Total Environ. 2010, 408, 5958–5965. [Google Scholar] [CrossRef]
- Bennett, J.E. Electrodes for generation of hydrogen and oxygen from seawater. Int. J. Hydrog. Energy 1980, 5, 401–408. [Google Scholar] [CrossRef]
- Kirk, D.W.; Ledas, A.E. Precipitate formation during sea water electrolysis. Int. J. Hydrog. Energy 1982, 7, 925–932. [Google Scholar] [CrossRef]
- Cuartero, M.; Crespo, G.; Cherubini, T.; Pankratova, N.; Confalonieri, F.; Massa, F.; Tercier-Waeber, M.-L.; Abdou, M.; Schäfer, J.; Bakker, E. In Situ detection of macronutrients and chloride in seawater by submersible electrochemical sensors. Anal. Chem. 2018, 90, 4702–4710. [Google Scholar] [CrossRef] [Green Version]
- Ji, S.M.; Jun, H.; Jang, J.S.; Son, H.C.; Borse, P.H.; Lee, J.S. Photocatalytic hydrogen production from natural seawater. J. Photochem. Photobiol. A 2007, 189, 141–144. [Google Scholar] [CrossRef]
- Kester, D.R.; Duedall, I.W.; Connors, D.N.; Pytkowicz, R.M. Praparation of artifical seawater. Limnol. Oceanogr. 1967, 12, 176–179. [Google Scholar] [CrossRef]
- Maeda, K.; Masuda, H.; Domen, K. Effect of electrolyte addition on activity of (Ga1−xZnx)(N1−xOx) photocatalyst for overall water splitting under visible light. Catal. Today 2009, 147, 173–178. [Google Scholar] [CrossRef]
- Lu, X.; Pan, J.; Lovell, E.; Tan, T.H.; Ng, Y.H.; Amal, R. A sea-change: Manganese doped nickel/nickel oxide electrocatalysts for hydrogen generation from seawater. Energy Environ. Sci. 2018, 11, 1898–1910. [Google Scholar] [CrossRef]
- Ha, H.; Jin, K.; Park, S.; Lee, K.-G.; Cho, K.H.; Seo, H.; Ahn, H.-Y.; Lee, Y.H.; Nam, K.T. Highly selective active chlorine generation electrocatalyzed by Co3O4 nanoparticles: Mechanistic investigation through in situ electrokinetic and spectroscopic analyses. J. Phys. Chem. Lett. 2019, 10, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Hu, Z.; Xu, X.; He, J.; Dai, J.; Lin, H.-J.; Chan, T.-S.; Chen, C.-T.; Tjeng, L.H.; Zhou, W.; et al. Ternary phase diagram-facilitated rapid screening of double perovskites as electrocatalysts for the oxygen evolution reaction. Chem. Mater. 2019, 31, 5919–5926. [Google Scholar] [CrossRef]
- Dionigi, F.; Reier, T.; Pawolek, Z.; Gliech, M.; Strasser, P. Design criteria, operating conditions, and nickel–iron hydroxide catalyst materials for selective seawater electrolysis. ChemSusChem 2016, 9, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Luo, Y.; Yu, Q.; Zhang, Z.; Zhang, S.; Liu, Z.; Ren, W.; Cheng, H.-M.; Li, J.; Liu, B. A durable and efficient electrocatalyst for saline water splitting with current density exceeding 2000 mA cm−2. Adv. Funct. Mater. 2021, 31, 2010367. [Google Scholar] [CrossRef]
- Wu, D.; Chen, D.; Zhu, J.; Mu, S. Ultralow Ru incorporated amorphous cobalt-based oxides for high-current-density overall water splitting in alkaline and seawater media. Small 2021, 17, 2102777. [Google Scholar] [CrossRef]
- Phan Khanh Linh, T.; Duy Thanh, T.; Malhotra, D.; Prabhakaran, S.; Kim, D.H.; Kim, N.H.; Lee, J.H. Highly effective freshwater and seawater electrolysis enabled by atomic Rh-modulated Co-CoO lateral heterostructures. Small 2021, 17, 2103826. [Google Scholar] [CrossRef]
- Li, L.; Wang, B.; Zhang, G.; Yang, G.; Yang, T.; Yang, S.; Yang, S. Electrochemically modifying the electronic structure of IrO2 nanoparticles for overall electrochemical water splitting with extensive adaptability. Adv. Energy Mater. 2020, 10, 2001600. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, W.; Liu, S.; Chen, X.; Ma, T.; Wang, G.; Lu, Z.; Sun, J. Enhanced interface interaction in Cu2S@Ni core-shell nanorod arrays as hydrogen evolution reaction electrode for alkaline seawater electrolysis. J. Power Sources 2021, 506, 230235. [Google Scholar] [CrossRef]
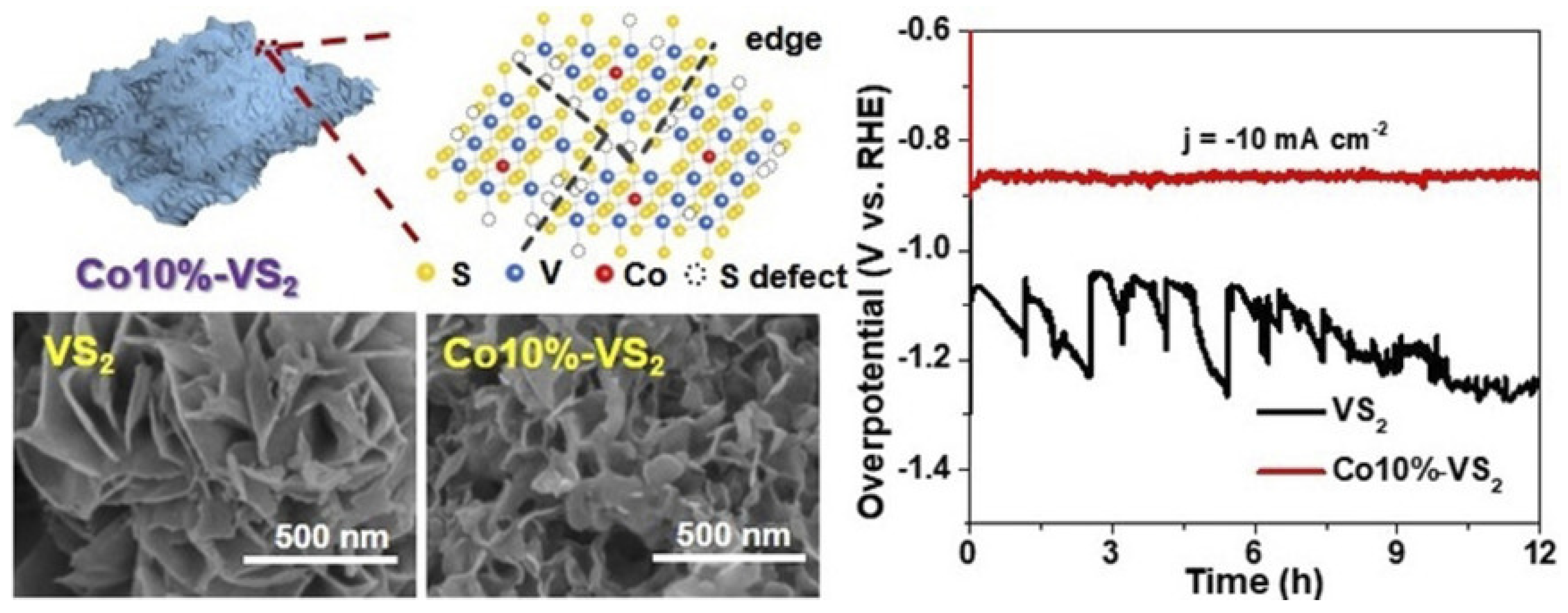
- Zhao, M.; Yang, M.; Huang, W.; Liao, W.; Bian, H.; Chen, D.; Wang, L.; Tang, J.; Liu, C. Synergism on electronic structures and active edges of metallic vanadium disulfide nanosheets via Co doping for efficient hydrogen evolution reaction in seawater. ChemCatChem 2021, 13, 2138–2144. [Google Scholar] [CrossRef]
- Zhao, Y.; Jin, B.; Zheng, Y.; Jin, H.; Jiao, Y.; Qiao, S.-Z. Charge state manipulation of cobalt selenide catalyst for overall seawater electrolysis. Adv. Energy Mater. 2018, 8, 1801926. [Google Scholar] [CrossRef]
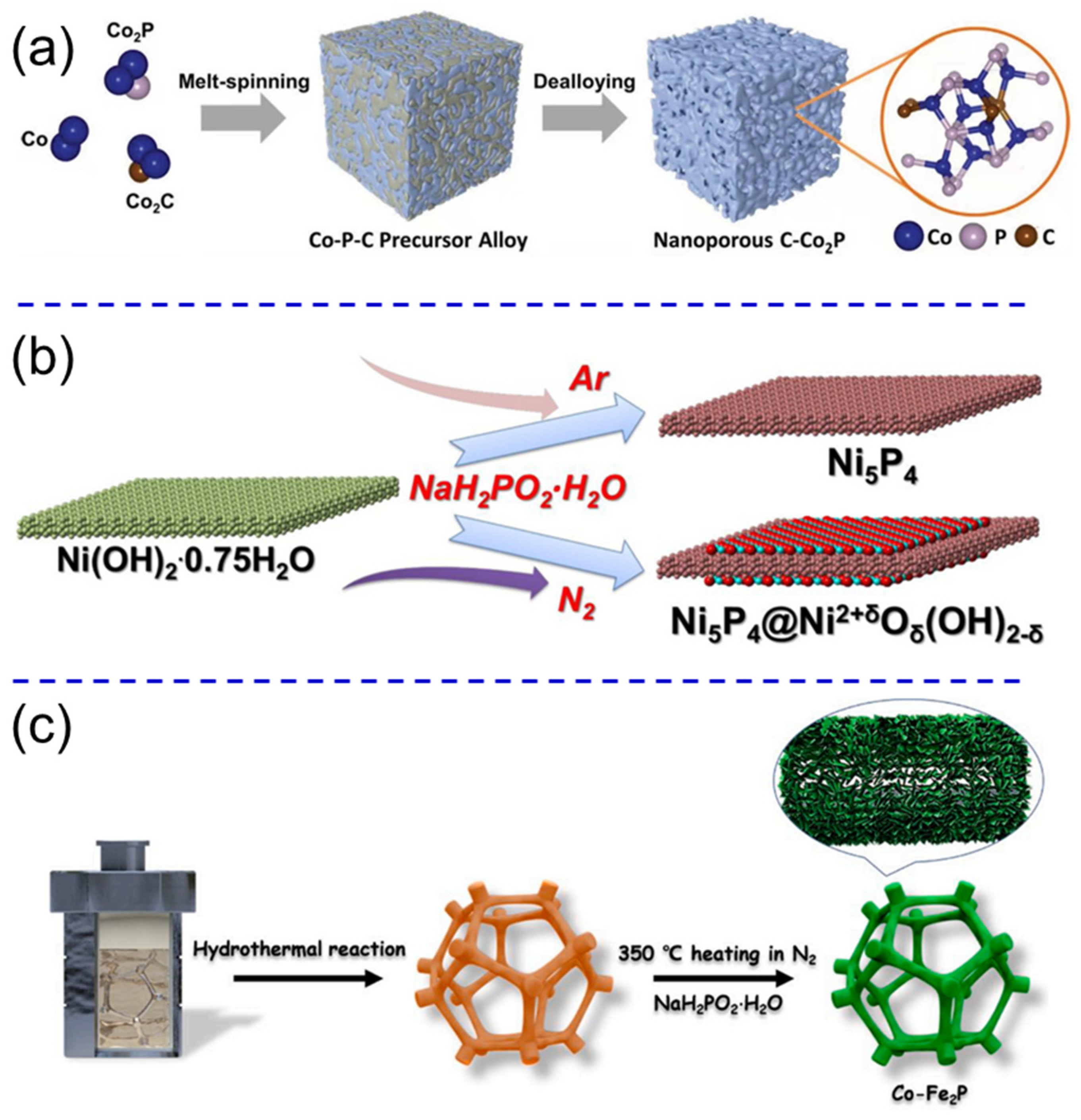
- Xu, W.; Fan, G.; Zhu, S.; Liang, Y.; Cui, Z.; Li, Z.; Jiang, H.; Wu, S.; Cheng, F. Electronic structure modulation of nanoporous cobalt phosphide by carbon doping for alkaline hydrogen evolution reaction. Adv. Funct. Mater. 2021, 31, 2107333. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, L.; Liu, R.; Hu, Y.; Xiong, T.; Qiu, W.; Balogun, M.S.; Pan, A.; Tong, Y. Nitrogen treatment generates tunable nanohybridization of Ni5P4 nanosheets with nickel hydr(oxy)oxides for efficient hydrogen production in alkaline, seawater and acidic media. Appl. Catal. B 2019, 251, 181–194. [Google Scholar] [CrossRef]
- Wang, S.; Yang, P.; Sun, X.; Xing, H.; Hu, J.; Chen, P.; Cui, Z.; Zhu, W.; Ma, Z. Synthesis of 3D heterostructure Co-doped Fe2P electrocatalyst for overall seawater electrolysis. Appl. Catal. B Environ. 2021, 297, 120386. [Google Scholar] [CrossRef]
- Yu, L.; Wu, L.; Song, S.; McElhenny, B.; Zhang, F.; Chen, S.; Ren, Z. Hydrogen generation from seawater electrolysis over a sandwich-like NiCoN|NixP|NiCoN microsheet array catalyst. ACS Energy Lett. 2020, 5, 2681–2689. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, S.; Wang, Z.; Liu, J.; Pei, W.; Yang, P.; Zhao, J.; Qiu, J. Engineering multifunctional collaborative catalytic interface enabling efficient hydrogen evolution in all pH range and seawater. Adv. Energy Mater. 2019, 9, 1901333. [Google Scholar] [CrossRef]
- Zang, W.; Sun, T.; Yang, T.; Xi, S.; Waqar, M.; Kou, Z.; Lyu, Z.; Feng, Y.P.; Wang, J.; Pennycook, S.J. Efficient hydrogen evolution of oxidized Ni-N3 defective sites for alkaline freshwater and seawater electrolysis. Adv. Mater. 2021, 33, 2003846. [Google Scholar] [CrossRef]
- Sun, F.; Qin, J.; Wang, Z.; Yu, M.; Wu, X.; Sun, X.; Qiu, J. Energy-saving hydrogen production by chlorine-free hybrid seawater splitting coupling hydrazine degradation. Nat. Commun. 2021, 12, 4182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Li, F.; Easton, C.D.; Bond, A.M.; Zhang, J. Oxomolybdate anchored on copper for electrocatalytic hydrogen production over the entire pH range. Appl. Catal. B 2019, 249, 227–234. [Google Scholar] [CrossRef]
- Yuan, W.; Cui, Z.; Zhu, S.; Li, Z.; Wu, S.; Liang, Y. Structure engineering of electrodeposited NiMo films for highly efficient and durable seawater splitting. Electrochim. Acta 2021, 365, 137366. [Google Scholar] [CrossRef]
- Ros, C.; Murcia-Lopez, S.; Garcia, X.; Rosado, M.; Arbiol, J.; Llorca, J.; Morante, J.R. Facing seawater splitting challenges by regeneration with Ni-Mo-Fe bifunctional electrocatalyst for hydrogen and oxygen evolution. ChemSusChem 2021, 14, 2872–2881. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Sun, W.; Hu, Y.; Liao, J.; Tian, X.; Gao, H.; Ge, C. IrOx nanoclusters modified by BaCO3 enable “Two Birds with One Stone” in solar-driven direct unbuffered seawater electrolysis. ACS Appl. Mater. Interfaces 2021, 13, 61088–61097. [Google Scholar] [CrossRef]
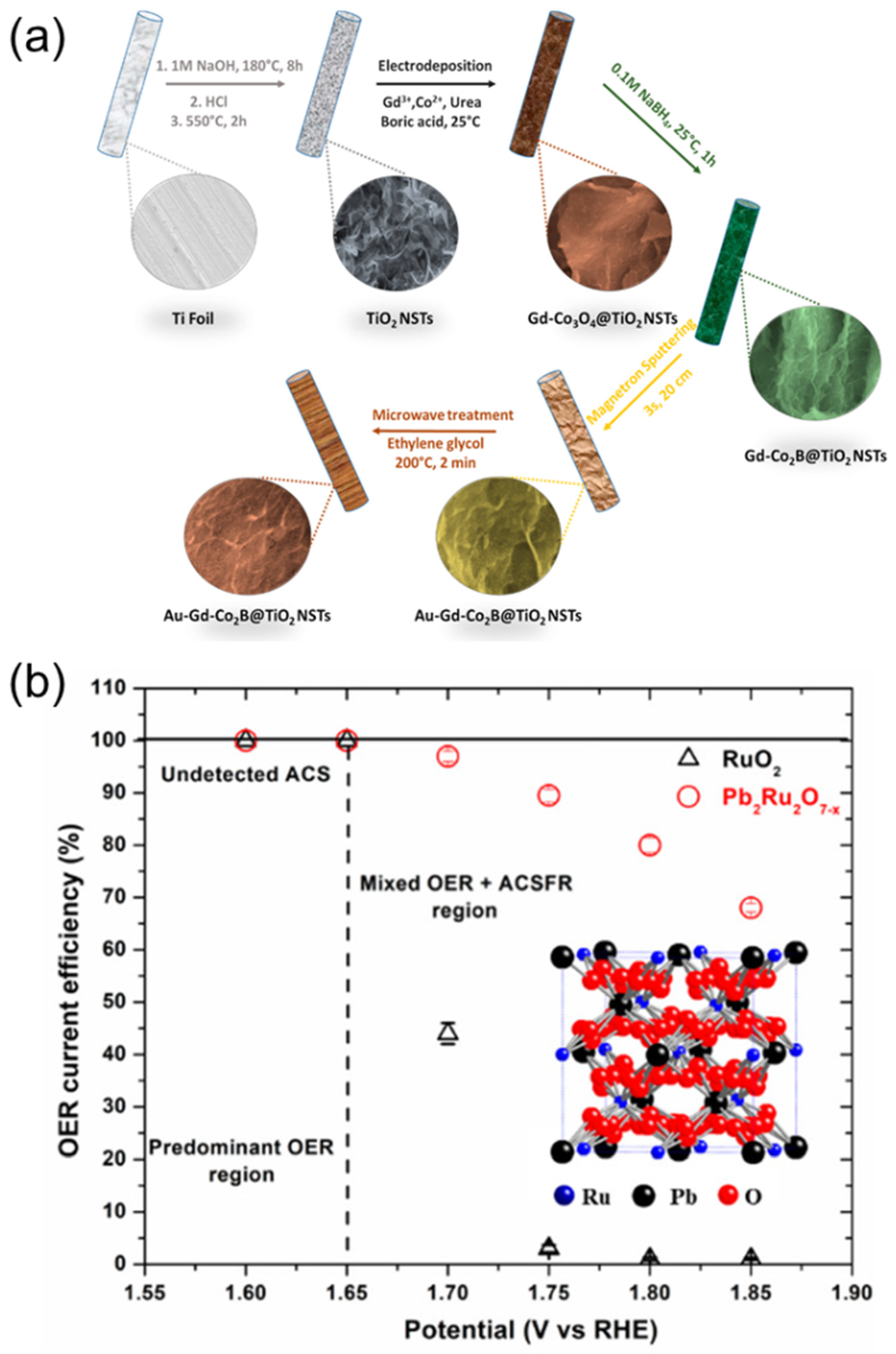
- ul Haq, T.; Pasha, M.; Tong, Y.; Mansour, S.A.; Haik, Y. Au nanocluster coupling with Gd-Co2B nanoflakes embedded in reduced TiO2 nanosheets: Seawater electrolysis at low cell voltage with high selectivity and corrosion resistance. Appl. Catal. B 2022, 301, 120836. [Google Scholar] [CrossRef]
- Ko, J.S.; Johnson, J.K.; Johnson, P.I.; Xia, Z. Decoupling oxygen and chlorine evolution reactions in seawater using iridium-based electrocatalysts. ChemCatChem 2020, 12, 4526–4532. [Google Scholar] [CrossRef]
- Gayen, P.; Saha, S.; Ramani, V. Selective seawater splitting using pyrochlore electrocatalyst. ACS Appl. Energy Mater. 2020, 3, 3978–3983. [Google Scholar] [CrossRef]
- Ning, M.; Wu, L.; Zhang, F.; Wang, D.; Song, S.; Tong, T.; Bao, J.; Chen, S.; Yu, L.; Ren, Z. One-step spontaneous growth of NiFe layered double hydroxide at room temperature for seawater oxygen evolution. Mater. Today Phys. 2021, 19, 100419. [Google Scholar] [CrossRef]
- Badreldin, A.; Nabeeh, A.; Youssef, E.; Mubarak, N.; ElSayed, H.; Mohsen, R.; Ahmed, F.; Wubulikasimu, Y.; Elsaid, K.; Abdel-Wahab, A. Adapting early transition metal and nonmetallic dopants on CoFe oxyhydroxides for enhanced alkaline and neutral pH saline water oxidation. ACS Appl. Energy Mater. 2021, 4, 6942–6956. [Google Scholar] [CrossRef]
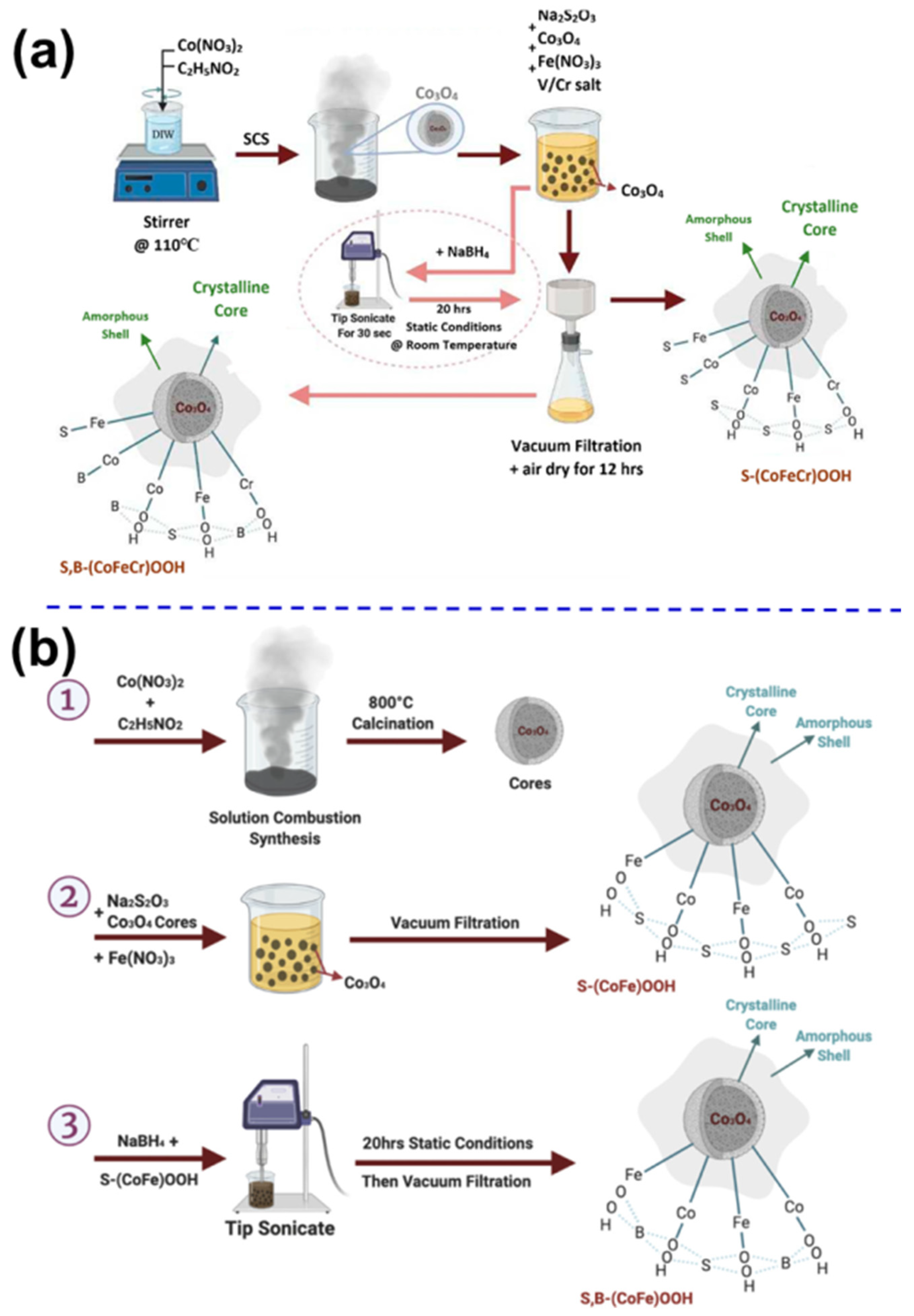
- Badreldin, A.; Youssef, K.; El Ghenymy, A.; Wubulikasimu, Y.; Ghouri, Z.K.; Elsaid, K.; Kumar, D.; Abdel-Wahab, A. Solution combustion synthesis of novel S,B-codoped CoFe oxyhydroxides for the oxygen evolution reaction in saline water. ACS Omega 2022, 7, 5521–5536. [Google Scholar] [CrossRef]
- Li, L.; Zhang, G.; Wang, B.; Zhu, D.; Liu, D.; Liu, Y.; Yang, S. Fe2O3/NiO interface for the electrochemical oxygen evolution in seawater and domestic sewage. ACS Appl. Mater. Interfaces 2021, 13, 37152–37161. [Google Scholar] [CrossRef]
- Ul Haq, T.; Mansour, S.; Haik, Y. Electronic and structural modification of Mn3O4 nanosheets for selective and sustained seawater oxidation. ACS Appl. Mater. Interfaces 2022, 14, 20443–20454. [Google Scholar] [CrossRef]
- Abe, H.; Murakami, A.; Tsunekawa, S.; Okada, T.; Wakabayashi, T.; Yoshida, M.; Nakayama, M. Selective catalyst for oxygen evolution in neutral brine electrolysis: An oxygen-deficient manganese oxide film. ACS Catal. 2021, 11, 6390–6397. [Google Scholar] [CrossRef]
- Dresp, S.; Dionigi, F.; Loos, S.; de Araujo, J.F.; Spoeri, C.; Gliech, M.; Dau, H.; Strasser, P. Direct Electrolytic splitting of seawater: Activity, selectivity, degradation, and recovery studied from the molecular catalyst structure to the electrolyzer cell level. Adv. Energy Mater. 2018, 8, 1800338. [Google Scholar] [CrossRef]
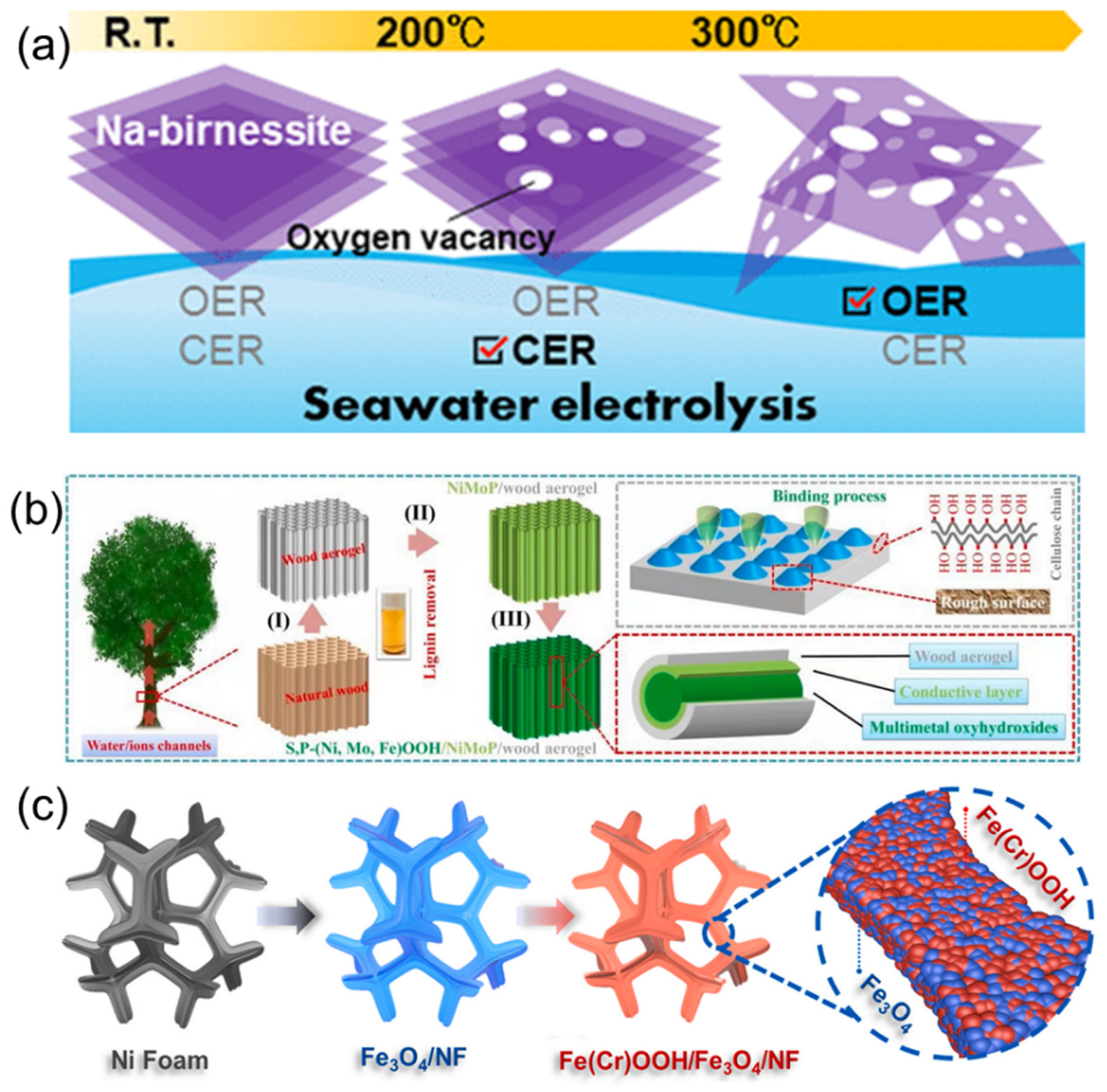
- Chen, H.; Zou, Y.; Li, J.; Zhang, K.; Xia, Y.; Hui, B.; Yang, D. Wood aerogel-derived sandwich-like layered nanoelectrodes for alkaline overall seawater electrosplitting. Appl. Catal. B 2021, 293, 120215. [Google Scholar] [CrossRef]
- Li, L.; Zhang, G.; Wang, B.; Yang, S. Constructing the Fe/Cr double (oxy)hydroxides on Fe3O4 for boosting the electrochemical oxygen evolution in alkaline seawater and domestic sewage. Appl. Catal. B 2022, 302, 120847. [Google Scholar] [CrossRef]
- Wu, L.; Yu, L.; Zhu, Q.; McElhenny, B.; Zhang, F.; Wu, C.; Xing, X.; Bao, J.; Chen, S.; Ren, Z. Boron-modified cobalt iron layered double hydroxides for high efficiency seawater oxidation. Nano Energy 2021, 83, 105838. [Google Scholar] [CrossRef]
- Jung, S.Y.; Kang, S.; Kim, K.M.; Mhin, S.; Kim, J.C.; Kim, S.J.; Enkhtuvshin, E.; Choi, S.; Han, H. Sulfur-incorporated nickel-iron layered double hydroxides for effective oxygen evolution reaction in seawater. Appl. Surf. Sci. 2021, 568, 150965. [Google Scholar] [CrossRef]
- Gao, X.; Chen, Y.; Sun, T.; Huang, J.; Zhang, W.; Wang, Q.; Cao, R. Karst landform-featured monolithic electrode for water electrolysis in neutral media. Energy Environ. Sci. 2020, 13, 174–182. [Google Scholar] [CrossRef]
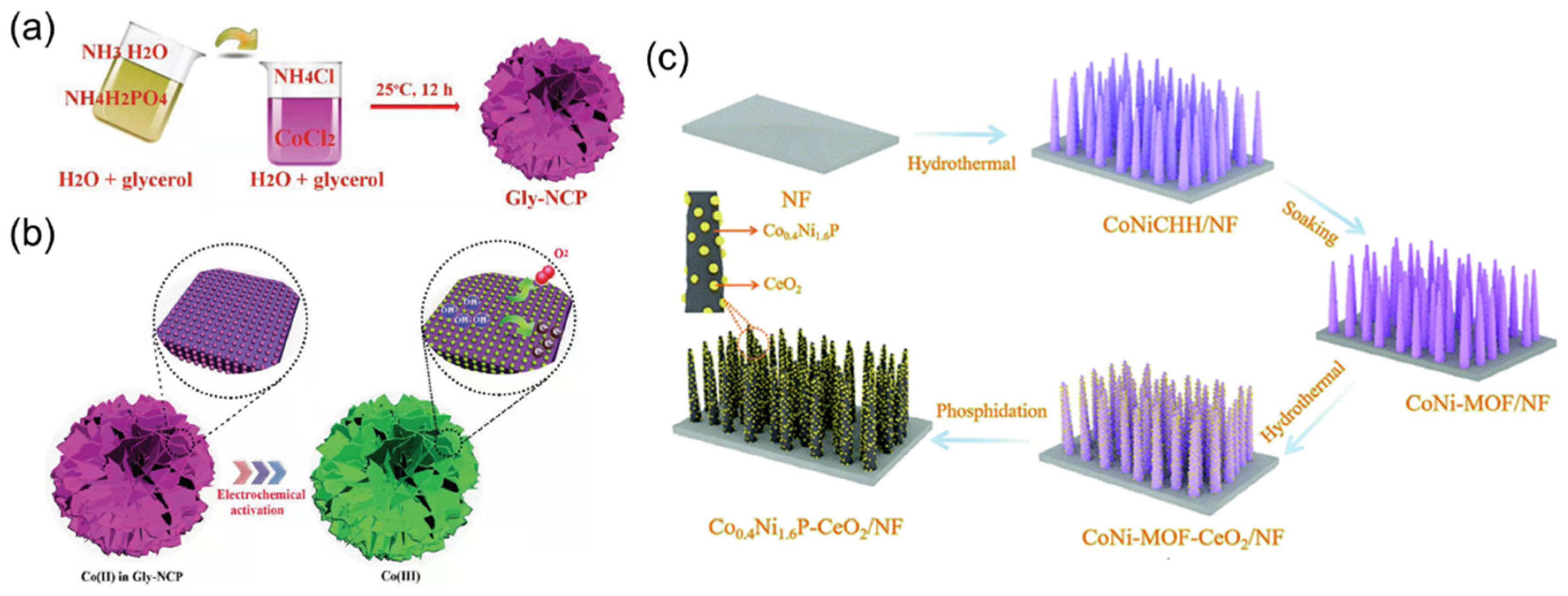
- Song, Z.; Wang, K.; Sun, Q.; Zhang, L.; Li, J.; Li, D.; Sze, P.-W.; Liang, Y.; Sun, X.; Fu, X.Z.; et al. High-performance ammonium cobalt phosphate nanosheet electrocatalyst for alkaline saline water oxidation. Adv. Sci. 2021, 8, 2100498. [Google Scholar] [CrossRef]
- Cong, Y.; Chen, X.; Mei, Y.; Ye, J.; Li, T.-T. CeO2 decorated bimetallic phosphide nanowire arrays for enhanced oxygen evolution reaction electrocatalysis via interface engineering. Dalton Trans. 2022, 51, 2923–2931. [Google Scholar] [CrossRef]
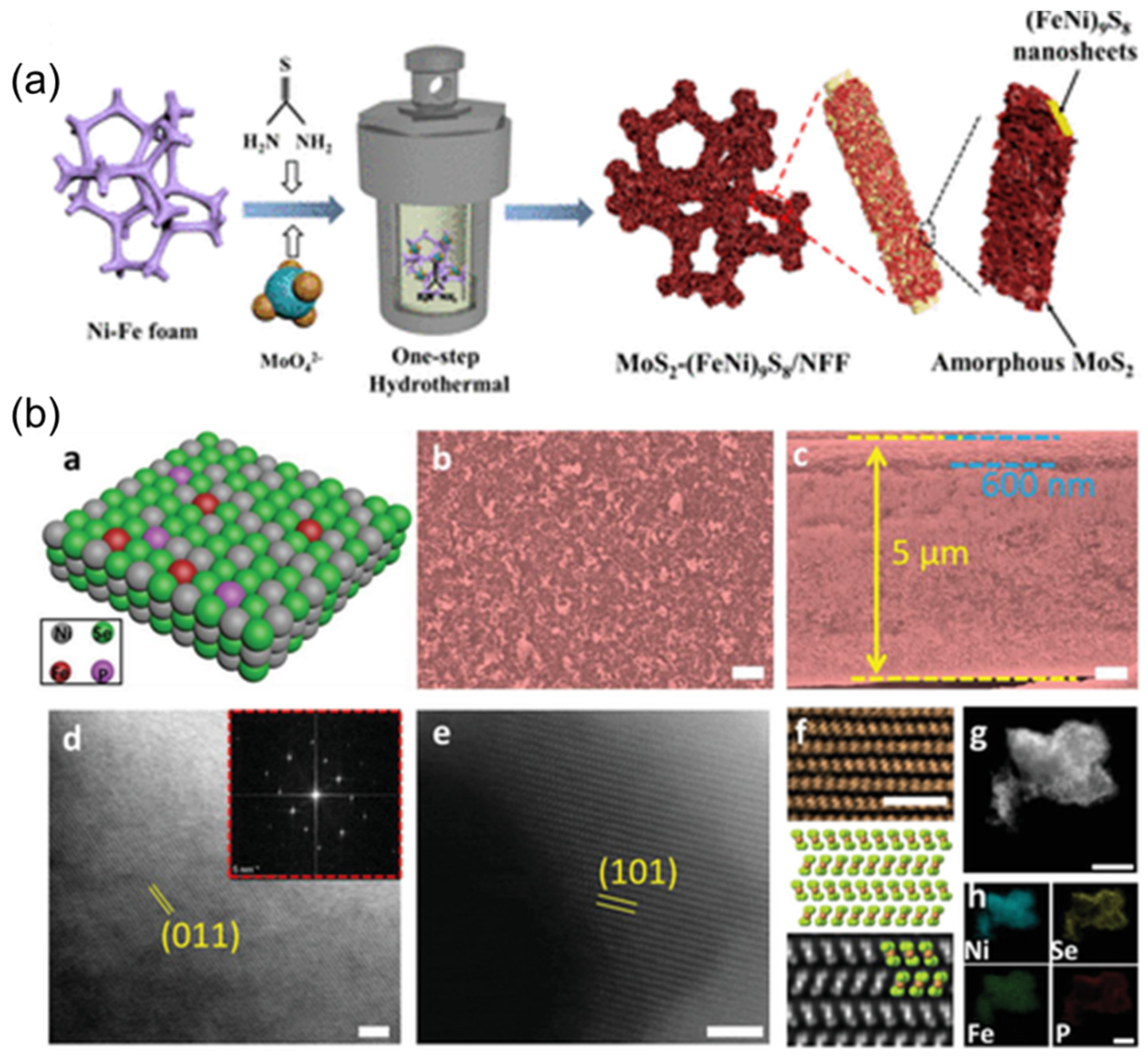
- Song, S.; Wang, Y.; Zhou, S.; Gao, H.; Tian, X.; Yuan, Y.; Li, W.; Zang, J.B. One-step synthesis of heterostructural MoS2-(FeNi)9S8 on Ni-Fe foam synergistically boosting for efficient fresh/seawater electrolysis. ACS Appl. Energy Mater. 2022, 5, 1810–1821. [Google Scholar] [CrossRef]
- Chang, J.; Wang, G.; Yang, Z.; Li, B.; Wang, Q.; Kuliiev, R.; Orlovskaya, N.; Gu, M.; Du, Y.; Wang, G.; et al. Dual-doping and synergism toward high-performance seawater electrolysis. Adv. Mater. 2021, 33, 2101425. [Google Scholar] [CrossRef]
- Cui, B.; Hu, Z.; Liu, C.; Liu, S.; Chen, F.; Hu, S.; Zhang, J.; Zhou, W.; Deng, Y.; Qin, Z.; et al. Heterogeneous lamellar-edged Fe-Ni(OH)2/Ni3S2 nanoarray for efficient and stable seawater oxidation. Nano Res. 2021, 14, 1149–1155. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, M.; Cao, Z.; Zhu, P.; Cao, Y.; Xu, X.; Xu, C.; Yin, Z. Heterogeneous bimetallic sulfides based seawater electrolysis towards stable industrial-level large current density. Appl. Catal. B 2021, 291, 120071. [Google Scholar] [CrossRef]
- Yu, L.; Wu, L.; McElhenny, B.; Song, S.; Luo, D.; Zhang, F.; Yu, Y.; Chen, S.; Ren, Z. Ultrafast room-temperature synthesis of porous S-doped Ni/Fe (oxy)hydroxide electrodes for oxygen evolution catalysis in seawater splitting. Energy Environ. Sci. 2020, 13, 3439–3446. [Google Scholar] [CrossRef]
- Wu, L.; Yu, L.; Zhang, F.; McElhenny, B.; Luo, D.; Karim, A.; Chen, S.; Ren, Z. Heterogeneous bimetallic phosphide Ni2P-Fe2P as an efficient bifunctional catalyst for water/seawater splitting. Adv. Funct. Mater. 2021, 31, 2006484. [Google Scholar] [CrossRef]
- Qi, L.; Li, A.; Wang, M.; Zhang, Y.; Zhang, K.; Li, X. Stable and efficient oxygen evolution from seawater enabled by graphene-supported sub-nanometer arrays of transition metal phosphides. Adv. Mater. Interfaces 2022, 9, 2101720. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, B.; Zhu, J.; Li, Y.; Tsiakaras, P.; Shen, P.K. Electronic modulation of cobalt phosphide nanosheet arrays via copper doping for highly efficient neutral-pH overall water splitting. Appl. Catal. B 2020, 265, 118555. [Google Scholar] [CrossRef]
- Yu, L.; Zhu, Q.; Song, S.; McElhenny, B.; Wang, D.; Wu, C.; Qin, Z.; Bao, J.; Yu, Y.; Chen, S.; et al. Non-noble metal-nitride based electrocatalysts for high-performance alkaline seawater electrolysis. Nat. Commun. 2019, 10, 5106. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.; Xu, W.; Li, B.; Chen, X.; Zhao, J.; Wan, S.; Jiang, K.; Zhang, S.; Wang, Z.; Tian, Z.; et al. The critical role of additive sulfate for stable alkaline seawater oxidation on nickel-based electrodes. Angew. Chem. Int. Ed. 2021, 60, 22740–22744. [Google Scholar] [CrossRef]
- Zhuang, L.; Li, J.; Wang, K.; Li, Z.; Zhu, M.; Xu, Z. Structural buffer engineering on metal oxide for long-term stable seawater splitting. Adv. Funct. Mater. 2022, 2201127. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Chen, H.; Zhang, Z.; Zou, X. Design of a multilayered oxygen-evolution electrode with high catalytic activity and corrosion resistance for saline water splitting. Adv. Funct. Mater. 2021, 31, 2101820. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, L.; Wang, C.; Feng, S.; Zhang, N.; Zhang, K.; Yang, B. Utilizing tannic acid and polypyrrle to induce reconstruction to optimize the activity of MOF-derived electrocatalyst for water oxidation in seawater. Chem. Eng. J. 2022, 430, 132632. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, L.; Li, S.; Li, J.; Wang, K.; Guan, Z.; Liang, C.; Xu, Z. Recent Advances on Hydrogen Evolution and Oxygen Evolution Catalysts for Direct Seawater Splitting. Coatings 2022, 12, 659. https://doi.org/10.3390/coatings12050659
Zhuang L, Li S, Li J, Wang K, Guan Z, Liang C, Xu Z. Recent Advances on Hydrogen Evolution and Oxygen Evolution Catalysts for Direct Seawater Splitting. Coatings. 2022; 12(5):659. https://doi.org/10.3390/coatings12050659
Chicago/Turabian StyleZhuang, Linzhou, Shiyi Li, Jiankun Li, Keyu Wang, Zeyu Guan, Chen Liang, and Zhi Xu. 2022. "Recent Advances on Hydrogen Evolution and Oxygen Evolution Catalysts for Direct Seawater Splitting" Coatings 12, no. 5: 659. https://doi.org/10.3390/coatings12050659
APA StyleZhuang, L., Li, S., Li, J., Wang, K., Guan, Z., Liang, C., & Xu, Z. (2022). Recent Advances on Hydrogen Evolution and Oxygen Evolution Catalysts for Direct Seawater Splitting. Coatings, 12(5), 659. https://doi.org/10.3390/coatings12050659





