UV Blocking and Oxygen Barrier Coatings Based on Polyvinyl Alcohol and Zinc Oxide Nanoparticles for Packaging Applications
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
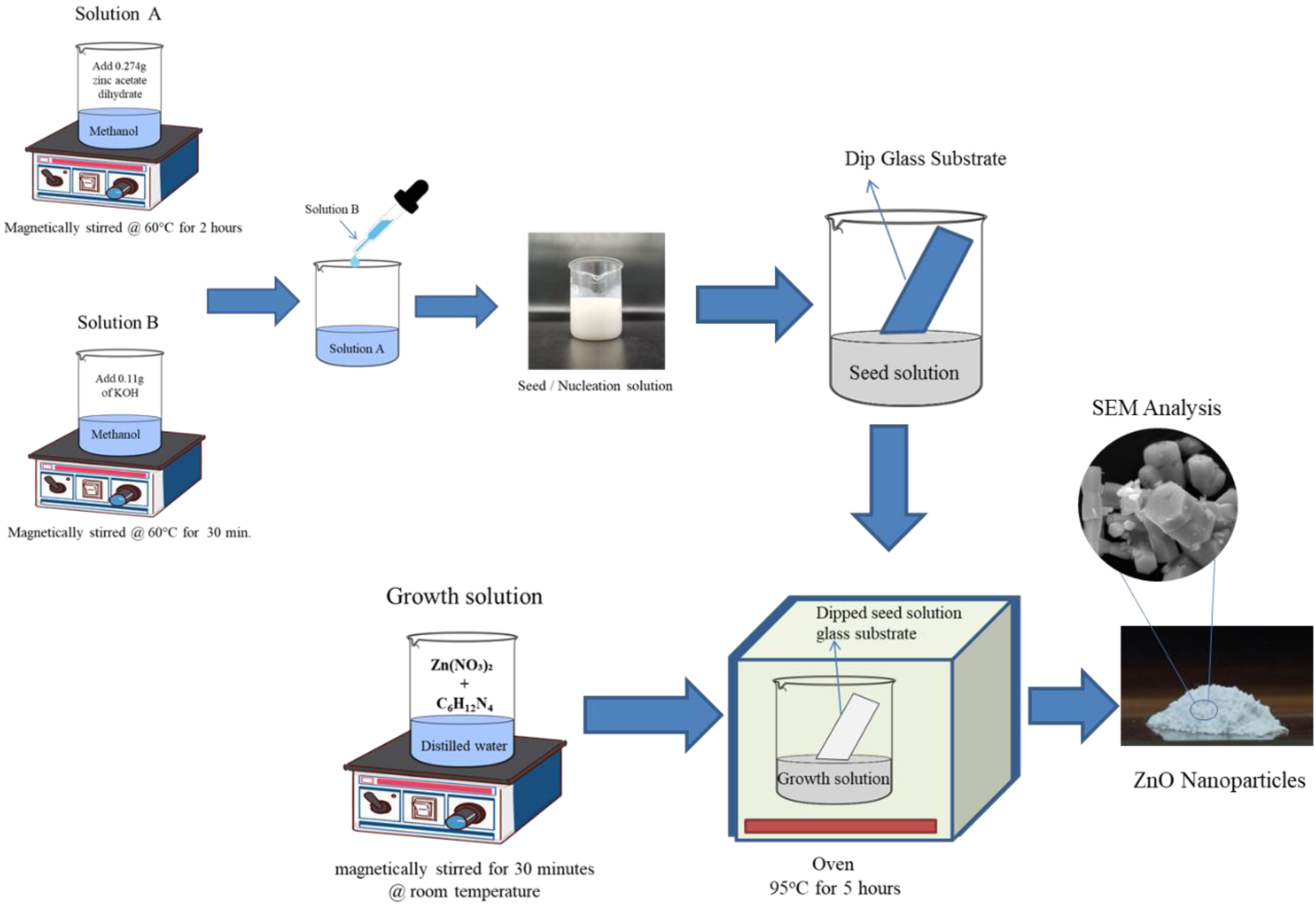
2.2.1. Synthesis of ZnO Nanoparticles
2.2.2. Synthesis of PVA/ZnO-Based Nanocomposite Films
2.3. Characterization
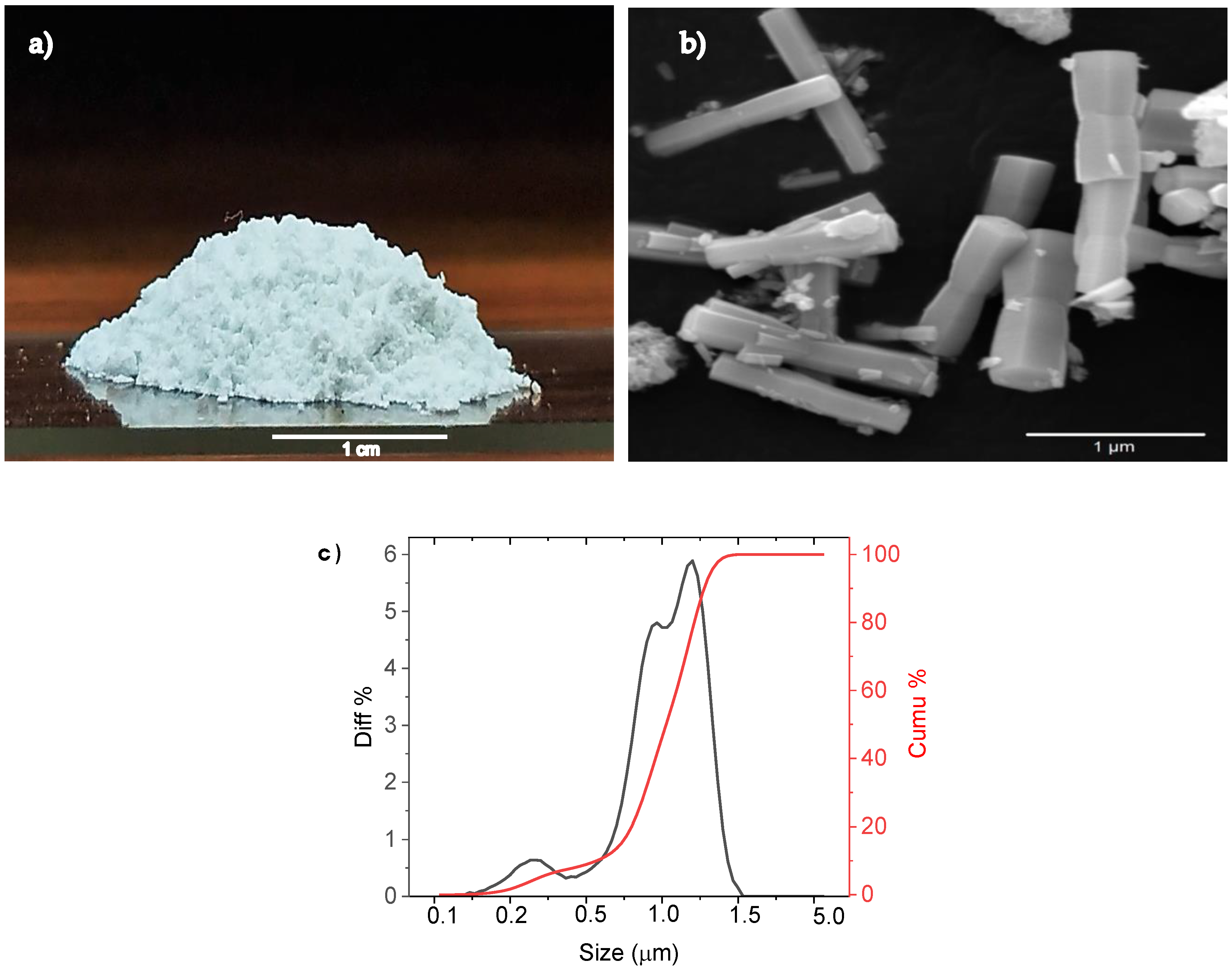
2.3.1. SEM of ZnO Nanoparticle
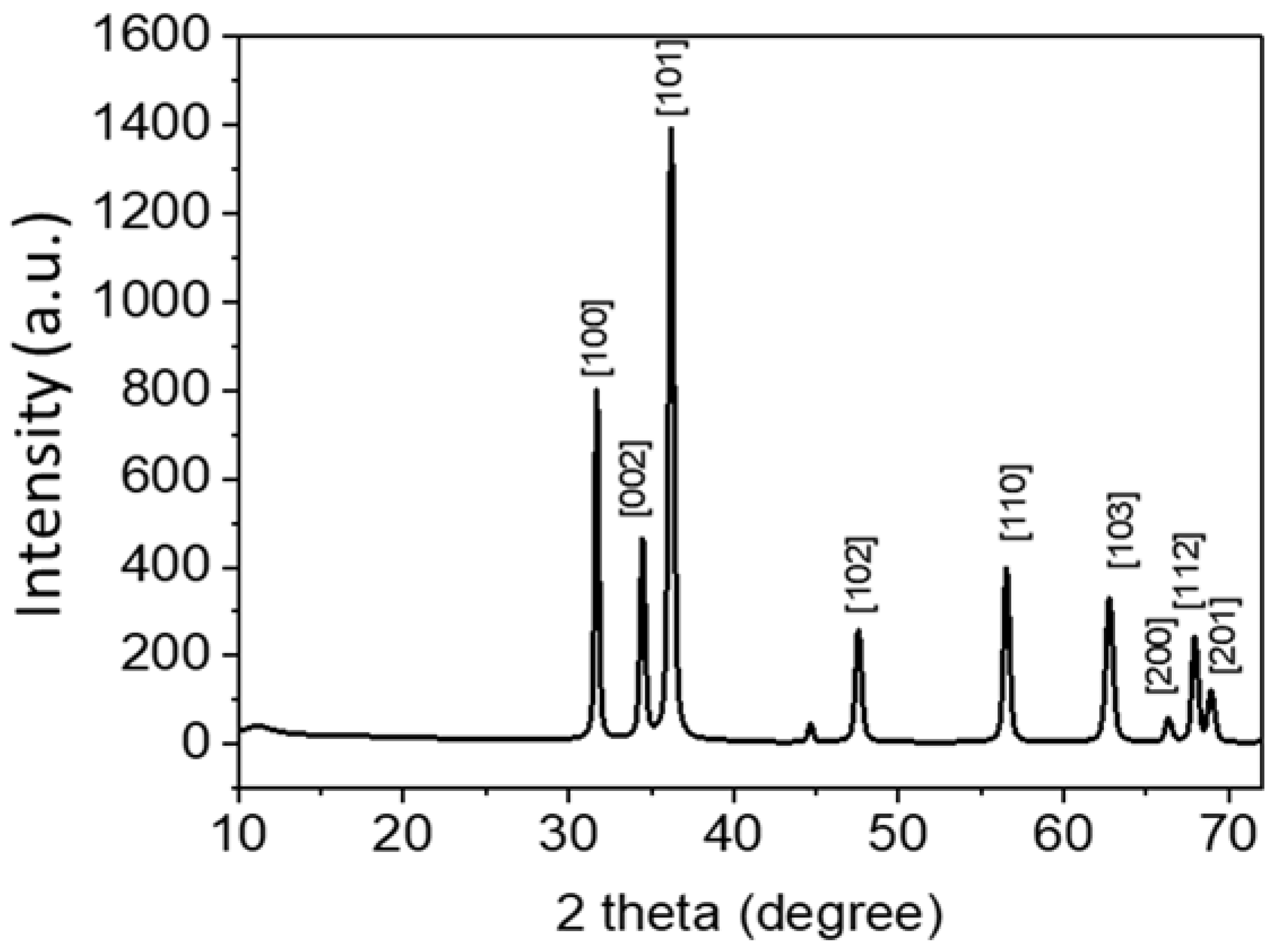
2.3.2. XRD of ZnO Nanoparticle
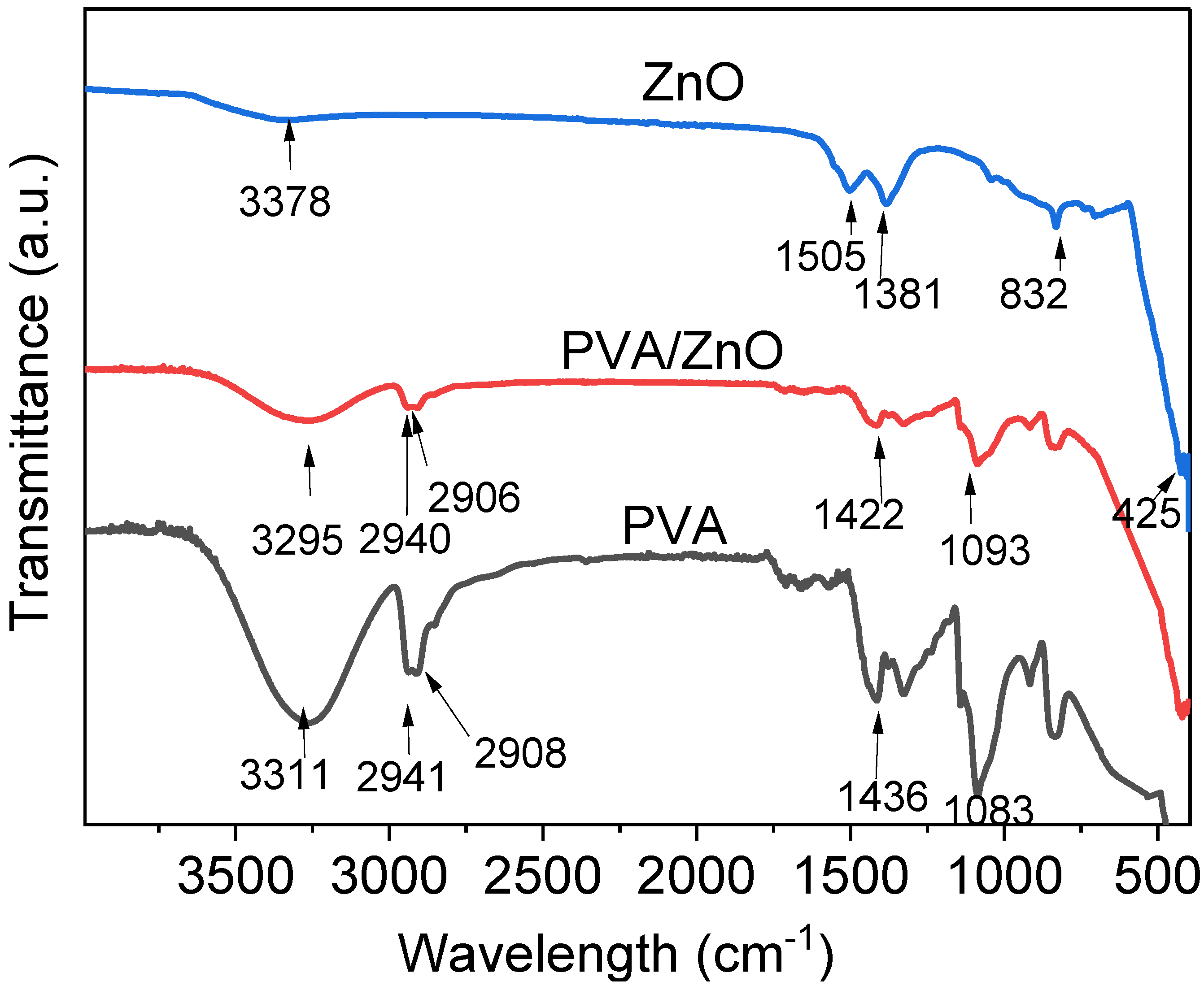
2.3.3. FTIR of ZnO Nanoparticle
2.3.4. TGA of ZnO Nanoparticle
2.3.5. Contact Angle
2.3.6. UV–VIS of PVA/ZnO Films
2.3.7. Oxygen Transmission Rate Measurement of Films
2.3.8. Water Vapor Transmission Rate
2.3.9. Bending of the Films
2.3.10. Tensile Strength
2.3.11. Hardness
3. Result and Discussion
3.1. SEM Analysis of ZnO Nanoparticle
3.2. XRD Analysis of ZnO Powder
3.3. FTIR Analysis of Films and Composites
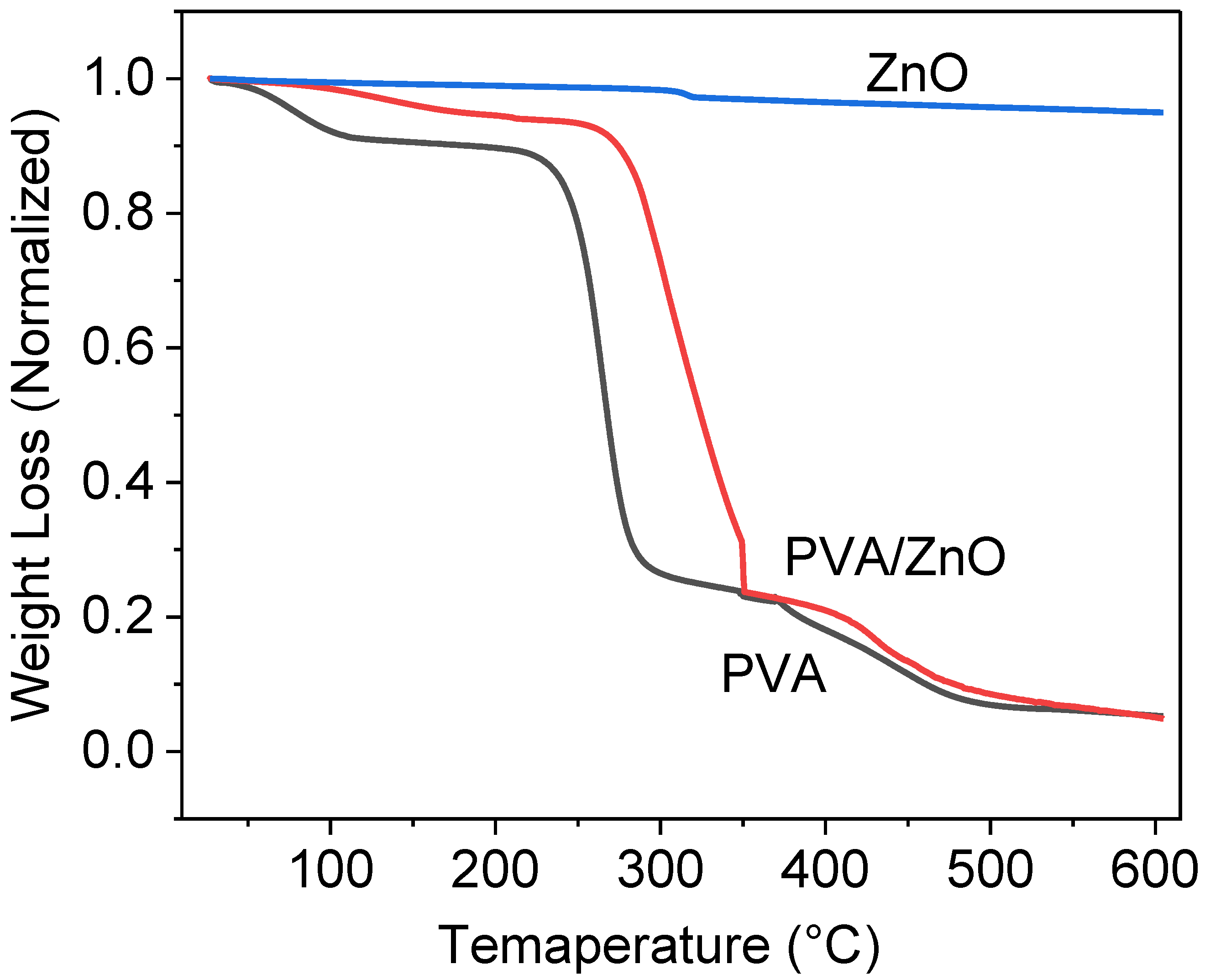
3.4. Thermal Stability of Produced Nanocomposites
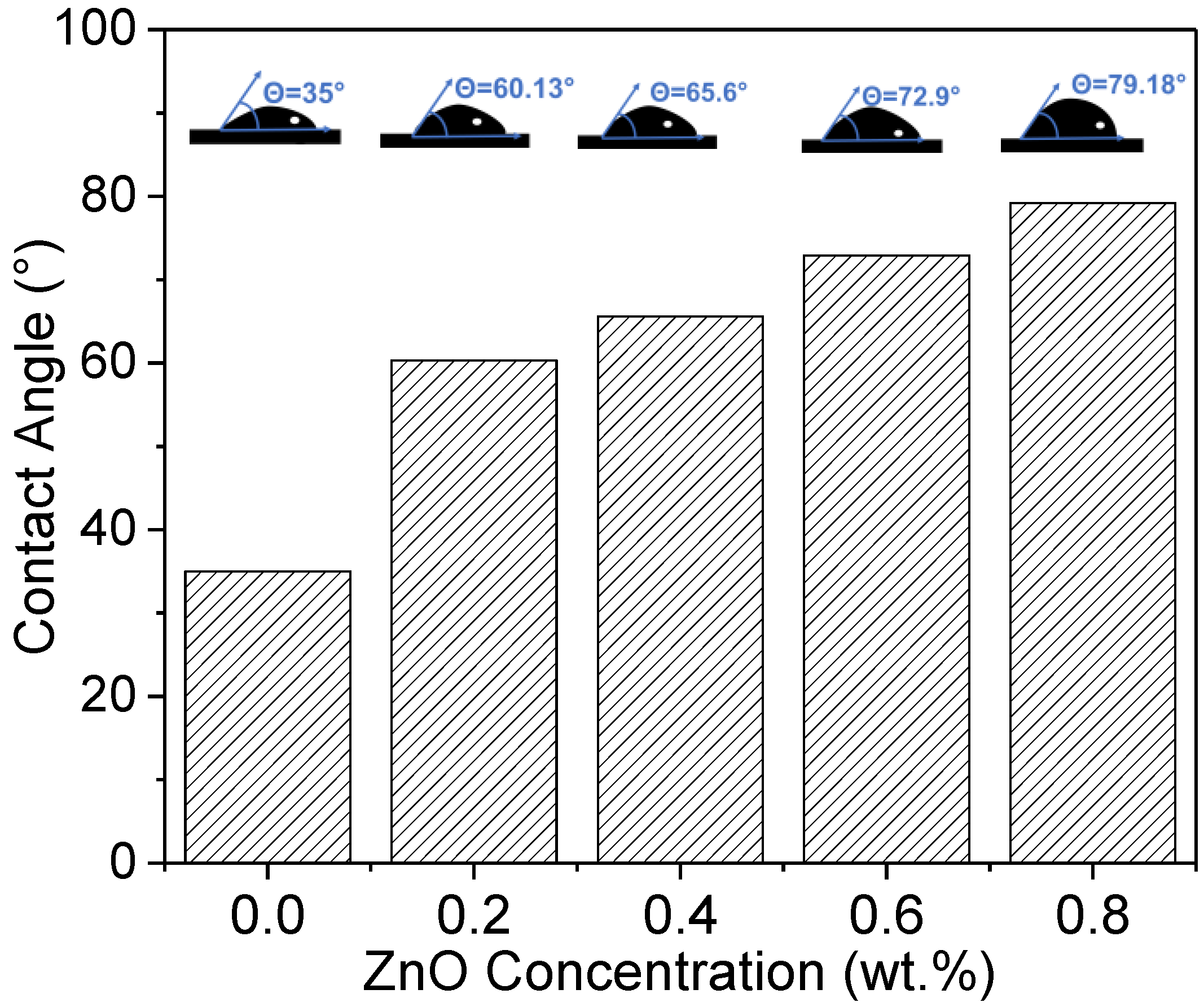
3.5. Water Contact Angle
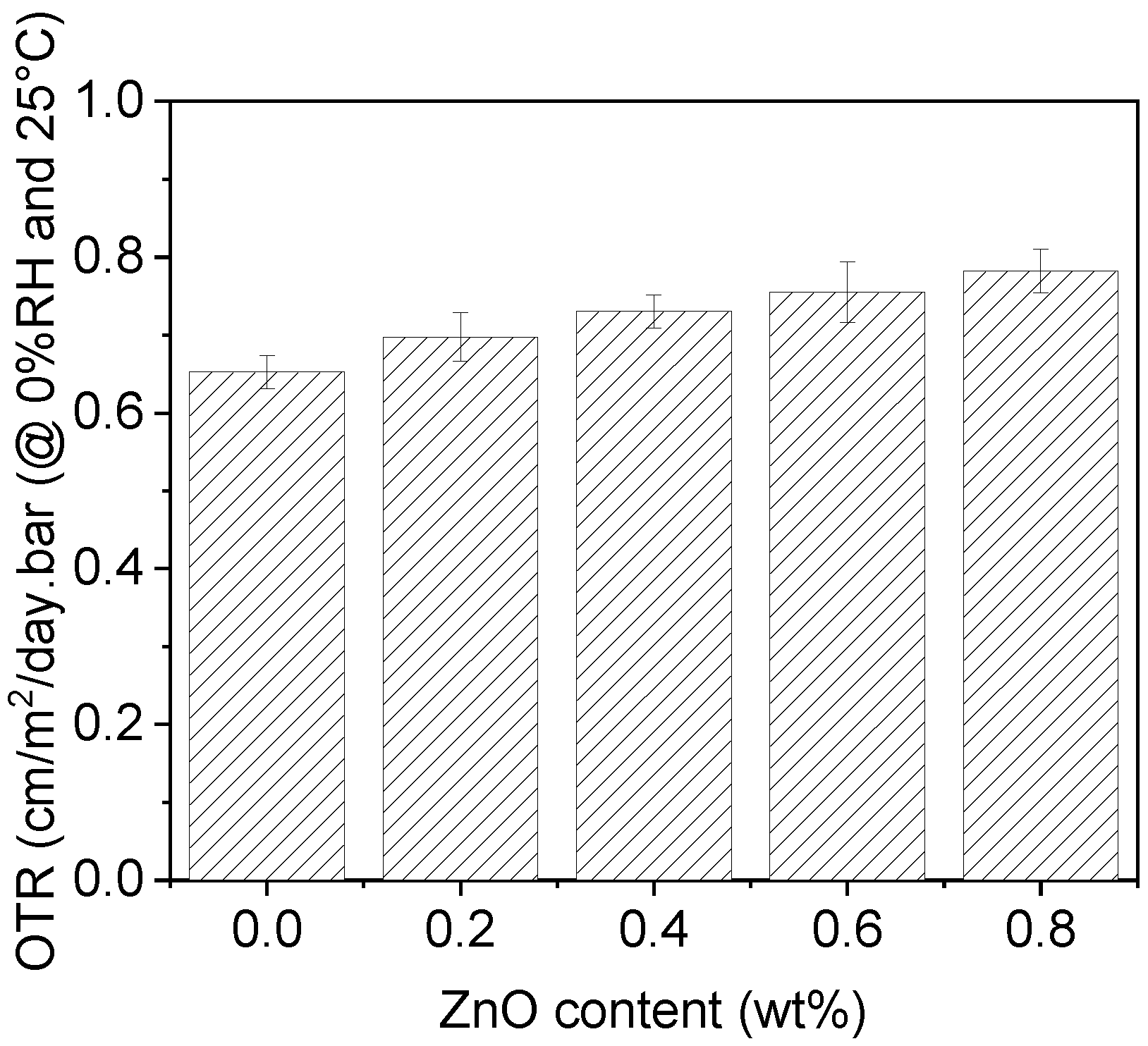
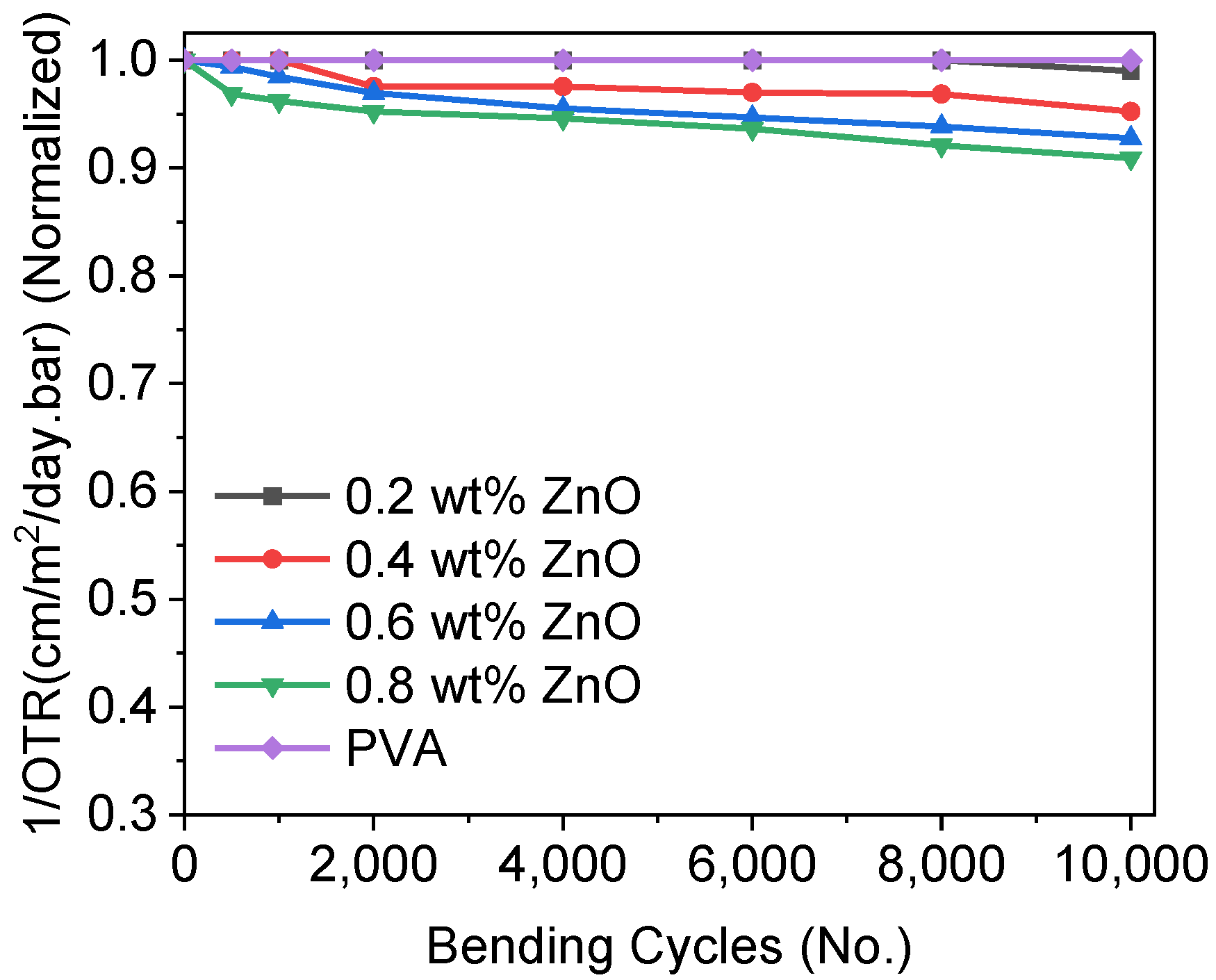
3.6. Oxygen Transmission Rate (OTR)
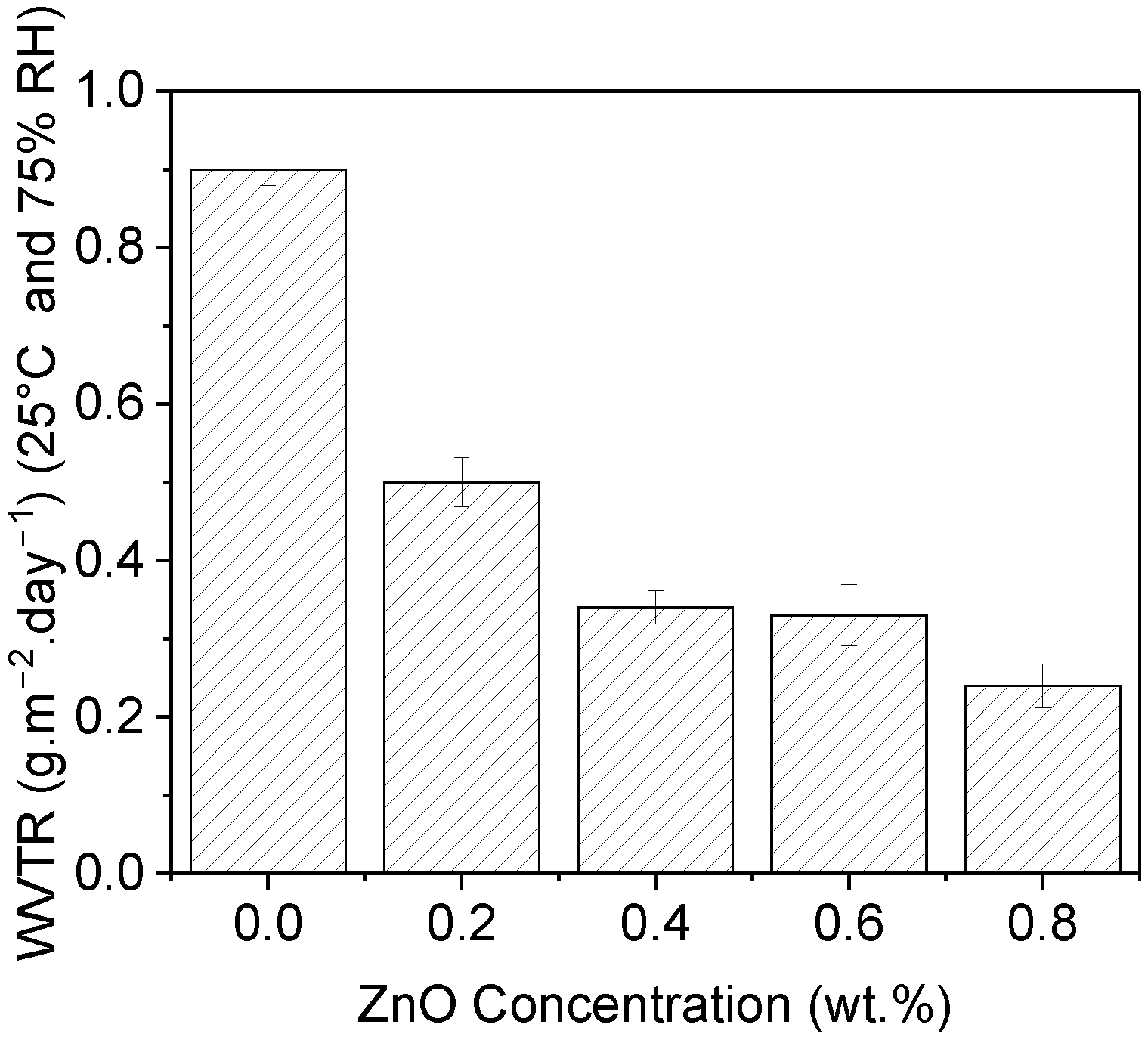
3.7. Water Vapor Transmission Rate (WVTR)
3.8. Bendability
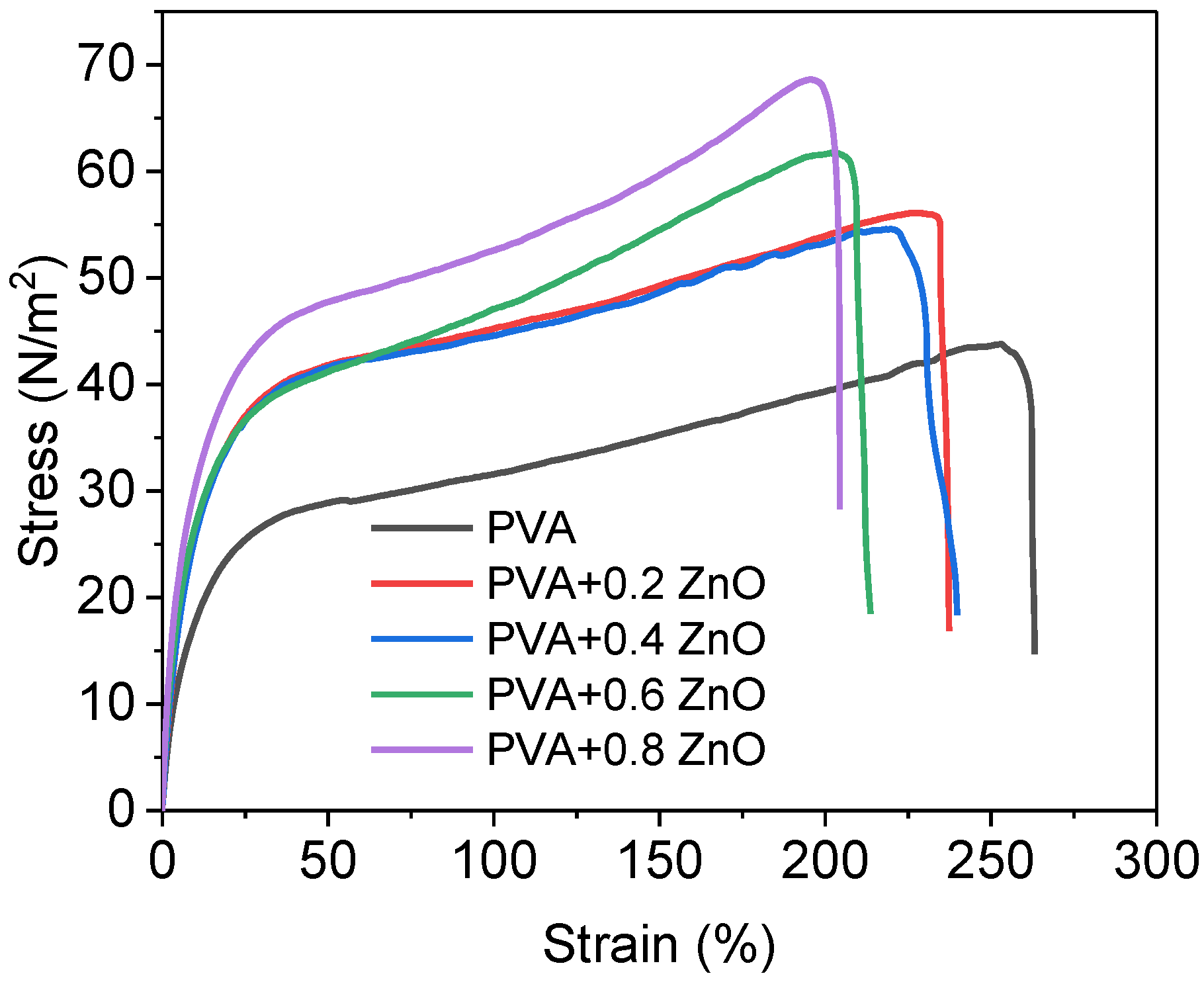
3.9. Tensile Strength
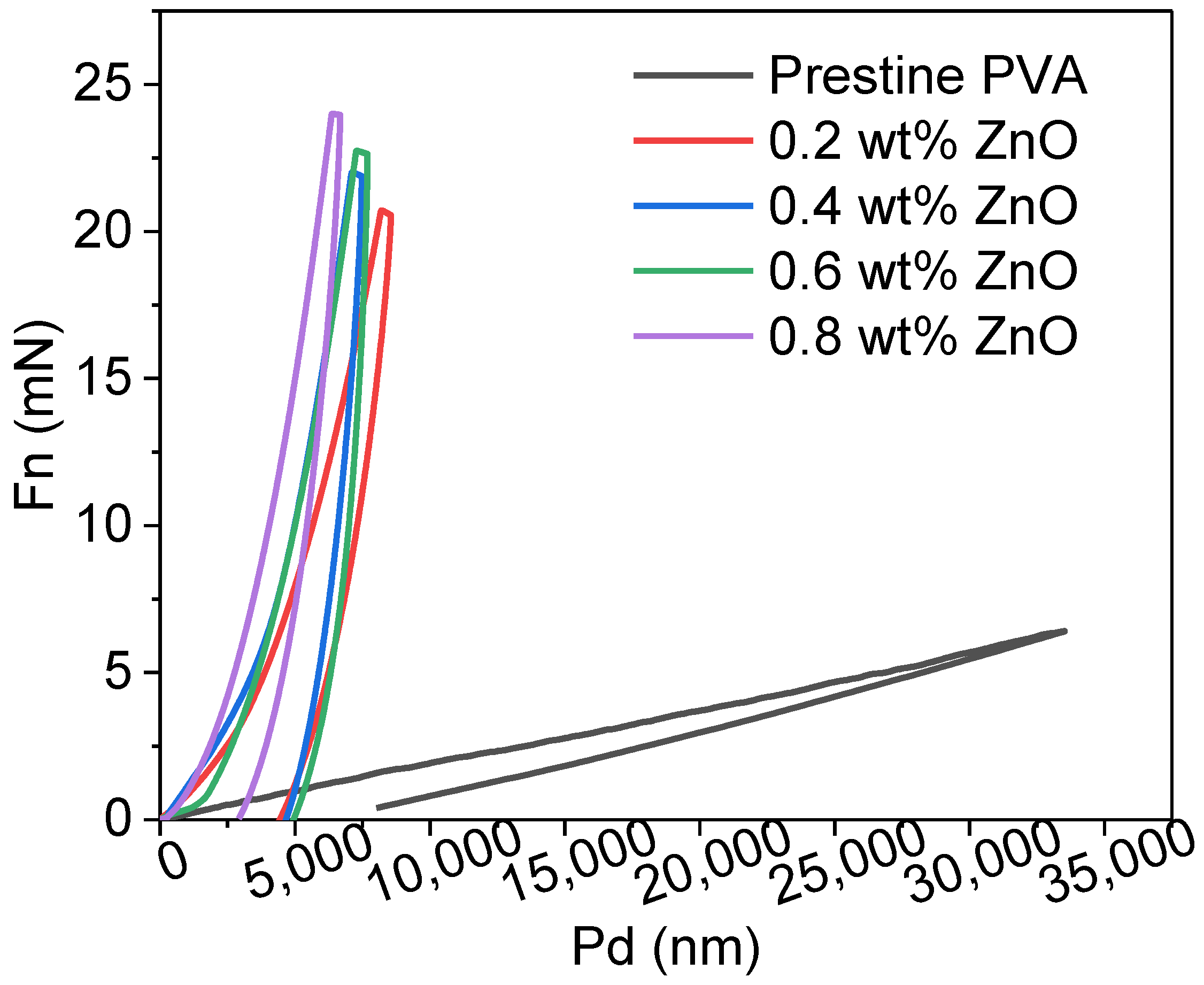
3.10. Hardness of the Films
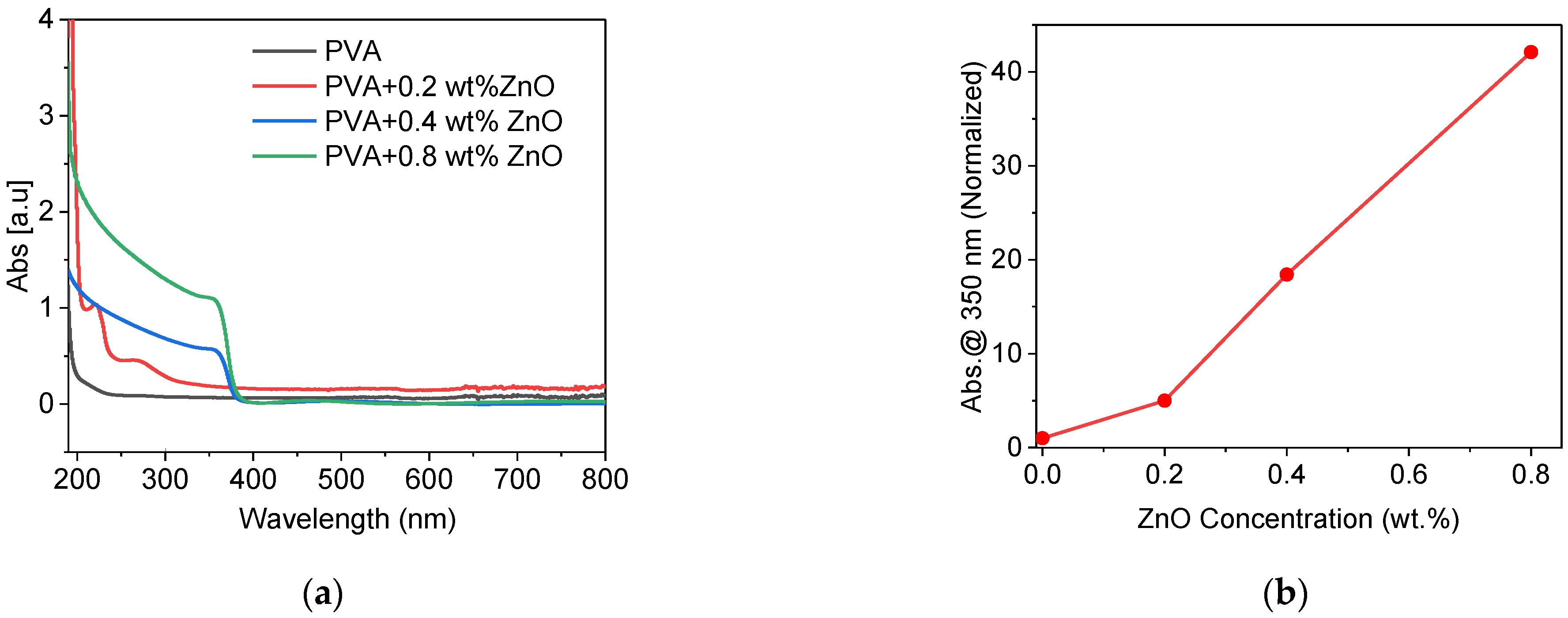
3.11. UV Blocking Effect
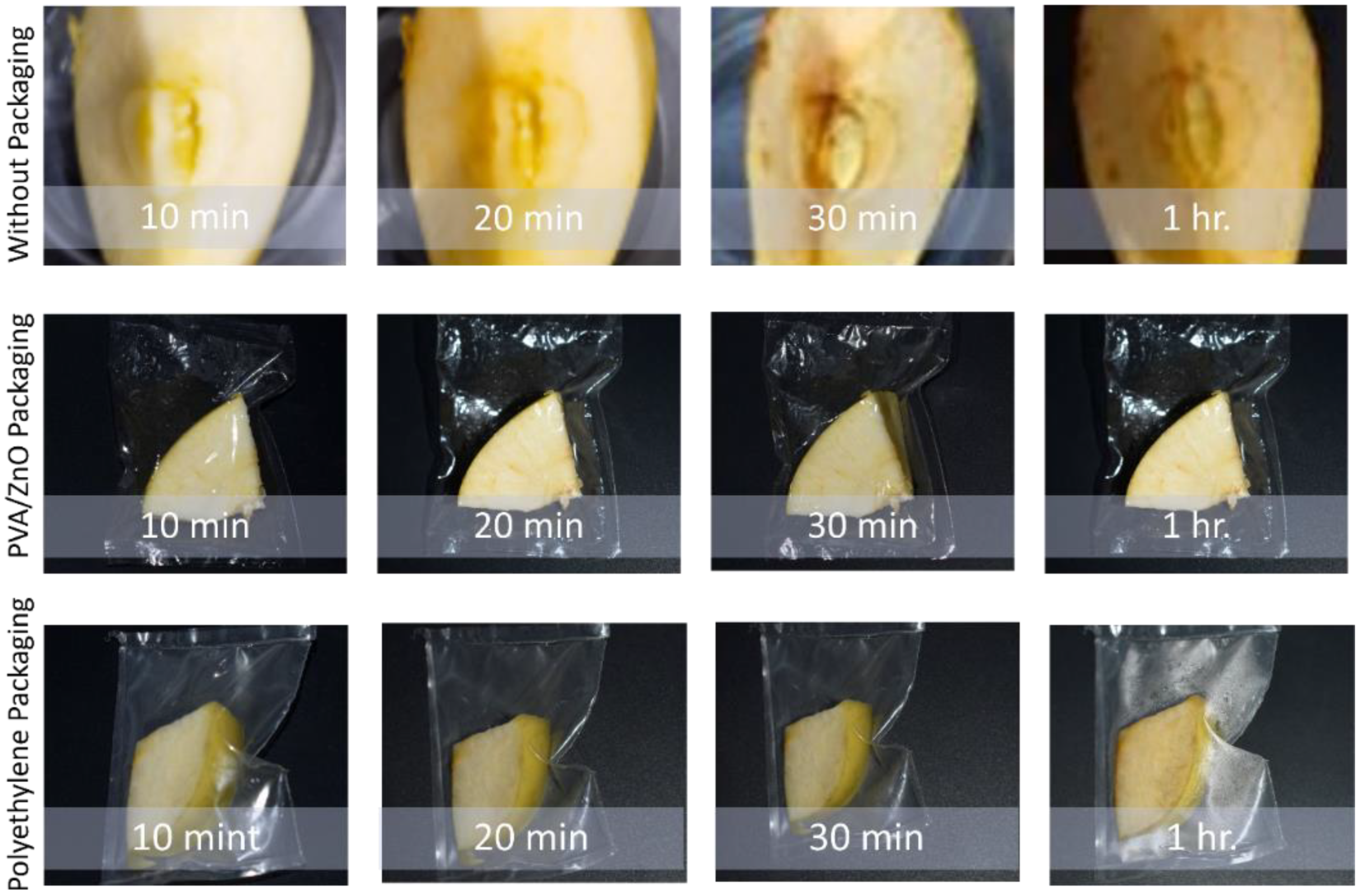
3.12. Degradation Test against Oxidation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krehula, L.K.; Papić, A.; Krehula, S.; Gilja, V.; Foglar, L.; Hrnjak-Murgić, Z. Properties of UV protective films of poly(vinyl-chloride)/TiO2 nanocomposites for food packaging. Polym. Bull. 2017, 74, 1387–1404. [Google Scholar] [CrossRef]
- Kwon, S.; Orsuwan, A.; Bumbudsanpharoke, N.; Yoon, C.; Choi, J.; Ko, S. A Short Review of Light Barrier Materials for Food and Beverage Packaging. Korean J. Packag. Sci. Technol. 2018, 24, 141–148. [Google Scholar] [CrossRef]
- Ashfaq, J.; Channa, I.A.; Shaikh, A.A.; Chandio, A.D.; Shah, A.A.; Bughio, B.; Birmahani, A.; Alshehri, S.; Ghoneim, M.M. Gelatin- and Papaya-Based Biodegradable and Edible Packaging Films to Counter Plastic Waste Generation. Materials 2022, 15, 1046. [Google Scholar] [CrossRef] [PubMed]
- Geueke, B.; Groh, K.; Muncke, J. Food packaging in the circular economy: Overview of chemical safety aspects for commonly used materials. J. Clean. Prod. 2018, 193, 491–505. [Google Scholar] [CrossRef]
- Janani, N.; Zare, E.N.; Salimi, F.; Makvandi, P. Antibacterial tragacanth gum-based nanocomposite films carrying ascorbic acid antioxidant for bioactive food packaging. Carbohydr. Polym. 2020, 247, 116678. [Google Scholar] [CrossRef]
- Channa, I.A.; Chandio, A.D.; Rizwan, M.; Shah, A.A.; Bhatti, J.; Shah, A.K.; Hussain, F.; Shahr, M.A.; AlHazaa, A. Solution Processed PVB / Mica Flake Coatings for the Encapsulation of Organic Solar Cells. Materials 2021, 14, 2496. [Google Scholar] [CrossRef]
- Islamipour, Z.; Zare, E.N.; Salimi, F.; Ghomi, M.; Makvandi, P. Biodegradable antibacterial and antioxidant nanocomposite films based on dextrin for bioactive food packaging. J. Nanostruct. Chem. 2022, 1–16. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [Green Version]
- Llorens, A.; Lloret, E.; Picouet, P.A.; Trbojevich, R.; Fernandez, A. Metallic-based micro and nanocomposites in food contact materials and active food packaging. Trends Food Sci. Technol. 2012, 24, 19–29. [Google Scholar] [CrossRef]
- Satoh, K. Poly(vinyl alcohol) (PVA) BT—Encyclopedia of Polymeric Nanomaterials; Kobayashi, S., Müllen, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–6. [Google Scholar]
- Dejen, K.D.; Zereffa, E.A.; Murthy, H.C.A.; Merga, A. Synthesis of ZnO and ZnO/PVA nanocomposite using aqueous Moringa Oleifeira leaf extract template: Antibacterial and electrochemical activities. Rev. Adv. Mater. Sci. 2020, 59, 464–476. [Google Scholar] [CrossRef]
- Channa, I.A.; Distler, A.; Egelhaaf, H.; Brabec, C.J. Solution Coated Barriers for Flexible Electronics. In Organic Flexible Electronics, Fundamentals, Devices, and Applications; Cosseddu, P., Caironi, M., Eds.; Woodhead Publishing: Cambridge, UK, 2020; ISBN 9780128188903. [Google Scholar]
- Chandio, A.D.; Channa, I.A.; Rizwan, M.; Akram, S.; Javed, M.S.; Siyal, S.H.; Saleem, M.; Makhdoom, A.; Ashfaq, T.; Khan, S.; et al. Polyvinyl alcohol and nano-clay based solution processed packaging coatings. Coatings 2021, 11, 942. [Google Scholar] [CrossRef]
- Channa, I.A.; Distler, A.; Zaiser, M.; Brabec, C.J.; Egelhaaf, H. Thin Film Encapsulation of Organic Solar Cells by Direct Deposition of Polysilazanes from Solution. Adv. Energy Mater. 2019, 9, 1900598. [Google Scholar] [CrossRef]
- Kim, J.; Kang, T.; Kim, H.; Shin, H.J.; Oh, S.-G. Preparation of PVA/PAA nanofibers containing thiol-modified silica particles by electrospinning as an eco-friendly Cu (II) adsorbent. J. Ind. Eng. Chem. 2019, 77, 273–279. [Google Scholar] [CrossRef]
- Channa, I.A. Development of Solution Processed Thin Film Barriers for Encapsulating Thin Film Electronics Entwicklung von lösungsprozessierten Dünnschichtbarrieren für die Verpackung von Dünnschichtelektronik; Friedrich Alexander University of Erlangen Nuremberg: Erlangen, Germany, 2019. [Google Scholar]
- Channa, I.A.; Distler, A.; Scharfe, B.; Feroze, S.; Forberich, K.; Lipovšek, B.; Brabec, C.J.; Egelhaaf, H.-J. Solution processed oxygen and moisture barrier based on glass flakes for encapsulation of organic (opto-) electronic devices. Flex. Print. Electron. 2021, 6, 025006. [Google Scholar] [CrossRef]
- Barman, A.; De, A.; Das, M. Stabilization and Dispersion of ZnO Nanoparticles in PVA Matrix. J. Inorg. Organomet. Polym. Mater. 2019, 30, 2248–2257. [Google Scholar] [CrossRef]
- Ujjan, Z.A.; Bhatti, M.A.; Shah, A.A.; Tahira, A.; Shaikh, N.M.; Kumar, S.; Mugheri, A.Q.; Medany, S.S.; Nafady, A.; Alnjiman, F. Simultaneous doping of sulfur and chloride ions into ZnO nanorods for improved photocatalytic properties towards degradation of methylene blue. Ceram. Int. 2021, 48, 5535–5545. [Google Scholar] [CrossRef]
- Cole, C.; Shyr, T.; Ou-Yang, H. Metal oxide sunscreens protect skin by absorption, not by reflection or scattering. Photodermatol. Photoimmunol. Photomed. 2016, 32, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Smijs, T.G.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95–112. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.A.; Bhatti, M.A.; Tahira, A.; Chandio, A.; Channa, I.A.; Sahito, A.G.; Chalangar, E.; Willander, M.; Nur, O.; Ibupoto, Z.H. Facile synthesis of copper doped ZnO nanorods for the efficient photo degradation of methylene blue and methyl orange. Ceram. Int. 2019, 46, 9997–10005. [Google Scholar] [CrossRef]
- Sawai, J. Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. J. Microbiol. Methods 2003, 54, 177–182. [Google Scholar] [CrossRef]
- Verbič, A.; Gorjanc, M.; Simončič, B. Zinc Oxide for Functional Textile Coatings: Recent Advances. Coatings 2019, 9, 550. [Google Scholar] [CrossRef] [Green Version]
- Channa, I.A.; Shah, A.A.; Rizwan, M.; Makhdoom, M.A.; Chandio, A.D.; Shar, M.A.; Mahmood, A. Process Parameter Optimization of a Polymer Derived Ceramic Coatings for Producing Ultra-High Gas Barrier. Materials 2021, 14, 7000. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.; Hassan, S.E.-D.; Salem, S.S.; Shaheen, T.I. In-Vitro cytotoxicity, antibacterial, and UV protection properties of the biosynthesized Zinc oxide nanoparticles for medical textile applications. Microb. Pathog. 2018, 125, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, M.A.; Shah, A.A.; Almani, K.F.; Tahira, A.; Chalangar, S.E.; Chandio, A.D.; Nur, O.; Willander, M.; Ibupoto, Z.H. Efficient photo catalysts based on silver doped ZnO nanorods for the photo degradation of methyl orange. Ceram. Int. 2019, 45, 23289–23297. [Google Scholar] [CrossRef]
- Chandio, A.D.; Saleem, M.; Khan, H.R.; Hyder, I.N.; Ali, M. Modified Zinc Oxide Nanoparticles for Corrosion Resistance Applications. J. Chem. Soc. Pak. 2020, 42, 705. [Google Scholar]
- Alrajhi, A.; Ahmed, N.M.; Al Shafouri, M.; Almessiere, M.A.; Al-Ghamdi, A.A.M. Green synthesis of zinc oxide nanoparticles using salvia officials extract. Mater. Sci. Semicond. Process. 2021, 125, 105641. [Google Scholar] [CrossRef]
- Khoirunnisa, A.R.; Joni, I.M.; Panatarani, C.; Rochima, E.; Praseptiangga, D. UV-screening, transparency and water barrier properties of semi refined iota carrageenan packaging film incorporated with ZnO nanoparticles. AIP Conf. Proc. 2018, 1927, 030041. [Google Scholar] [CrossRef]
- Elgharyani, N. Oxide Polyvinylalcohol/Zinc Properties of Mechanical Some Nanoparticles as Thin Films and Solutions. J. Univ. Anbar Pure Sci. 2021, 15, 87–93. [Google Scholar] [CrossRef]
- Al-Karam, L.Q.; Majeed, S.M. Preparation and Study Characterize of ZnO-PVA Nano Composite and Its Preparation and Study to Characterize the ZnO-PVA Nano Composite and Its Antibacterial Effects. Biochem. Cell Arch. 2019, 19, 3641–3646. [Google Scholar]
- Shamhari, N.M.; Wee, B.S.; Chin, S.F.; Kok, K.Y. Synthesis and Characterization of Zinc Oxide Nanoparticles with Small Particle Size Distribution. Acta Chim. Slov. 2018, 65, 578–585. [Google Scholar] [CrossRef]
- Handore, K.; Bhavsar, S.; Horne, A.; Chhattise, P.; Mohite, K.; Ambekar, J.; Pande, N.; Chabukswar, V. Novel Green Route of Synthesis of ZnO Nanoparticles by Using Natural Biodegradable Polymer and Its Application as a Catalyst for Oxidation of Aldehydes. J. Macromol. Sci. Part A Pure Appl. Chem. 2014, 51, 941–947. [Google Scholar] [CrossRef]
- Jayarambabu, Y.T.; Kumari, N.; Rao, B.S.; Prabhu, K.V. Germination and growth characteristics of mungbean seeds (Vigna radiata L.) affected by synthesized zinc oxide nanoparticles. International Journal of Current Engineering and Technology. Int. J. Curr. Eng. Technol. 2014, 4, 2347–5161. [Google Scholar]
- Sharma, V.; Banait, J.S.; LaRock, R.C.; Kundu, P.P. Synthesis and characterization of styrene-co-divinylbenzene-graft-linseed oil by free radical polymerization. Express Polym. Lett. 2008, 2, 265–276. [Google Scholar] [CrossRef]
- Wen, Y.-H.; Tsou, C.-H.; de Guzman, M.R.; Huang, D.; Yu, Y.-Q.; Gao, C.; Zhang, X.-M.; Du, J.; Zheng, Y.-T.; Zhu, H.; et al. Antibacterial nanocomposite films of poly (vinyl alcohol) modified with zinc oxide-doped multiwalled carbon nanotubes as food packaging. Polym. Bull. 2022, 79, 3847–3866. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Channa, I.A.; Ashfaq, J.; Gilani, S.J.; Shah, A.A.; Chandio, A.D.; Jumah, M.N.b. UV Blocking and Oxygen Barrier Coatings Based on Polyvinyl Alcohol and Zinc Oxide Nanoparticles for Packaging Applications. Coatings 2022, 12, 897. https://doi.org/10.3390/coatings12070897
Channa IA, Ashfaq J, Gilani SJ, Shah AA, Chandio AD, Jumah MNb. UV Blocking and Oxygen Barrier Coatings Based on Polyvinyl Alcohol and Zinc Oxide Nanoparticles for Packaging Applications. Coatings. 2022; 12(7):897. https://doi.org/10.3390/coatings12070897
Chicago/Turabian StyleChanna, Iftikhar Ahmed, Jaweria Ashfaq, Sadaf Jamal Gilani, Aqeel Ahmed Shah, Ali Dad Chandio, and May Nasser bin Jumah. 2022. "UV Blocking and Oxygen Barrier Coatings Based on Polyvinyl Alcohol and Zinc Oxide Nanoparticles for Packaging Applications" Coatings 12, no. 7: 897. https://doi.org/10.3390/coatings12070897
APA StyleChanna, I. A., Ashfaq, J., Gilani, S. J., Shah, A. A., Chandio, A. D., & Jumah, M. N. b. (2022). UV Blocking and Oxygen Barrier Coatings Based on Polyvinyl Alcohol and Zinc Oxide Nanoparticles for Packaging Applications. Coatings, 12(7), 897. https://doi.org/10.3390/coatings12070897






