Synthesis of Biomolecule Functionalized Biocompatible Silver Nanoparticles for Antioxidant and Antibacterial Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Silver Nanoparticle
2.3. Characterization of Silver Nanoparticles
2.4. Biological Application
2.4.1. In Vitro Cytotoxicity
2.4.2. Antibacterial Assay
Well Diffusion Method
Minimum Inhibitory Concentration (MIC)
2.4.3. Antioxidant Assay
2.4.4. Hemolysis Assay
2.4.5. CAM Assay
2.5. Statistical Analysis
3. Results and Discussion
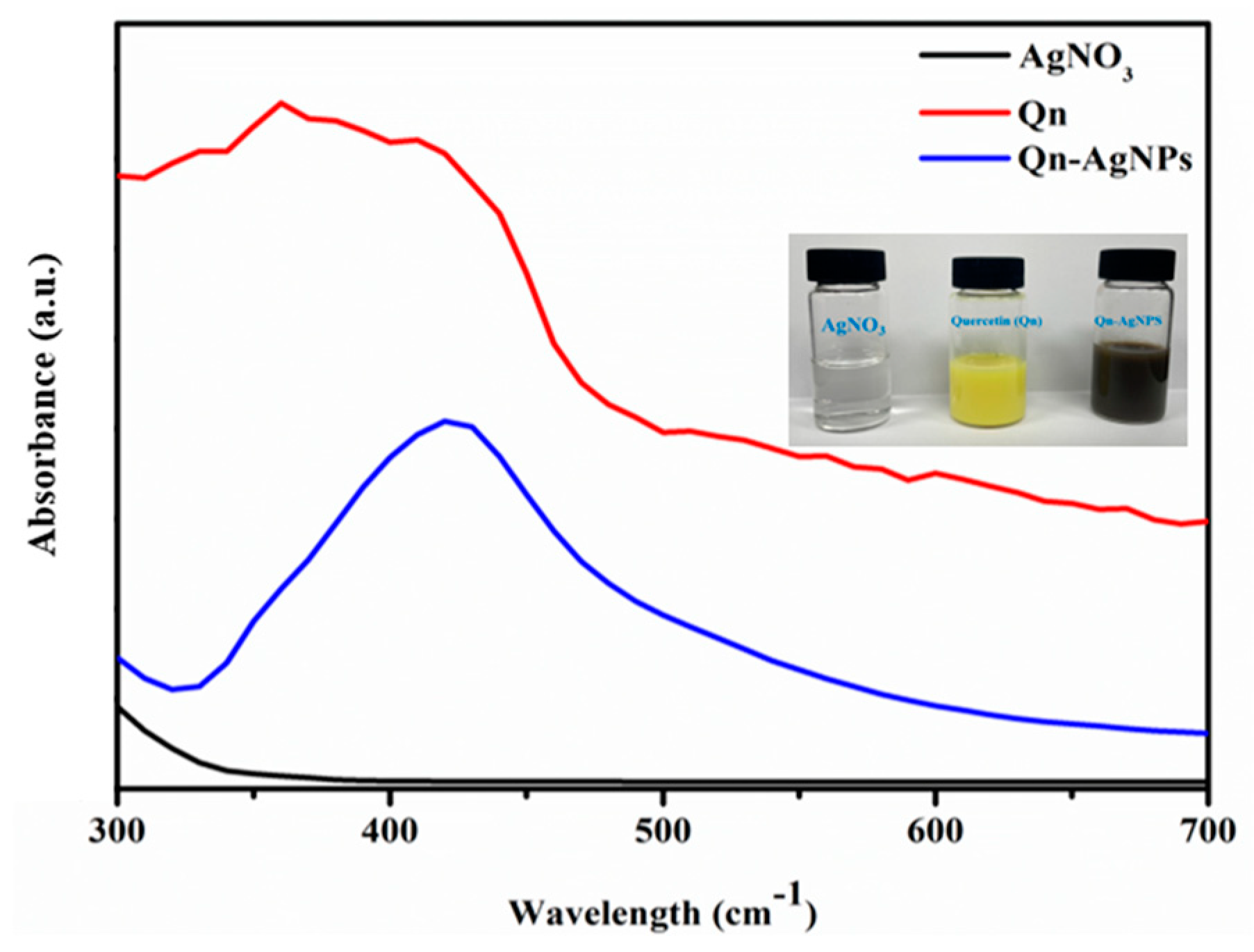
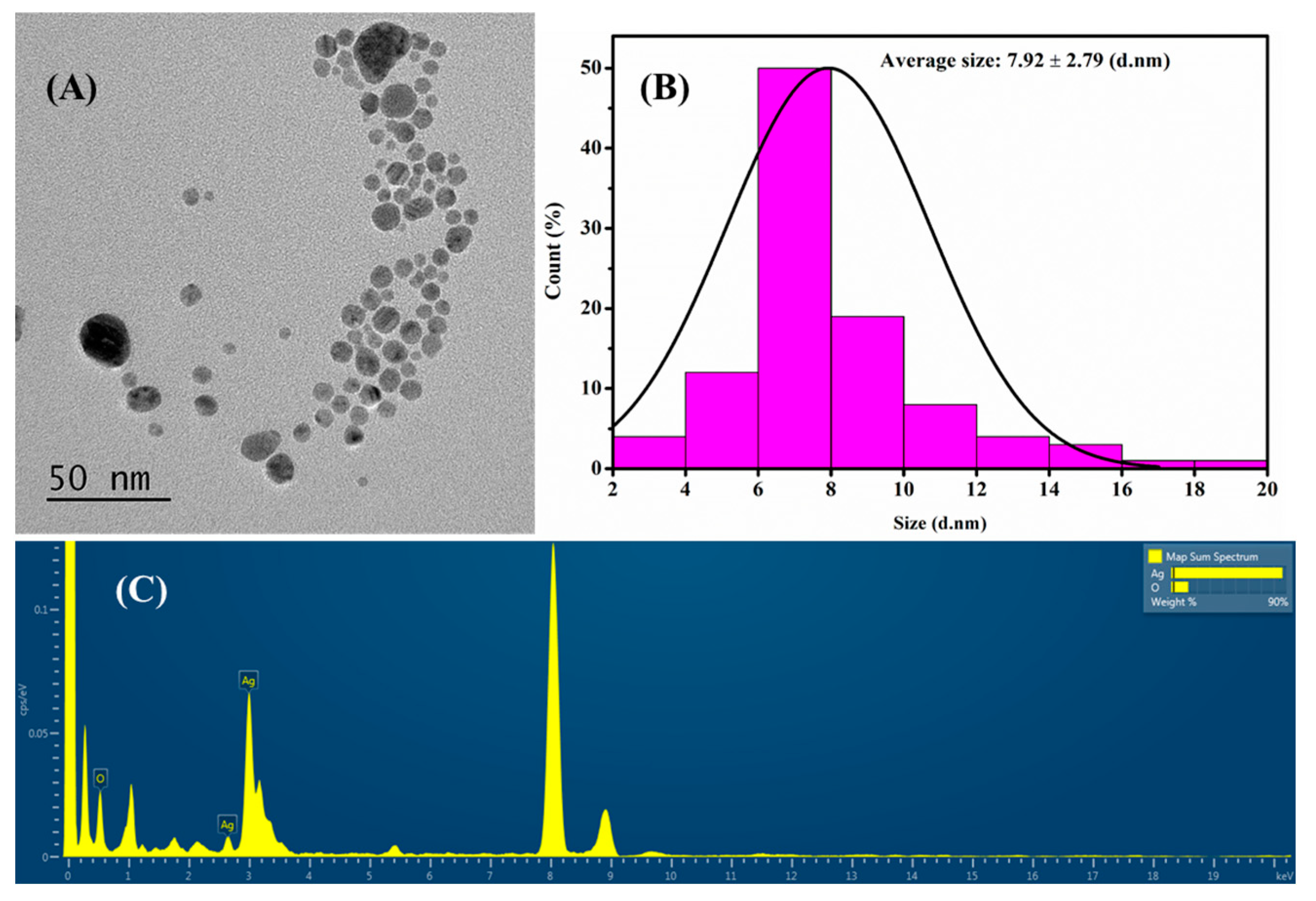
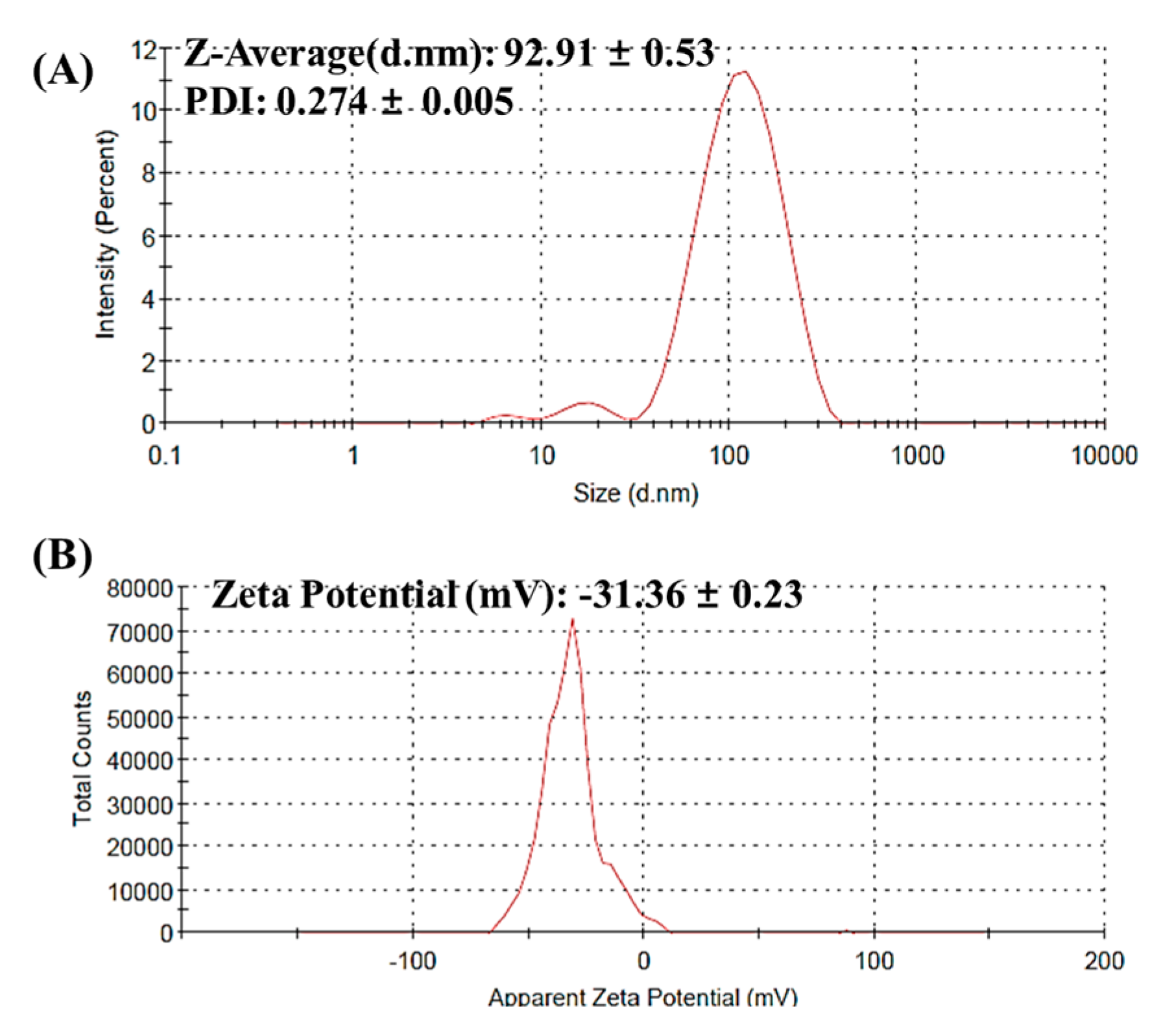
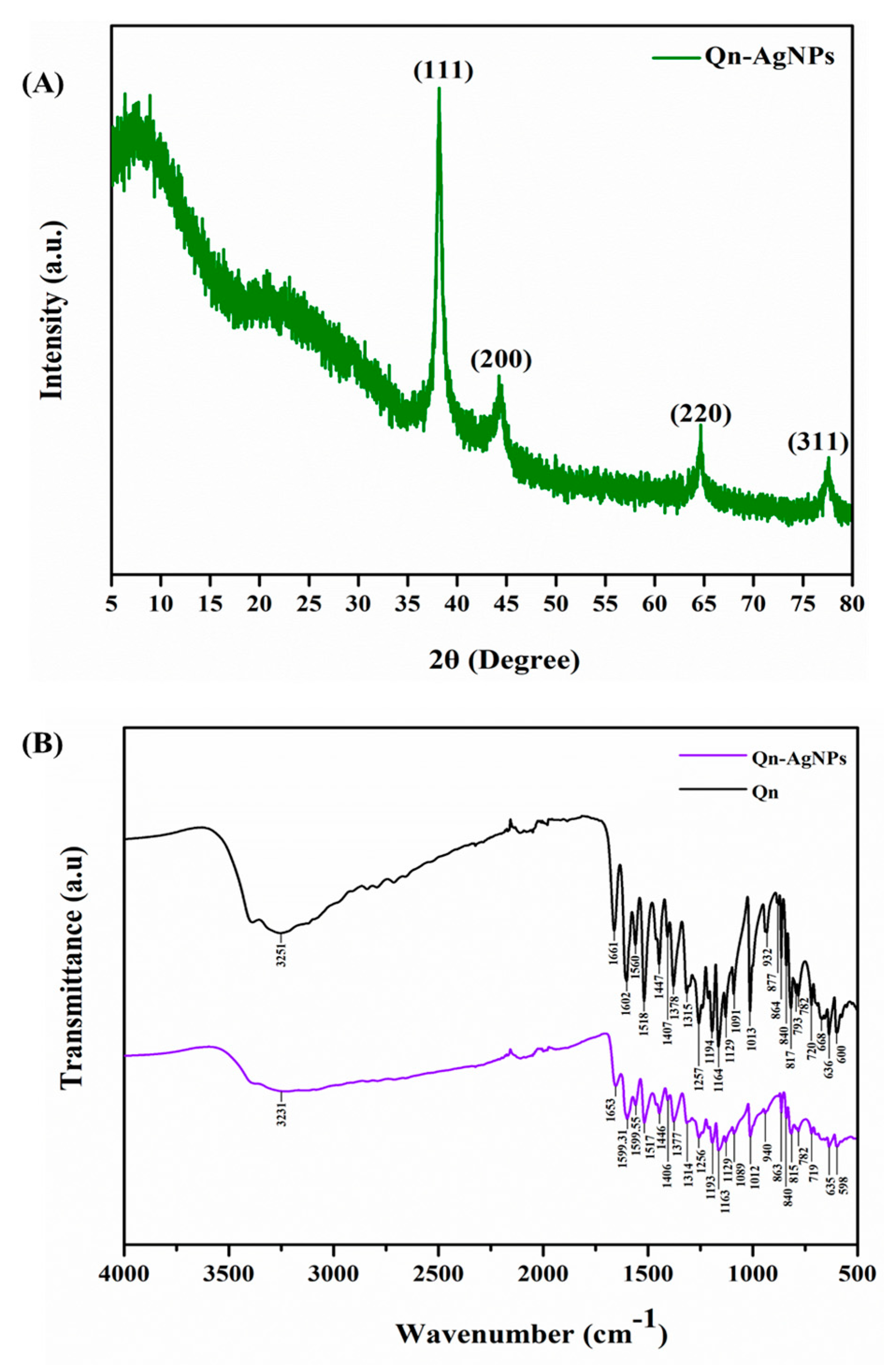
3.1. Synthesis and Characterization AgNPs
3.2. Biological Assays
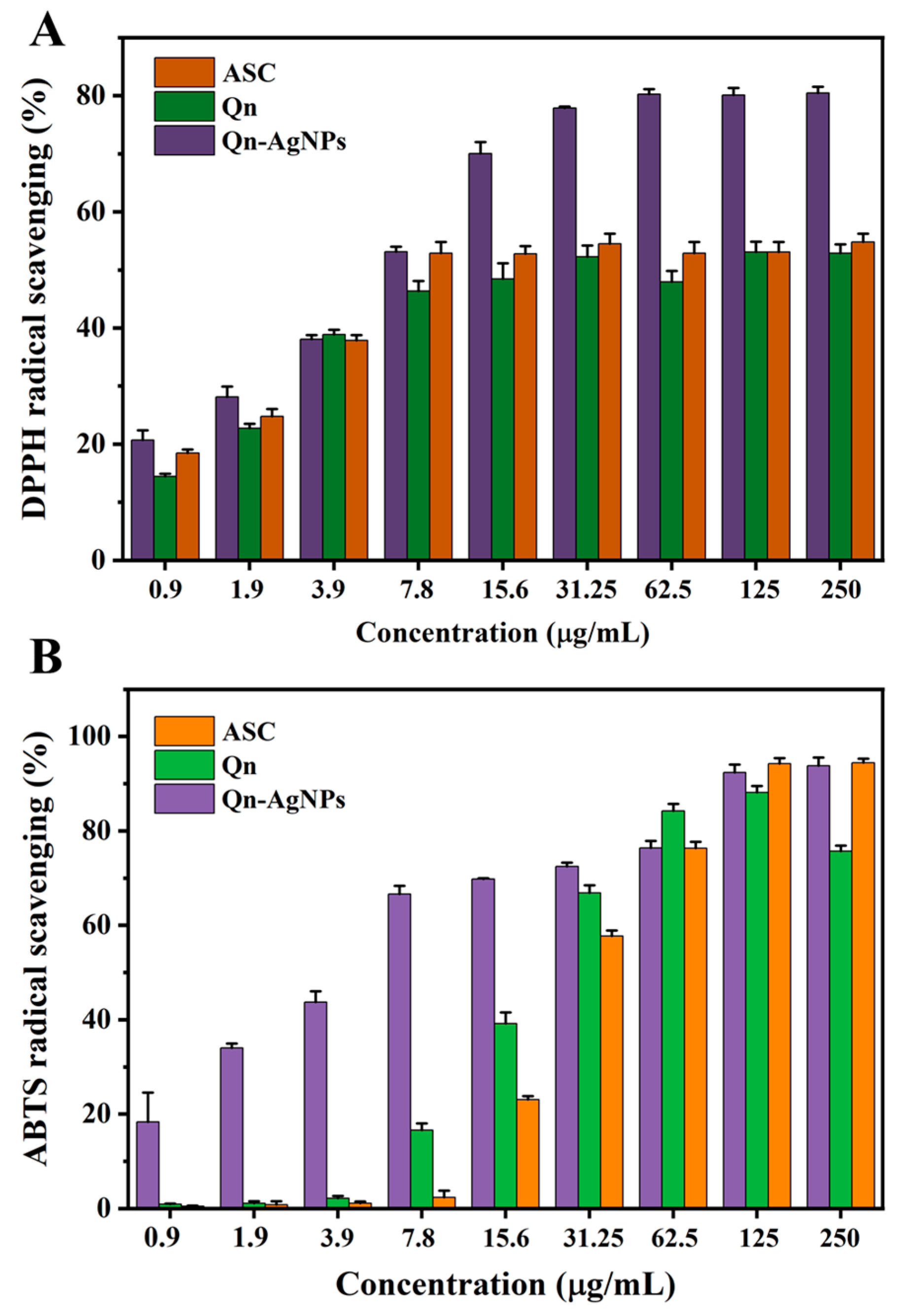
3.2.1. Antioxidant Assay
3.2.2. Antibacterial Assay
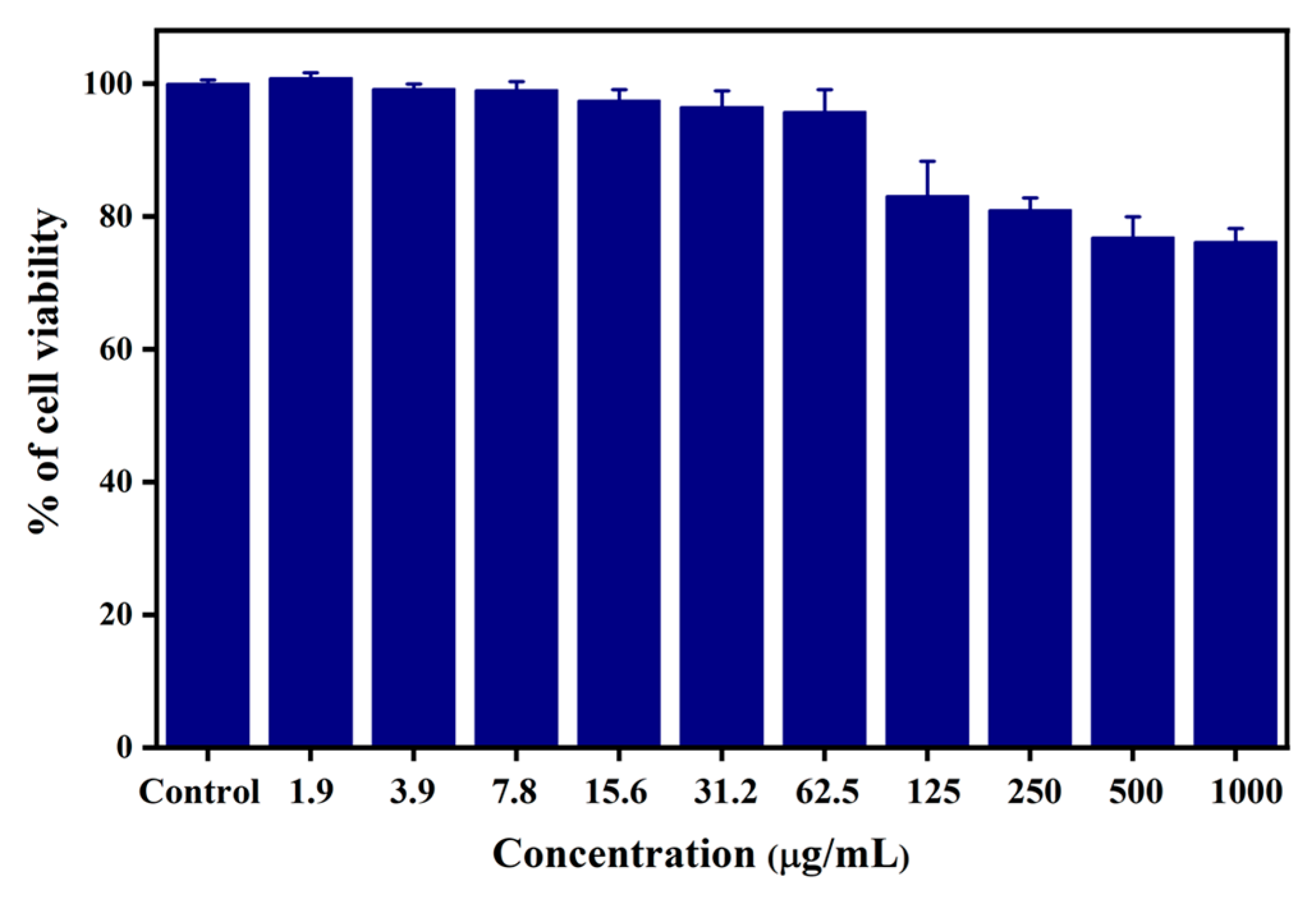
3.2.3. In Vitro Cytotoxicity
3.2.4. Hemolysis Assay
3.2.5. CAM Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Payal; Pandey, P. Role of Nanotechnology in Electronics: A Review of Recent Developments and Patents. Recent Pat. Nanotechnol. 2022, 16, 45–66. [Google Scholar] [CrossRef]
- Ikumapayi, O.M.; Akinlabi, E.T.; Adeoye, A.O.M.; Fatoba, S.O. Microfabrication and Nanotechnology in Manufacturing System–An Overview. Mater. Today Proc. 2021, 44, 1154–1162. [Google Scholar] [CrossRef]
- Ashfaq, A.; Khursheed, N.; Fatima, S.; Anjum, Z.; Younis, K. Application of Nanotechnology in Food Packaging: Pros and Cons. J. Agric. Food Res. 2022, 7, 100270. [Google Scholar] [CrossRef]
- Alghamdi, M.A.; Fallica, A.N.; Virzì, N.; Kesharwani, P.; Pittalà, V.; Greish, K. The Promise of Nanotechnology in Personalized Medicine. J. Pers. Med. 2022, 12, 673. [Google Scholar] [CrossRef]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A.K. Antimicrobial Activity of Metal and Metal-Oxide Based Nanoparticles. Adv. Ther. 2018, 1, 1700033. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal Nanoparticles Synthesis: An Overview on Methods of Preparation, Advantages and Disadvantages, and Applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Marei, I.; Crovella, S.; Abou-Saleh, H. Recent Developments in Nanomaterials-Based Drug Delivery and Upgrading Treatment of Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 1404. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Manoharan, M. Research on the Frontiers of Materials Science: The Impact of Nanotechnology on New Material Development. Technol. Soc. 2008, 30, 401–404. [Google Scholar] [CrossRef]
- Barkalina, N.; Charalambous, C.; Jones, C.; Coward, K. Nanotechnology in Reproductive Medicine: Emerging Applications of Nanomaterials. Nanomed. Nanotechnol. Biol. Med. 2014, 10, e921–e938. [Google Scholar] [CrossRef]
- Jha, D.; Thiruveedula, P.K.; Pathak, R.; Kumar, B.; Gautam, H.K.; Agnihotri, S.; Sharma, A.K.; Kumar, P. Multifunctional Biosynthesized Silver Nanoparticles Exhibiting Excellent Antimicrobial Potential against Multi-Drug Resistant Microbes along with Remarkable Anticancerous Properties. Mater. Sci. Eng. C 2017, 80, 659–669. [Google Scholar] [CrossRef]
- Prosposito, P.; Burratti, L.; Venditti, I. Silver Nanoparticles as Colorimetric Sensors for Water Pollutants. Chemosensors 2020, 8, 26. [Google Scholar] [CrossRef]
- Zayed, D.G.; Ebrahim, S.M.; Helmy, M.W.; Khattab, S.N.; Bahey-El-Din, M.; Fang, J.Y.; Elkhodairy, K.A.; Elzoghby, A.O. Combining Hydrophilic Chemotherapy and Hydrophobic Phytotherapy via Tumor-Targeted Albumin-QDs Nano-Hybrids: Covalent Coupling and Phospholipid Complexation Approaches. J. Nanobiotechnol. 2019, 17, 1–19. [Google Scholar] [CrossRef]
- Egger, S.; Lehmann, R.P.; Height, M.J.; Loessner, M.J.; Schuppler, M. Antimicrobial Properties of a Novel Silver-Silica Nanocomposite Material. Appl. Environ. Microbiol. 2009, 75, 2973–2976. [Google Scholar] [CrossRef]
- Yaqoob, S.B.; Adnan, R.; Rameez Khan, R.M.; Rashid, M. Gold, Silver, and Palladium Nanoparticles: A Chemical Tool for Biomedical Applications. Front. Chem. 2020, 8, 376. [Google Scholar] [CrossRef]
- Sakamoto, J.H.; van de Ven, A.L.; Godin, B.; Blanco, E.; Serda, R.E.; Grattoni, A.; Ziemys, A.; Bouamrani, A.; Hu, T.; Ranganathan, S.I.; et al. Enabling Individualized Therapy through Nanotechnology. Pharmacol. Res. 2010, 62, 57–89. [Google Scholar] [CrossRef]
- Nam, N.H.; Luong, N.H. Nanoparticles: Synthesis and applications. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 211–240. [Google Scholar] [CrossRef]
- Anbazhagan, S.; Azeez, S.; Morukattu, G.; Rajan, R.; Venkatesan, K.; Thangavelu, K.P. Synthesis, Characterization and Biological Applications of Mycosynthesized Silver Nanoparticles. Biotech 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Duan, H.; Wang, D.; Li, Y. Green Chemistry for Nanoparticle Synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef]
- Rónavári, A.; Igaz, N.; Adamecz, D.I.; Szerencsés, B.; Molnar, C.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Green Silver and Gold Nanoparticles: Biological Synthesis Approaches and Potentials for Biomedical Applications. Molecules 2021, 26, 844. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Shajahan, A.; Kalaichelvan, P.; Kaviyarasan, V. Fungal Chitosan Based Nanocomposites Sponges—An Alternative Medicine for Wound Dressing. Int. J. Biol. Macromol. 2017, 104, 1905–1915. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Saravanakumar, K.; Manikandan, M.; Shajahan, A.; Mariadoss, A.V.A.; Wang, M.-H. Core-Shell Silver Nanoparticles: Synthesis, Characterization, and Applications. In Green Synthesis of Silver Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 75–97. [Google Scholar] [CrossRef]
- Diniz, F.R.; Maia, R.C.A.P.; Rannier, L.; Andrade, L.N.; Chaud, M.V.; da Silva, C.F.; Corrêa, C.B.; de Albuquerque Junior, R.L.C.; da Costa, L.P.; Shin, S.R.; et al. Silver Nanoparticles-Composing Alginate/Gelatine Hydrogel Improves Wound Healing in Vivo. Nanomaterials 2020, 10, 390. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef]
- Mittal, A.K.; Kaler, A.; Banerjee, U.C. Free Radical Scavenging and Antioxidant Activity of Silver Nanoparticles Synthesized from Flower Extract of Rhododendron Dauricum. Nano Biomed. Eng. 2012, 4, 118–124. [Google Scholar] [CrossRef]
- Das, P.; Dutta, T.; Manna, S.; Loganathan, S.; Basak, P. Facile Green Synthesis of Non-Genotoxic, Non-Hemolytic Organometallic Silver Nanoparticles Using Extract of Crushed, Wasted, and Spent Humulus Lupulus (Hops): Characterization, Anti-Bacterial, and Anti-Cancer Studies. Environ. Res. 2022, 204, 111962. [Google Scholar] [CrossRef]
- Bar, H.; Bhui, D.K.; Sahoo, G.P.; Sarkar, P.; Pyne, S.; Misra, A. Green Synthesis of Silver Nanoparticles Using Seed Extract of Jatropha Curcas. Colloids Surfaces A Physicochem. Eng. Asp. 2009, 348, 212–216. [Google Scholar] [CrossRef]
- Amini, S.M. Preparation of Antimicrobial Metallic Nanoparticles with Bioactive Compounds. Mater. Sci. Eng. C 2019, 103, 109809. [Google Scholar] [CrossRef]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” Nanotechnologies: Synthesis of Metal Nanoparticles Using Plants. Acta Naturae 2014, 6, 35. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Biosynthesis of Metallic Nanoparticles Using Plant Derivatives and Their New Avenues in Pharmacological Applications–An Updated Report. Saudi Pharm. J. 2016, 24, 473–484. [Google Scholar] [CrossRef]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84. [Google Scholar] [CrossRef]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Klllç, C.S.; Sytar, O.; et al. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef]
- Ansari, M.A.; Abdul, H.M.; Joshi, G.; Opii, W.O.; Butterfield, D.A. Protective Effect of Quercetin in Primary Neurons against Aβ(1-42): Relevance to Alzheimer’s Disease. J. Nutr. Biochem. 2009, 20, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Dok-Go, H.; Lee, K.H.; Kim, H.J.; Lee, E.H.; Lee, J.; Song, Y.S.; Lee, Y.H.; Jin, C.; Lee, Y.S.; Cho, J. Neuroprotective Effects of Antioxidative Flavonoids, Quercetin, (+)-Dihydroquercetin and Quercetin 3-Methyl Ether, Isolated from Opuntia Ficus-Indica Var. Saboten. Brain Res. 2003, 965, 130–136. [Google Scholar] [CrossRef]
- Deepika; Maurya, P.K. Health Benefits of Quercetin in Age-Related Diseases. Molecules 2022, 27, 2498. [Google Scholar] [CrossRef]
- Tasca, F.; Antiochia, R. Biocide Activity of Green Quercetin-Mediated Synthesized Silver Nanoparticles. Nanomaterials 2020, 10, 909. [Google Scholar] [CrossRef] [PubMed]
- Pandian, S.R.K.; Kunjiappan, S.; Ravishankar, V.; Sundarapandian, V. Synthesis of Quercetin-Functionalized Silver Nanoparticles by Rapid One-Pot Approach. BioTechnologia 2021, 102, 75–84. [Google Scholar] [CrossRef]
- Lee, J.H.; Lim, J.M.; Velmurugan, P.; Park, Y.J.; Park, Y.J.; Bang, K.S.; Oh, B.T. Photobiologic-Mediated Fabrication of Silver Nanoparticles with Antibacterial Activity. J. Photochem. Photobiol. B Biol. 2016, 162, 93–99. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Saravanakumar, K.; Mariadoss, A.V.A.; Wang, M.-H. Antimicrobial and Wound Healing Properties of FeO Fabricated Chitosan/PVA Nanocomposite Sponge. Antibiotics 2021, 10, 524. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Saravanakumar, K.; Mariadoss, A.V.A.; Wang, M.H. Biocompatible Fungal Chitosan Encapsulated Phytogenic Silver Nanoparticles Enhanced Antidiabetic, Antioxidant and Antibacterial Activity. Int. J. Biol. Macromol. 2020, 153, 63–71. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Saravanakumar, K.; Mariadoss, A.V.A.; Ramachandran, C.; Hu, X.; Oh, D.H.; Wang, M.H. Chitosan-Tea Tree Oil Nanoemulsion and Calcium Chloride Tailored Edible Coating Increase the Shelf Life of Fresh Cut Red Bell Pepper. Prog. Org. Coat. 2020, 151, 106010. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Park, S.; Saravanakumar, K.; Mariadoss, A.V.A.; Wang, M.-H. Evaluation of Phytochemicals, Antioxidants, and Antidiabetic Efficacy of Various Solvent Fractions of Gynura Procumbens (Lour.) Merr. Process Biochem. 2021, 111, 51–62. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Saravanakumar, K.; Manivasagan, P.; Jeong, M.S.; Jang, E.S.; Wang, M.H. Folic Acid Conjugated Chitosan Encapsulated Palladium Nanoclusters for NIR Triggered Photothermal Breast Cancer Treatment. Carbohydr. Polym. 2022, 280, 119021. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lai, W.; Cui, M.; Liang, L.; Lin, Y.; Fang, Q.; Liu, Y.; Xie, L. An Evaluation of Blood Compatibility of Silver Nanoparticles. Sci. Rep. 2016, 6, 1–15. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, R.C. Facile and Eco-Friendly Fabrication of Colored and Bioactive Silk Materials Using Silver Nanoparticles Synthesized by Two Flavonoids. Polymers 2018, 10, 404. [Google Scholar] [CrossRef]
- Alqadi, M.K.; Abo Noqtah, O.A.; Alzoubi, F.Y.; Alzouby, J.; Aljarrah, K. PH Effect on the Aggregation of Silver Nanoparticles Synthesized by Chemical Reduction. Mater. Sci. 2014, 32, 107–111. [Google Scholar] [CrossRef]
- Gloria, E.C.; Ederley, V.; Gladis, M.; César, H.; Jaime, O.; Oscar, A.; José, I.U.; Franklin, J. Synthesis of Silver Nanoparticles (AgNPs) with Antibacterial Activity. J. Phys. Conf. Ser. 2017, 850, 012023. [Google Scholar] [CrossRef]
- Gupta, A.; Briffa, S.M.; Swingler, S.; Gibson, H.; Kannappan, V.; Adamus, G.; Kowalczuk, M.; Martin, C.; Radecka, I. Synthesis of Silver Nanoparticles Using Curcumin-Cyclodextrins Loaded into Bacterial Cellulose-Based Hydrogels for Wound Dressing Applications. Biomacromolecules 2020, 21, 1802–1811. [Google Scholar] [CrossRef]
- Zhang, X.F.; Huang, F.H.; Zhang, G.L.; Bai, D.P.; de Felici, M.; Huang, Y.F.; Gurunathan, S. Novel Biomolecule Lycopene-Reduced Graphene Oxide-Silver Nanoparticle Enhances Apoptotic Potential of Trichostatin A in Human Ovarian Cancer Cells (SKOV3). Int. J. Nanomed. 2017, 12, 7551. [Google Scholar] [CrossRef] [Green Version]
- Venil, C.K.; Malathi, M.; Velmurugan, P.; Renuka Devi, P. Green Synthesis of Silver Nanoparticles Using Canthaxanthin from Dietzia Maris AURCCBT01 and Their Cytotoxic Properties against Human Keratinocyte Cell Line. J. Appl. Microbiol. 2021, 130, 1730–1744. [Google Scholar] [CrossRef]
- Chahardoli, A.; Hajmomeni, P.; Ghowsi, M.; Qalekhani, F.; Shokoohinia, Y.; Fattahi, A. Optimization of Quercetin-Assisted Silver Nanoparticles Synthesis and Evaluation of Their Hemocompatibility, Antioxidant, Anti-Inflammatory, and Antibacterial Effects. Glob. Chall. 2021, 5, 2100075. [Google Scholar] [CrossRef]
- Lee, Y.J.; Park, Y. Green Synthetic Nanoarchitectonics of Gold and Silver Nanoparticles Prepared Using Quercetin and Their Cytotoxicity and Catalytic Applications. J. Nanosci. Nanotechnol. 2019, 20, 2781–2790. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.G.; Wang, Y.H.; Xing, H.H.; Gurunathan, S. Quercetin-Mediated Synthesis of Graphene Oxide–Silver Nanoparticle Nanocomposites: A Suitable Alternative Nanotherapy for Neuroblastoma. Int. J. Nanomed. 2017, 12, 5819. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Ninomiya, M.; Tanaka, K.; Koketsu, M. Effects of Functional Groups and Sugar Composition of Quercetin Derivatives on Their Radical Scavenging Properties. J. Nat. Prod. 2016, 79, 1808–1814. [Google Scholar] [CrossRef] [PubMed]
- Dian, L.; Yu, E.; Chen, X.; Wen, X.; Zhang, Z.; Qin, L.; Wang, Q.; Li, G.; Wu, C. Enhancing Oral Bioavailability of Quercetin Using Novel Soluplus Polymeric Micelles. Nanoscale Res. Lett. 2014, 9, 1–11. [Google Scholar] [CrossRef]
- Hu, B.; Liu, X.; Zhang, C.; Zeng, X. Food Macromolecule Based Nanodelivery Systems for Enhancing the Bioavailability of Polyphenols. J. Food Drug Anal. 2017, 25, 3–15. [Google Scholar] [CrossRef]
- Shabbir, U.; Rubab, M.; Daliri, E.B.M.; Chelliah, R.; Javed, A.; Oh, D.H. Curcumin, Quercetin, Catechins and Metabolic Diseases: The Role of Gut Microbiota. Nutrients 2021, 13, 206. [Google Scholar] [CrossRef]
- Vaiserman, A.; Koliada, A.; Zayachkivska, A.; Lushchak, O. Nanodelivery of Natural Antioxidants: An Anti-Aging Perspective. Front. Bioeng. Biotechnol. 2020, 7, 447. [Google Scholar] [CrossRef]
- Olszowy-Tomczyk, M. How to Express the Antioxidant Properties of Substances Properly? Chem. Pap. 2021, 75, 6157–6167. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Panda, S.K.; Jayabalan, R.; Sharma, N.; Bastia, A.K.; Mohanta, T.K. Antimicrobial, Antioxidant and Cytotoxic Activity of Silver Nanoparticles Synthesized by Leaf Extract of Erythrina Suberosa (Roxb.). Front. Mol. Biosci. 2017, 4, 14. [Google Scholar] [CrossRef]
- Salari, S.; Bahabadi, S.E.; Samzadeh-Kermani, A.; Yosefzaei, F. In-Vitro Evaluation of Antioxidant and Antibacterial Potential of GreenSynthesized Silver Nanoparticles Using Prosopis Farcta Fruit Extract. Iran. J. Pharm. Res. 2019, 18, 430. [Google Scholar]
- Wang, C.; Kim, Y.J.; Singh, P.; Mathiyalagan, R.; Jin, Y.; Yang, D.C. Green Synthesis of Silver Nanoparticles by Bacillus Methylotrophicus, and Their Antimicrobial Activity. Artif. Cells Nanomed. Biotechnol. 2015, 44, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.M. Determination of Minimum Inhibitory Concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Loo, Y.Y.; Rukayadi, Y.; Nor-Khaizura, M.A.R.; Kuan, C.H.; Chieng, B.W.; Nishibuchi, M.; Radu, S. In Vitro Antimicrobial Activity of Green Synthesized Silver Nanoparticles against Selected Gram-Negative Foodborne Pathogens. Front. Microbiol. 2018, 9, 1555. [Google Scholar] [CrossRef]
- Liao, S.; Zhang, Y.; Pan, X.; Zhu, F.; Jiang, C.; Liu, Q.; Cheng, Z.; Dai, G.; Wu, G.; Wang, L.; et al. Antibacterial Activity and Mechanism of Silver Nanoparticles against Multidrug-Resistant Pseudomonas aeruginosa. Int. J. Nanomed. 2019, 14, 1469–1487. [Google Scholar] [CrossRef]
- Parvekar, P.; Palaskar, J.; Metgud, S.; Maria, R.; Dutta, S. The Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Silver Nanoparticles against Staphylococcus Aureus. Biomater. Investig. Dent. 2020, 7, 105–109. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, C.; Tang, M.; Yang, Z.; Jia, W.; Ma, Y.; Jia, P.; Pei, D.; Wang, H. Nanotoxicity of Silver Nanoparticles on HEK293T Cells: A Combined Study Using Biomechanical and Biological Techniques. ACS Omega 2018, 3, 6770–6778. [Google Scholar] [CrossRef]
- Gliga, A.R.; Skoglund, S.; Odnevall Wallinder, I.; Fadeel, B.; Karlsson, H.L. Size-Dependent Cytotoxicity of Silver Nanoparticles in Human Lung Cells: The Role of Cellular Uptake, Agglomeration and Ag Release. Part. Fibre Toxicol. 2014, 11, 11. [Google Scholar] [CrossRef]
- Liu, X.; Shan, K.; Shao, X.; Shi, X.; He, Y.; Liu, Z.; Jacob, J.A.; Deng, L. Nanotoxic Effects of Silver Nanoparticles on Normal HEK-293 Cells in Comparison to Cancerous HeLa Cell Line. Int. J. Nanomed. 2021, 16, 753–761. [Google Scholar] [CrossRef]
- Chen, L.Q.; Fang, L.; Ling, J.; Ding, C.Z.; Kang, B.; Huang, C.Z. Nanotoxicity of Silver Nanoparticles to Red Blood Cells: Size Dependent Adsorption, Uptake, and Hemolytic Activity. Chem. Res. Toxicol. 2015, 28, 501–509. [Google Scholar] [CrossRef]
- Buhr, C.R.; Wiesmann, N.; Tanner, R.C.; Brieger, J.; Eckrich, J. The Chorioallantoic Membrane Assay in Nanotoxicological Research—An Alternative for In Vivo Experimentation. Nanomaterials 2020, 10, 2328. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Norbert, C.C.; Acharyya, R.; Mukherjee, S.; Kathirvel, M.; Patra, C.R. Biosynthesized Silver Nanoparticles for Cancer Therapy and in Vivo Bioimaging. Cancers 2021, 13, 6114. [Google Scholar] [CrossRef] [PubMed]
- Craciunescu, O.; Seciu, A.M.; Zarnescu, O. In Vitro and in Vivo Evaluation of a Biomimetic Scaffold Embedding Silver Nanoparticles for Improved Treatment of Oral Lesions. Mater. Sci. Eng. C 2021, 123, 112015. [Google Scholar] [CrossRef] [PubMed]

| Qn-AgNPs | B. cereus | S. aereus | E. coli | S. enterica | L. monocytogenes |
|---|---|---|---|---|---|
| Zone of Inhibation (mm) | |||||
| 7.8 | 9 ± 1.2 | 7 ± 0.8 | 8 ± 0.6 | 8 ± 1.6 | 8 ± 1.4 |
| 15.6 | 10 ± 1.4 | 8 ± 0.8 | 10 ± 0.8 | 9 ± 1.6 | 9 ± 1.4 |
| 31.2 | 11 ± 1.4 | 11 ± 1.0 | 12 ± 0.8 | 11 ± 1.2 | 11 ± 1.2 |
| 62.5 | 12 ± 1.2 | 13 ± 1.0 | 13 ± 0.8 | 12 ± 1.4 | 13 ± 1.6 |
| Qn | - | - | - | - | - |
| TCH | 11 ± 0.6 | 10 ± 0.4 | 10 ± 0.4 | 10 ± 0.6 | 10 ± 0.8 |
| Minimum inhibitory concentration (µg/mL) | |||||
| Qn-AgNPs | 3.9 | 3.9 | 7.8 | 3.9 | 3.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, K.; Sathiyaseelan, A.; Saravanakumar, K.; Wang, M.-H. Synthesis of Biomolecule Functionalized Biocompatible Silver Nanoparticles for Antioxidant and Antibacterial Applications. Coatings 2022, 12, 1292. https://doi.org/10.3390/coatings12091292
Han K, Sathiyaseelan A, Saravanakumar K, Wang M-H. Synthesis of Biomolecule Functionalized Biocompatible Silver Nanoparticles for Antioxidant and Antibacterial Applications. Coatings. 2022; 12(9):1292. https://doi.org/10.3390/coatings12091292
Chicago/Turabian StyleHan, Kiseok, Anbazhagan Sathiyaseelan, Kandasamy Saravanakumar, and Myeong-Hyeon Wang. 2022. "Synthesis of Biomolecule Functionalized Biocompatible Silver Nanoparticles for Antioxidant and Antibacterial Applications" Coatings 12, no. 9: 1292. https://doi.org/10.3390/coatings12091292






