Improving Hydrophilicity and Adhesion of PDMS through Dopamine Modification Assisted by Carbon Dioxide Plasma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PDMS Films
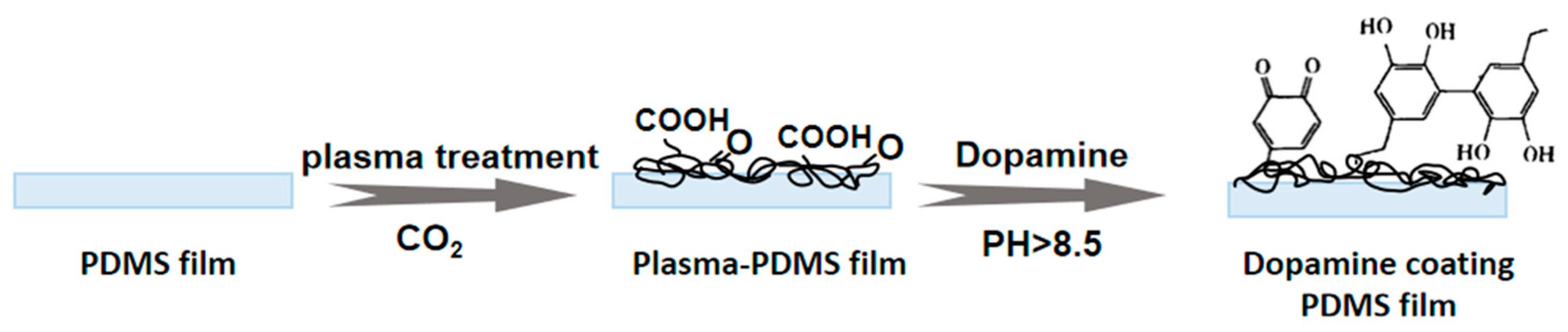
2.2. Carbon Dioxide Plasma Treatment
2.3. Modification of Dopamine
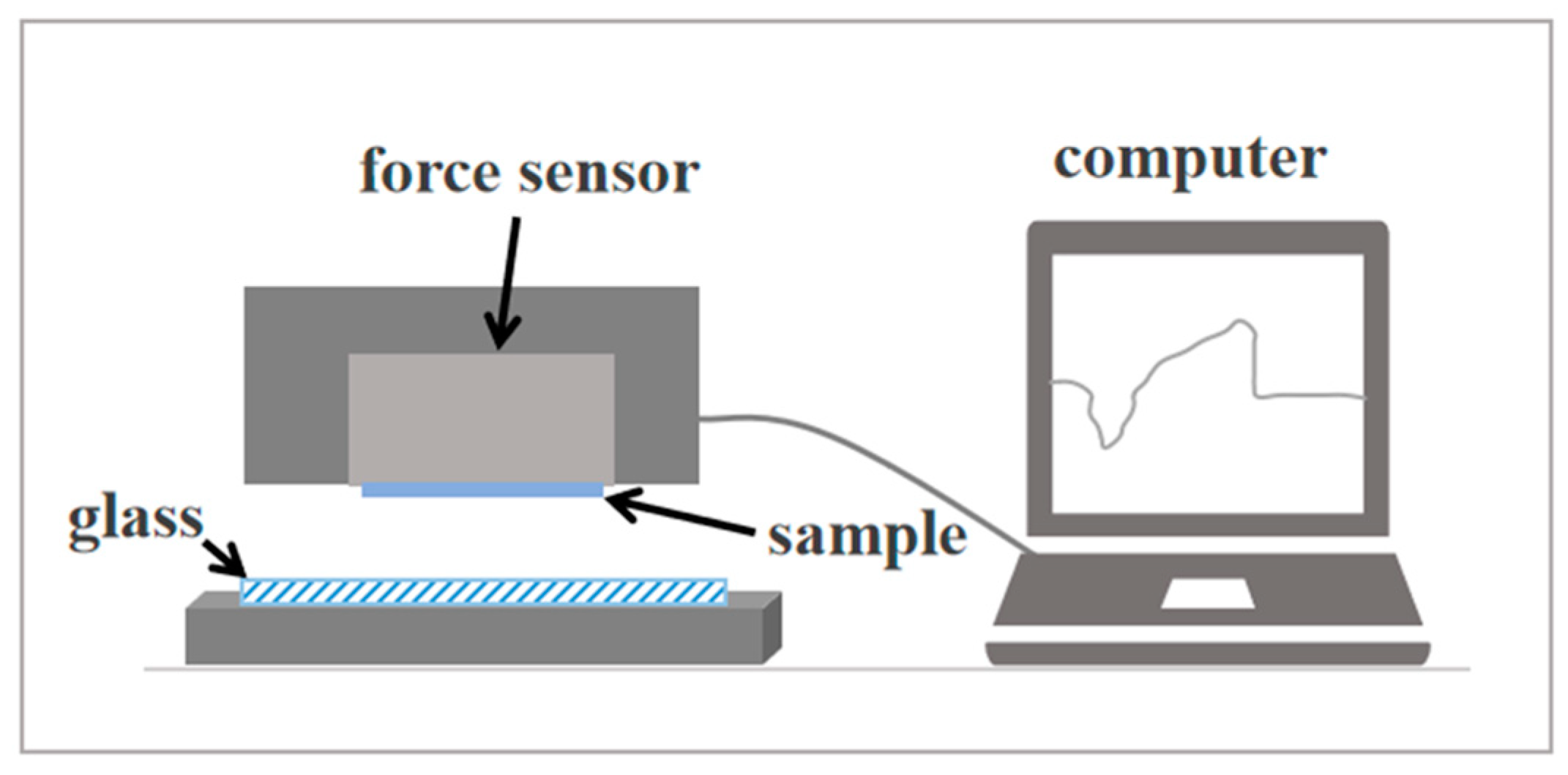
2.4. Characterization and Testing
3. Results and Discussion
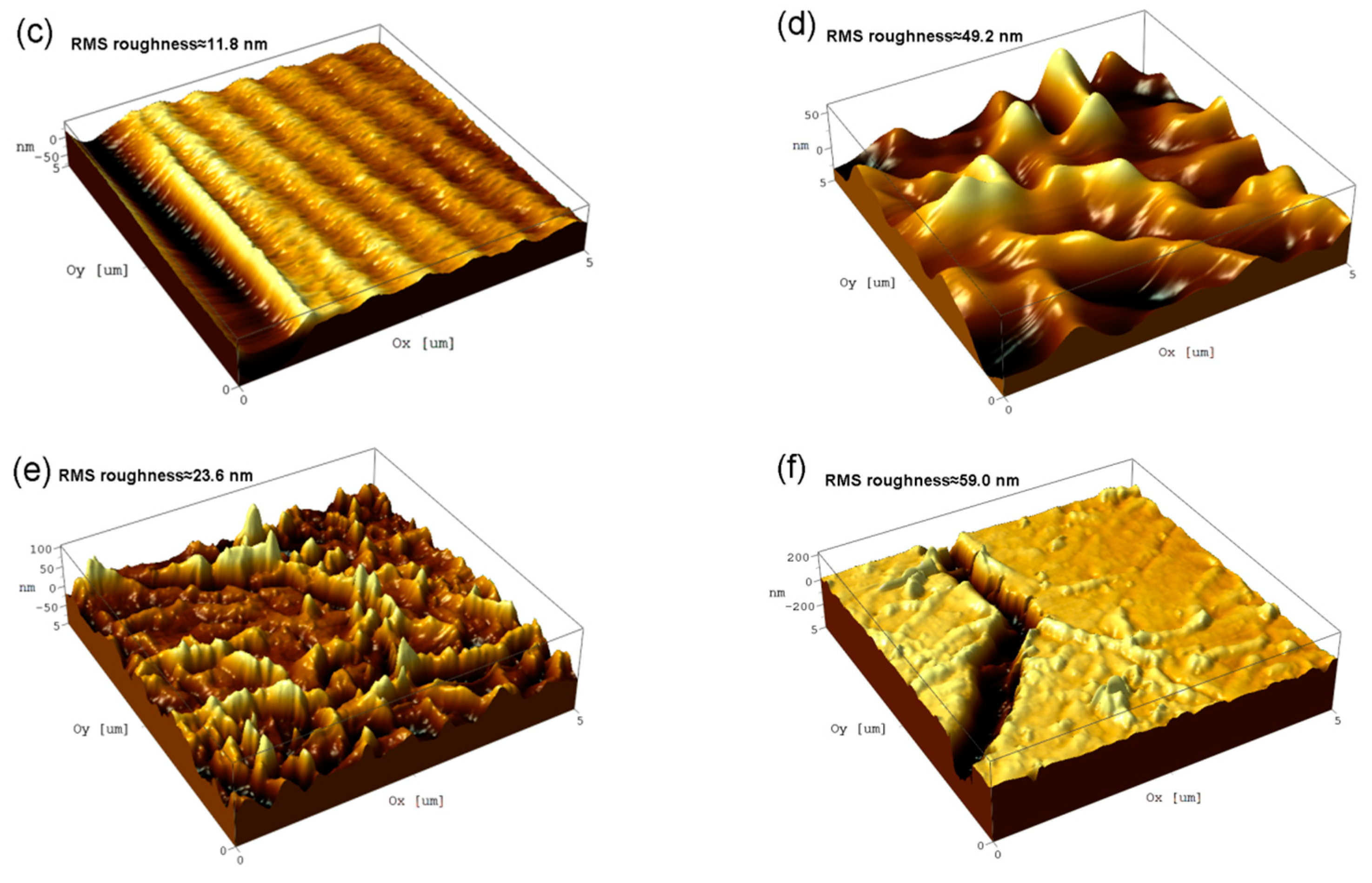
3.1. SEM Observation and EDS Test
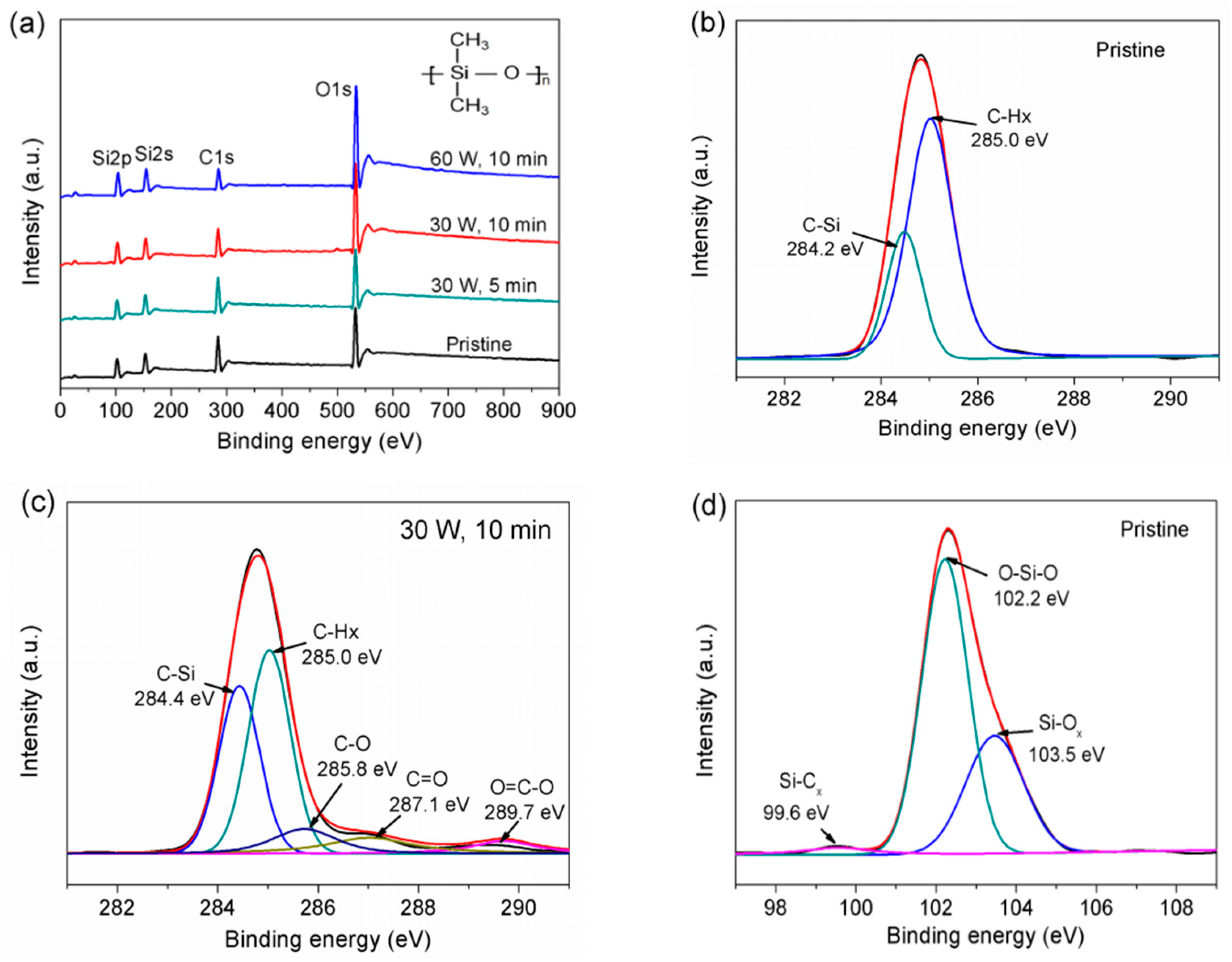
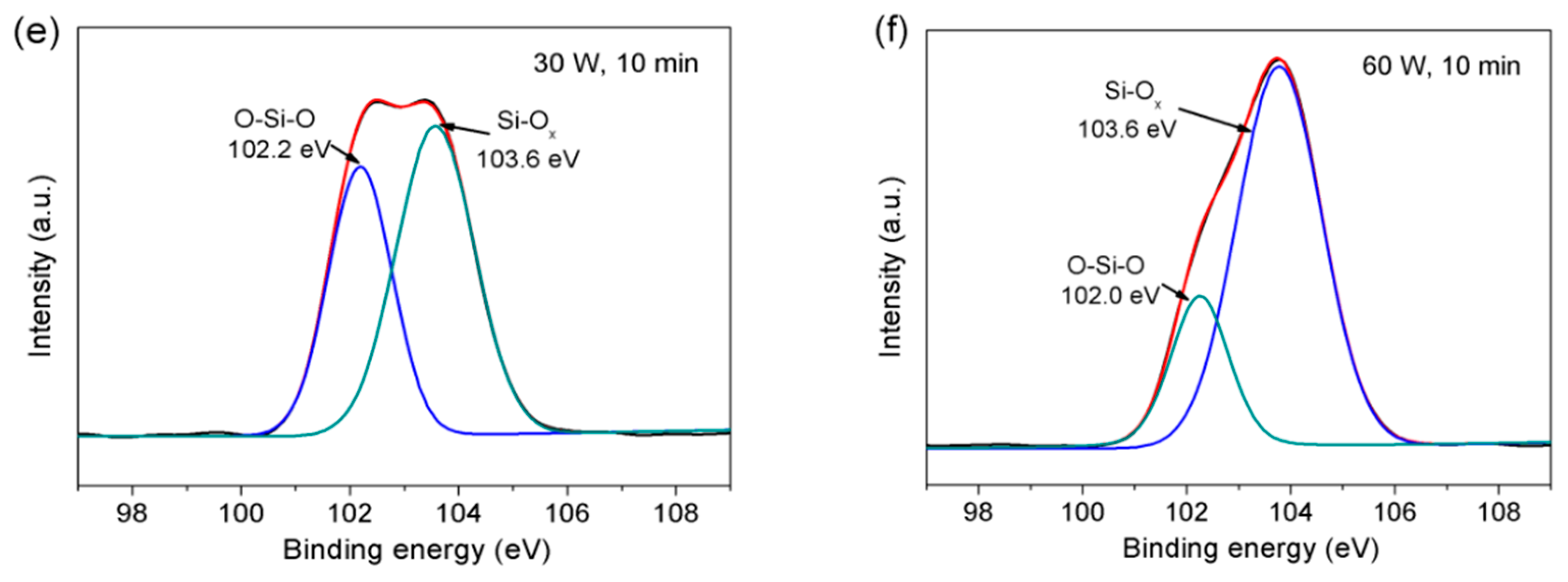
3.2. XPS Analysis
3.3. ATR-FTIR Analysis
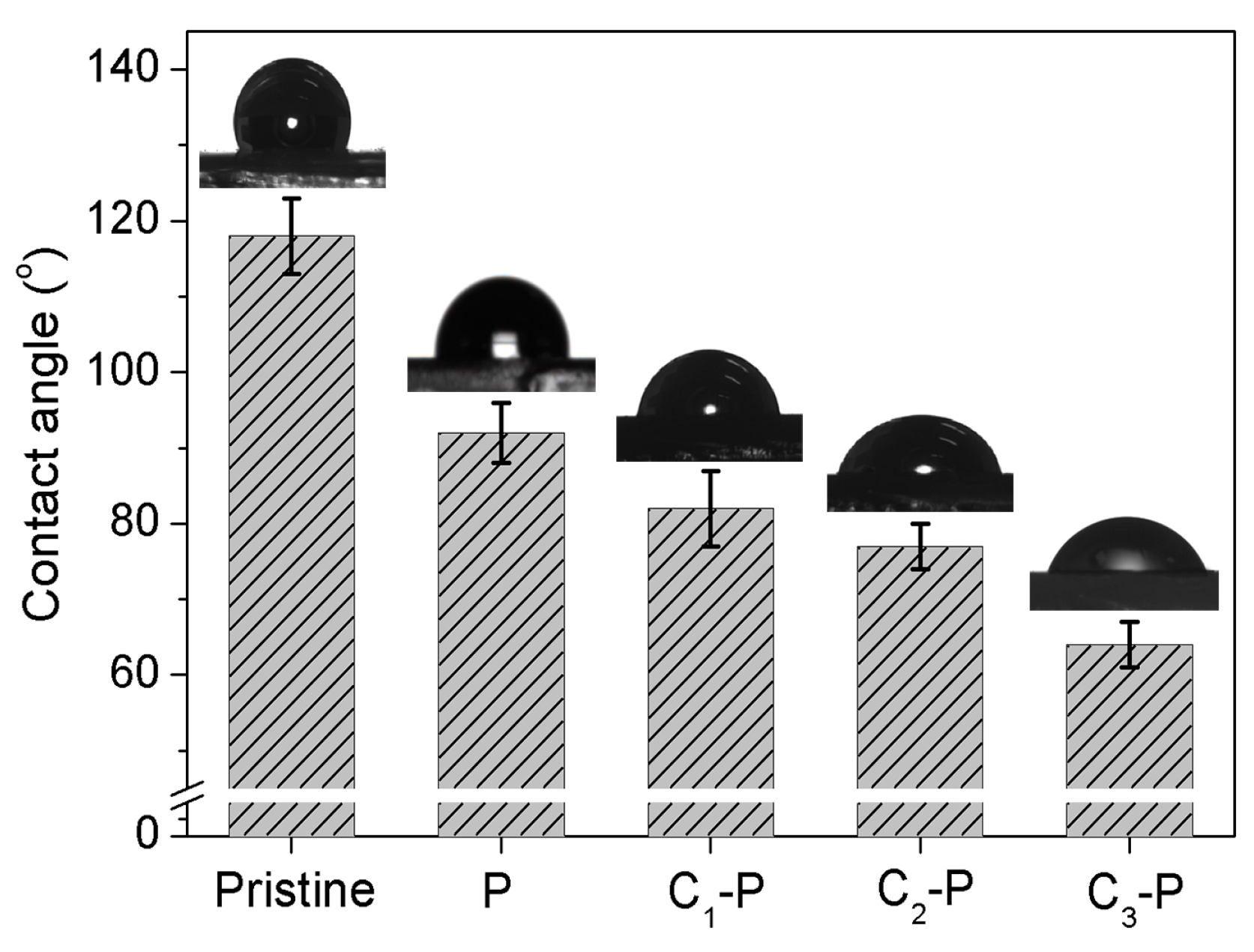
3.4. Contact Angle Tests
3.5. Adhesion Strength Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, S.; Chen, Y.; Tian, J.; Shi, J.; Geng, C.; Zou, H.; Liang, M.; Li, Z. Superior low-temperature reversible adhesion based on bio-inspired microfibrillar adhesives fabricated by phenyl containing polydimethylsiloxane elastomers. Adv. Funct. Mater. 2021, 31, 2101143. [Google Scholar] [CrossRef]
- Tsujioka, K.; Matsuo, Y.; Shimomura, M.; Hirai, Y. A new concept for an adhesive material inspired by clingfish sucker nanofilaments. Langmuir 2022, 38, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Pu, W.; Wei, P.; Kong, M. Molecular dynamics simulations on adhesion energy of PDMS-silica interface caused by molecular structures and temperature. Appl. Surf. Sci. 2022, 577, 151930. [Google Scholar] [CrossRef]
- Ibáñez-Ibáñez, P.F.; Ruiz-Cabello, F.J.M.; Cabrerizo-Vílchez, M.A.; Rodríguez-Valverde, M.A. Ice adhesion of PDMS surfaces with balanced elastic and water-repellent properties. J. Colloid Interf. Sci. 2022, 608, 792–799. [Google Scholar] [CrossRef]
- Liu, X.; Chen, K.; Zhang, D.; Guo, Z. One-step methods to fabricate durable superhydrophobic coatings for flexible electronic sensors. Coatings 2021, 11, 95. [Google Scholar] [CrossRef]
- Dirany, M.; Dies, L.; Restagno, F.; Léger, L.; Miquelard-Garnier, G. Chemical modification of PDMS surface without impacting the viscoelasticity: Model systems for a better understanding of elastomer/elastomer adhesion and friction. Colloids Surf. A 2015, 468, 174–183. [Google Scholar] [CrossRef]
- Jiang, B.; Guo, H.; Chen, D.; Zhou, M. Microscale investigation on the wettability and bonding mechanism of oxygen plasma-treated PDMS microfluidic chip. Appl. Surf. Sci. 2022, 574, 151704. [Google Scholar] [CrossRef]
- Yan, Y.; Qi, Y.; Chen, W. Strategies to hydrophilize silicones via spontaneous adsorption of poly(vinyl alcohol) from aqueous solution. Colloids Surf. A 2018, 546, 186–193. [Google Scholar] [CrossRef]
- Majhy, B.; Priyadarshini, P.; Sen, A.K. Effect of surface energy and roughness on cell adhesion and growth–facile surface modification for enhanced cell culture. RSC Adv. 2021, 11, 15467–15476. [Google Scholar] [CrossRef]
- Chang, K.Y.; Chou, Y.N.; Chen, W.Y.; Chen, C.Y.; Lin, H.R. Mussel-inspired adhesive and self-healing hydrogel as an injectable wound dressing. Polymers 2022, 14, 3346. [Google Scholar] [CrossRef]
- Yi, Z.; Zhao, B.; Liao, M.; Qin, Z. Fabrication of superhydrophobic wood surface by etching polydopamine coating with sodium hydroxide. Coatings 2020, 10, 847. [Google Scholar] [CrossRef]
- Tran, N.T.; Flanagan, D.P.; Orlicki, J.A.; Lenhart, J.L.; Proctor, K.L.; Knorr, D.B. Polydopamine and polydopamine-silane hybrid surface treatments in structural adhesive applications. Langmuir 2018, 34, 1274–1286. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tirrell, M.; Korba, G.A.; Poclus, A. Surface energy and adhesion studies on acrylic pressure sensitive adhesives. J. Adhes. 2001, 76, 307–334. [Google Scholar] [CrossRef]
- Dabaghi, M.; Shahriari, S.; Saraei, N.; Da, K.; Chandiramohan, A.; Selvaganapathy, P.R.; Hirota, J.A. Surface modification of PDMS-based microfluidic devices with collagen using polydopamine as a spacer to enhance primary human bronchial epithelial cell adhesion. Micromachines 2021, 12, 132. [Google Scholar] [CrossRef]
- Perikamana, S.K.M.; Shin, Y.M.; Lee, J.K.; Lee, Y.B.; Heo, Y.; Ahmad, T.; Park, S.Y.; Shin, J.; Park, K.M.; Jung, H.S.; et al. Graded functionalization of biomaterial surfaces using mussel-inspired adhesive coating of polydopamine. Colloids Surf. B 2017, 159, 546–556. [Google Scholar] [CrossRef]
- Zhang, F.T.; Xu, L.; Chen, J.H.; Zhao, B.; Fu, X.Z.; Sun, R.; Chen, Q.; Wong, C.P. Electroless deposition metals on poly(dimethylsiloxane) with strong adhesion as flexible and stretchable conductive materials. Acs. Appl. Mater. Inter. 2017, 10, 2075–2082. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, Q.; Li, M.; Jin, S.; Fan, J.; Zhao, L.; Yao, Z. Surface modification of activated carbon fiber by low-temperature oxygen plasma: Textural property, surface chemistry, and the effect of water vapor adsorption. Chem. Eng. J. 2021, 418, 129474. [Google Scholar] [CrossRef]
- Compoint, F.; Fall, D.; Piombini, H.; Belleville, P.; Montouillout, Y.; Duquennoy, M.; Jenot, F.; Piwakowski, B.; Sanchez, C. Sol-gel-processed hybrid silica-PDMS layers for the optics of high-power laser flux systems. J. Mater. Sci. 2016, 51, 5031–5045. [Google Scholar] [CrossRef]
- Aymes-Chodur, C.; Salmi-Mani, H.; Dragoe, D.; Aubry-Barroca, N.; Buchotte, M.; Roger, P. Optimization of microwave plasma treatment conditions on polydimethylsiloxane films for further surface functionalization. Eur. Polym. J. 2021, 150, 110416. [Google Scholar] [CrossRef]
- Farrell, M.; Beaudoin, S. Surface forces and protein adsorption on dextran- and polyethylene glycol-modified polydimethylsiloxane. Colloids Surf. B 2010, 81, 468–475. [Google Scholar] [CrossRef]
- Xiong, L.; Chen, P.; Zhou, Q. Adhesion promotion between PDMS and glass by oxygen plasma pre-treatment. J. Adhes. Sci. Technol. 2014, 28, 1046–1054. [Google Scholar] [CrossRef]
- Meincken, M.; Berhane, T.A.; Mallon, P.E. Tracking the hydrophobicity recovery of PDMS compounds using the adhesive force determined by AFM force distance measurements. Polymer 2005, 46, 203–208. [Google Scholar] [CrossRef]
- Bodas, D. Surface modification and aging studies of addition-curing silicone rubbers by oxygen plasma. Eur. Polym. J. 2008, 44, 2130–2139. [Google Scholar] [CrossRef]
- Eddington, D.T.; Puccinelli, J.P.; Beebe, D.J. Thermal aging and reduced hydrophobic recovery of polydimethylsiloxane. Sensor. Actuators B Chem. 2006, 114, 170–172. [Google Scholar] [CrossRef]
- Brock, S.L.; Marquez, M.; Suib, S.L.; Hayashi, Y.; Matsumoto, H. Plasma decomposition of CO2 in the presence of metal catalysts. J. Catal. 1998, 180, 225–233. [Google Scholar] [CrossRef]
- Babu, D.J.; Yadav, S.; Heinlein, T.; Cherkashinin, G.; Schneider, J.J. Carbon dioxide plasma as a versatile medium for purification and functionalization of vertically aligned carbon nanotubes. J. Phys. Chem. C 2014, 118, 12028–12034. [Google Scholar] [CrossRef]
- Chen, L.; Ren, X.; Li, Y.; Hu, D.; Feng, X.; Li, W. Enhancing interface compatibility of UiO-66-NH2 and polyamide by incorporating dopamine into thin film nanocomposite membranes. J. Membr. Sci. 2022, 654, 120565. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, F.K.; Han, Y.; Gaikwad, R.; Leonenko, Z.; Zhao, B. Surface and tribological behaviors of the bioinspired polydopamine thin films under dry and wet conditions. Biomacromolecules 2013, 14, 394–405. [Google Scholar] [CrossRef]
- Wang, J.; Guo, H.; Shi, X.; Yao, Z.; Qing, W.; Liu, F.; Tang, C.Y. Fast polydopamine coating on reverse osmosis membrane: Process investigation and membrane performance study. J. Colloid Interface Sci. 2019, 535, 239–244. [Google Scholar] [CrossRef]
- Orishchin, N.; Crane, C.C.; Brownell, M.; Wang, T.; Jenkins, S.; Zou, M.; Nair, A.; Chen, J. Rapid deposition of uniform polydopamine coatings on nanoparticle surfaces with controllable thickness. Langmuir 2017, 33, 6046–6053. [Google Scholar] [CrossRef]
- Fang, M.; Zhang, H.; Chen, J.; Wang, T.; Liu, J.; Li, X.; Li, J.; Cao, X. A facile approach to construct hierarchical dense membranes via polydopamine for enhanced propylene/nitrogen separation. J. Membr. Sci. 2016, 499, 290–300. [Google Scholar] [CrossRef]
- Aerts, S.; Vanhulsel, A.; Buekenhoudt, A.; Weyten, H.; Kuypers, S.; Chen, H.; Bryjak, M.; Gevers, L.E.M.; Vankelecom, I.F.J.; Jacobs, P.A. Plasma-treated PDMS-membranes in solvent resistant nanofiltration: Characterization and study of transport mechanism. J. Membr. Sci. 2006, 275, 212–219. [Google Scholar] [CrossRef]
- Sharma, V.; Dhayal, M.; Shivaprasad, S.M.; Jain, S.C. Surface characterization of plasma-treated and PEG-grafted PDMS for micro fluidic applications. Vacuum 2007, 81, 1094–1100. [Google Scholar] [CrossRef]
- Al-Gamal, A.Q.; Falath, W.S.; Saleh, T.A. Enhanced efficiency of polyamide membranes by incorporating TiO2-Graphene oxide for water purification. J. Mol. Liq. 2021, 323, 114922. [Google Scholar] [CrossRef]
- Kim, H.; Park, S.S.; Seo, J.; Ha, C.S.; Moon, C.; Kim, Y. Stable protein device platform based on pyridine dicarboxylic acid-bound cubic-nanostructured mesoporous titania films. Acs. Appl. Mater. Interfaces 2013, 5, 6873–6878. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, C.; Mitschker, F.; Awakowicz, P.; Kirchheim, D.; Dahlmann, R.; de los Arcos, T.; Grundmeier, G. Adhesion of plasma-deposited silicon oxide barrier layers on PDMS containing polypropylene. Surf. Coat. Technol. 2018, 335, 25–31. [Google Scholar] [CrossRef]
- ButrÓN-GarcÍA, M.I.; Jofre-Reche, J.A. Use of statistical design of experiments in the optimization of Ar-O2 low-pressure plasma treatment conditions of polydimethylsiloxane (PDMS) for increasing polarity and adhesion, and inhibiting hydrophobic recovery. Appl. Surf. Sci. 2015, 332, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Jun, D.R.; Moon, S.K.; Choi, S.W. Uniform polydimethylsiloxane beads coated with polydopamine and their potential biomedical applications. Colloids Surf. B 2014, 121, 395–399. [Google Scholar] [CrossRef]
- Zhu, L.P.; Jiang, J.H.; Zhu, B.K.; Xu, Y.Y. Immobilization of bovine serum albumin onto porous polyethylene membranes using strongly attached polydopamine as a spacer. Colloids Surf. B 2011, 86, 111–118. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, Y.; Liu, X.; Wang, X.; Yang, B. Mussel-inspired modification of carbon fiber via polyethyleneimine/ polydopamine co-deposition for the improved interfacial adhesion. Compos. Sci. Technol. 2017, 151, 164–173. [Google Scholar] [CrossRef]



| Samples | Power (W) | Exposure Time (min) | PDA-Coating Time (h) |
|---|---|---|---|
| Pristine | 0 | 0 | 0 |
| C1 | 30 | 5 | 0 |
| C2 | 30 | 10 | 0 |
| C3 | 60 | 10 | 0 |
| P | 0 | 0 | 18 |
| C1-P | 30 | 5 | 18 |
| C2-P | 30 | 10 | 18 |
| C3-P | 60 | 10 | 18 |
| Samples | Pristine | P | C1-P | C2-P | C3-P |
|---|---|---|---|---|---|
| N (at.%) | 0 | 5.04 | 8.03 | 10.04 | 10.26 |
| Samples | C (at.%) | O (at.%) | Si (at.%) | O/C | O/Si |
|---|---|---|---|---|---|
| pristine | 45.64 | 28.93 | 25.43 | 0.63 | 1.13 |
| C1 | 44.66 | 29.58 | 25.76 | 0.66 | 1.15 |
| C2 | 33.42 | 40.39 | 26.19 | 1.2 | 1.54 |
| C3 | 25.09 | 46.43 | 28.48 | 1.85 | 1.63 |
| Components | Si-Cx (%) | O-Si-O (%) | Si-Ox (%) |
|---|---|---|---|
| pristine | 3.30 | 64.25 | 32.45 |
| C1 | 0 | 62.34 | 37.66 |
| C2 | 0 | 44.58 | 55.42 |
| C3 | 0 | 23.18 | 76.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, M.; Ding, L.; Zhong, T.; Dai, Z. Improving Hydrophilicity and Adhesion of PDMS through Dopamine Modification Assisted by Carbon Dioxide Plasma. Coatings 2023, 13, 126. https://doi.org/10.3390/coatings13010126
Lu M, Ding L, Zhong T, Dai Z. Improving Hydrophilicity and Adhesion of PDMS through Dopamine Modification Assisted by Carbon Dioxide Plasma. Coatings. 2023; 13(1):126. https://doi.org/10.3390/coatings13010126
Chicago/Turabian StyleLu, Mingyue, Li Ding, Tianci Zhong, and Zhendong Dai. 2023. "Improving Hydrophilicity and Adhesion of PDMS through Dopamine Modification Assisted by Carbon Dioxide Plasma" Coatings 13, no. 1: 126. https://doi.org/10.3390/coatings13010126
APA StyleLu, M., Ding, L., Zhong, T., & Dai, Z. (2023). Improving Hydrophilicity and Adhesion of PDMS through Dopamine Modification Assisted by Carbon Dioxide Plasma. Coatings, 13(1), 126. https://doi.org/10.3390/coatings13010126






