Effect of Mesogenic Phase and Structure of Liquid Crystals on Tribological Properties as Lubricant Additives
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals
2.2. Measurements
2.3. Synthesis of ethyl 6-(4-cyanobiphenyl-4-yloxy) Hexanoate (Intermediate 1)
2.4. Synthesis of 6-(4-cyanobiphenyl-4-yloxy) Hexanoate Acid (Intermediate 2)
2.5. Synthesis of (R)-octan-2-yl 4’-((methoxycarbonyl) oxy-[1,1-biphenyl]-4-carboxylate (Intermediate 3)
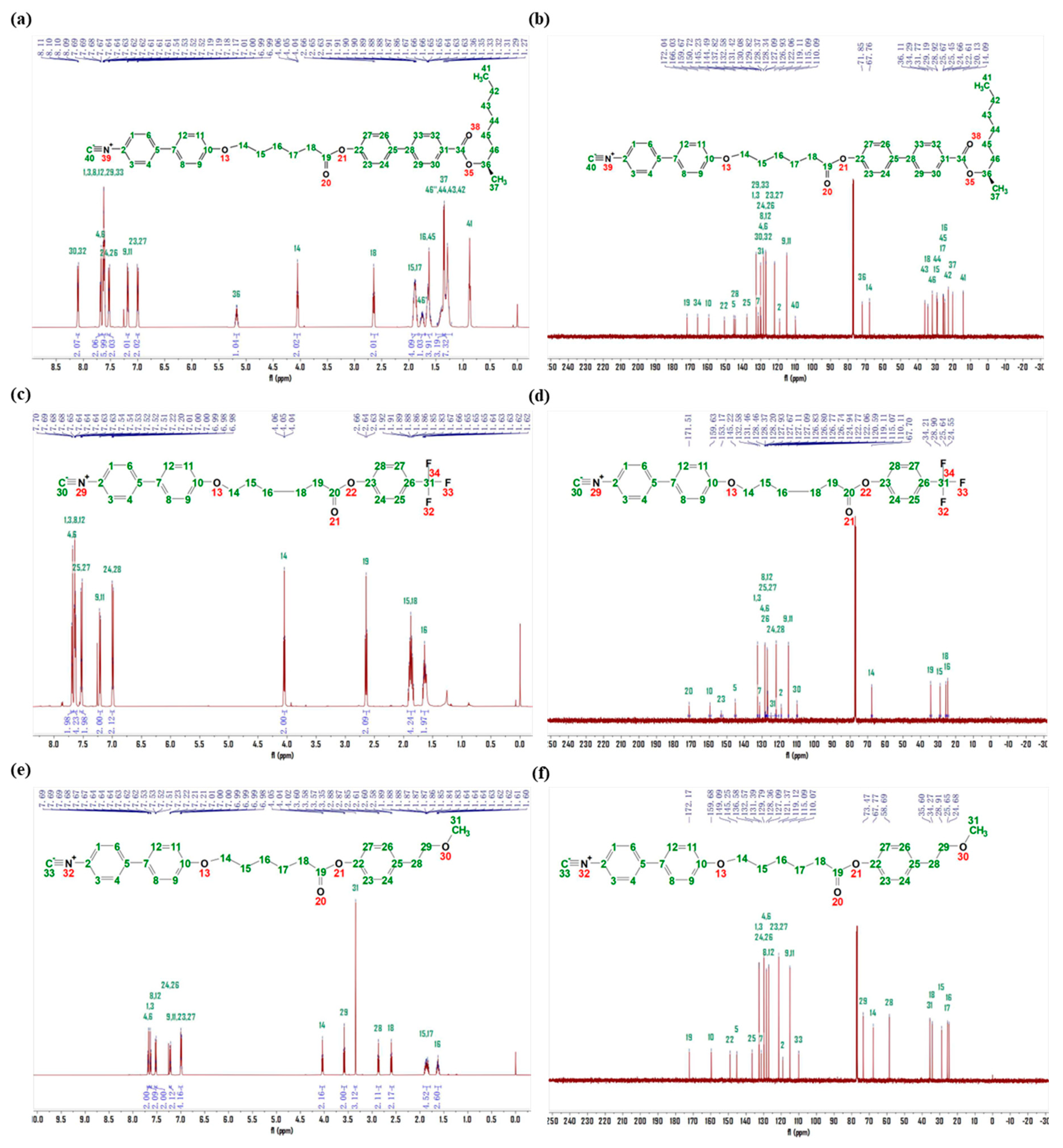
2.6. Synthesis of LCs
2.6.1. Synthesis of LC-A
2.6.2. Synthesis of LC-D
2.6.3. Synthesis of LC-Z
2.7. Tribological Test and Characterization of Worn Surface
3. Results and Discussion
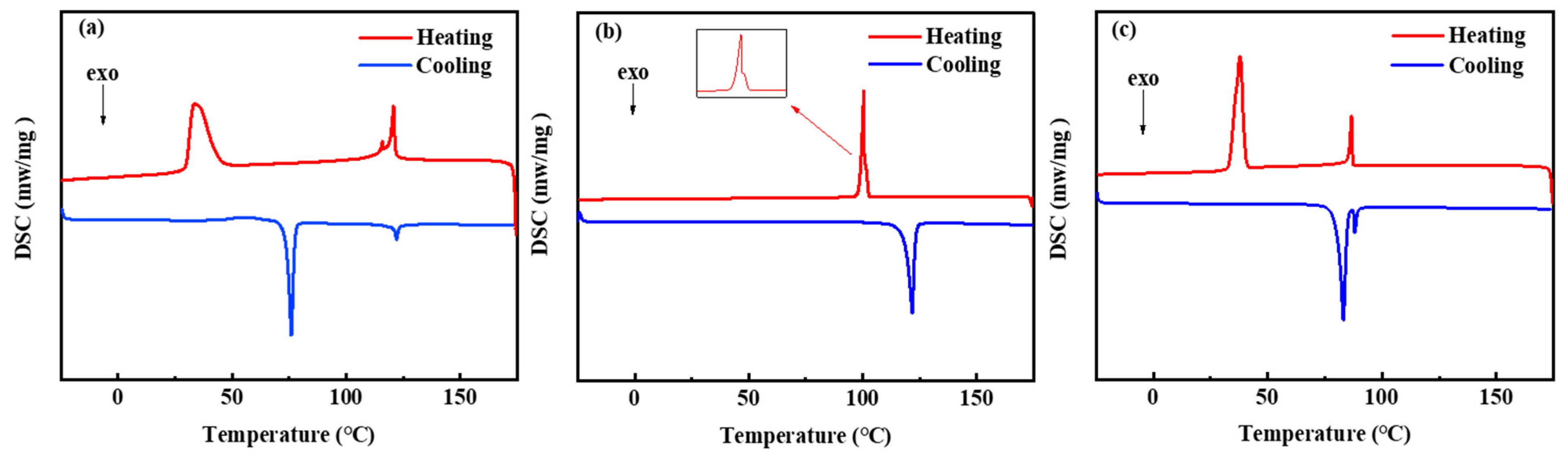
3.1. Mesogenic-Phase Behaviors of the Synthesized LCs
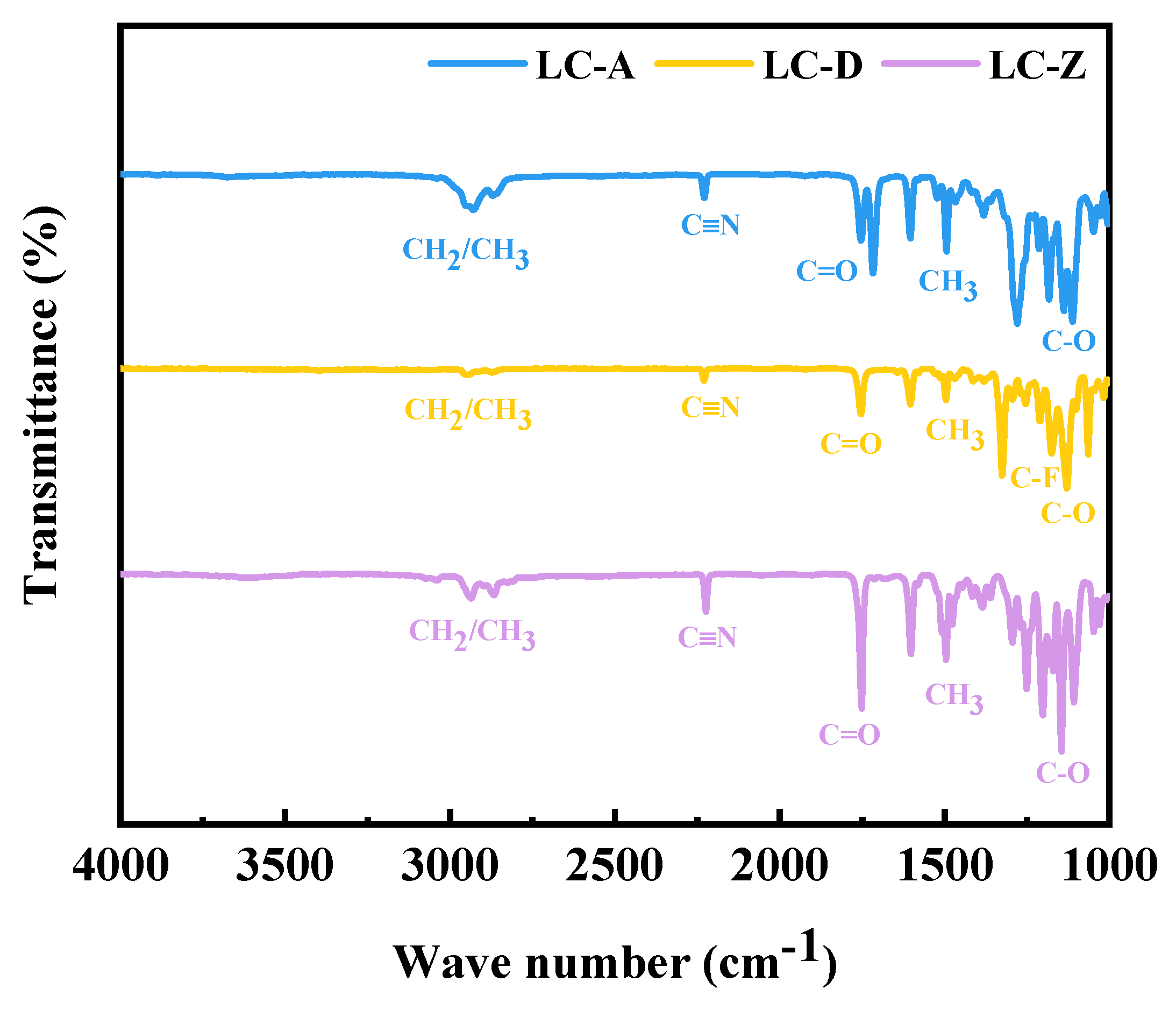
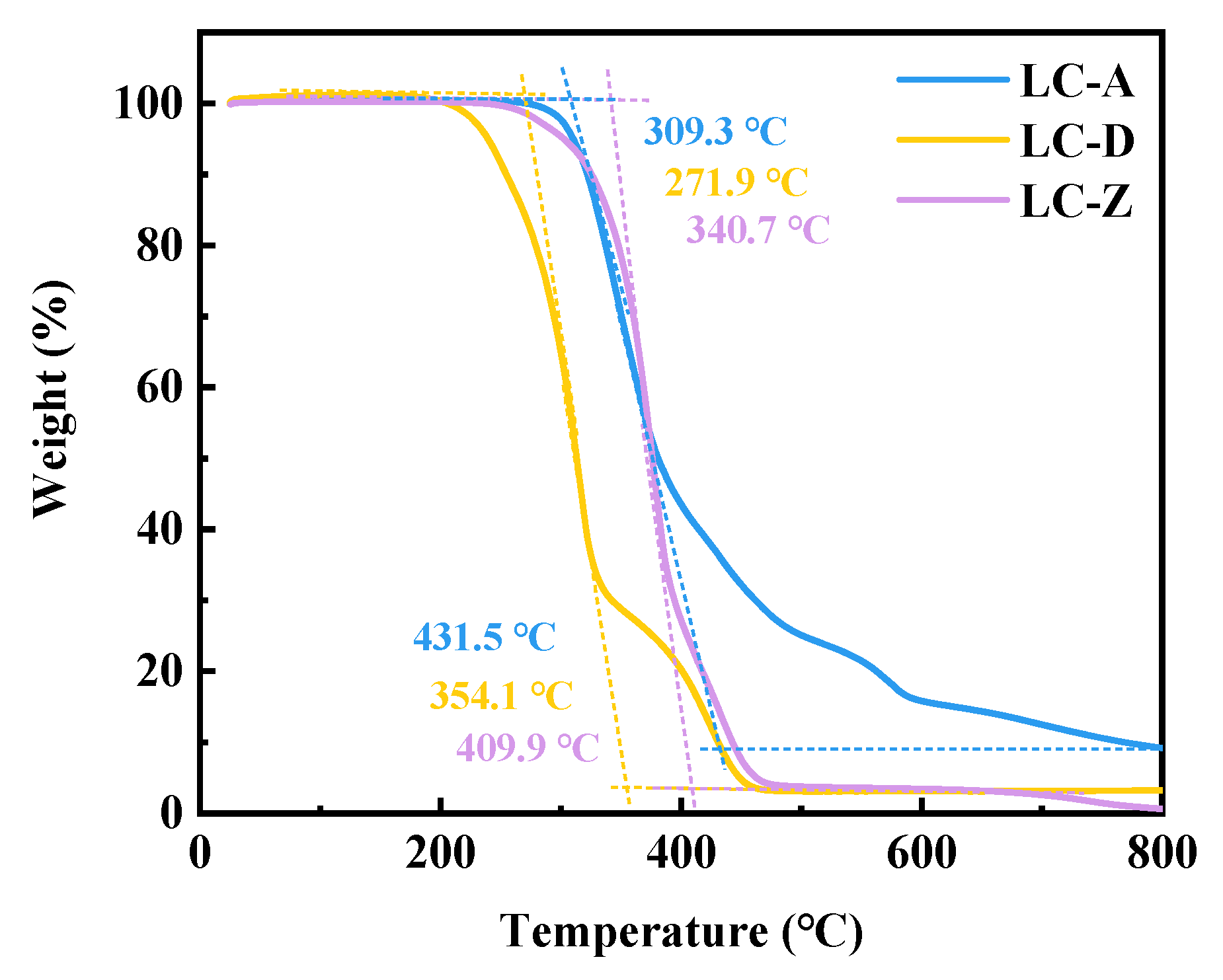
3.2. Analysis of Thermal Stability
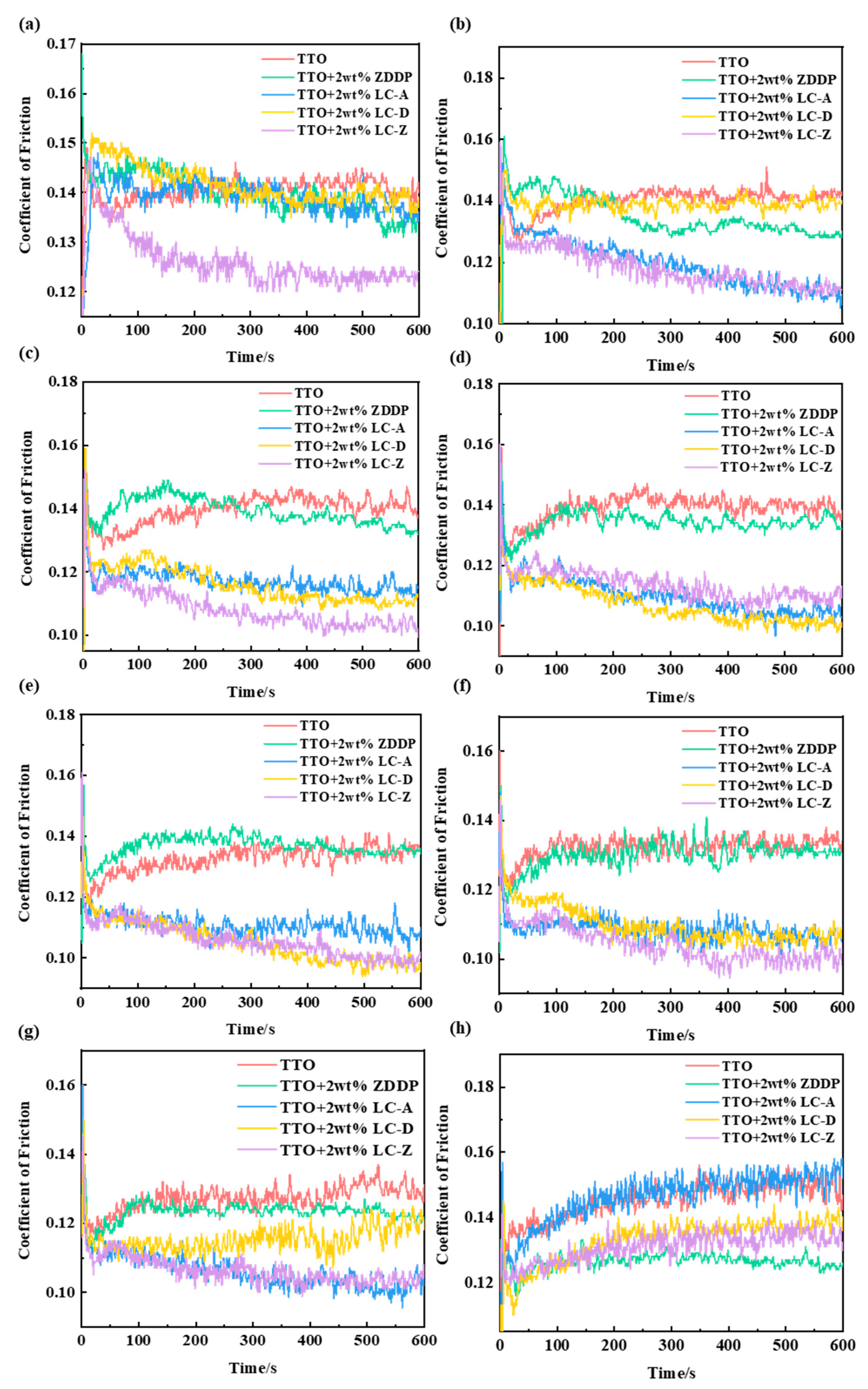
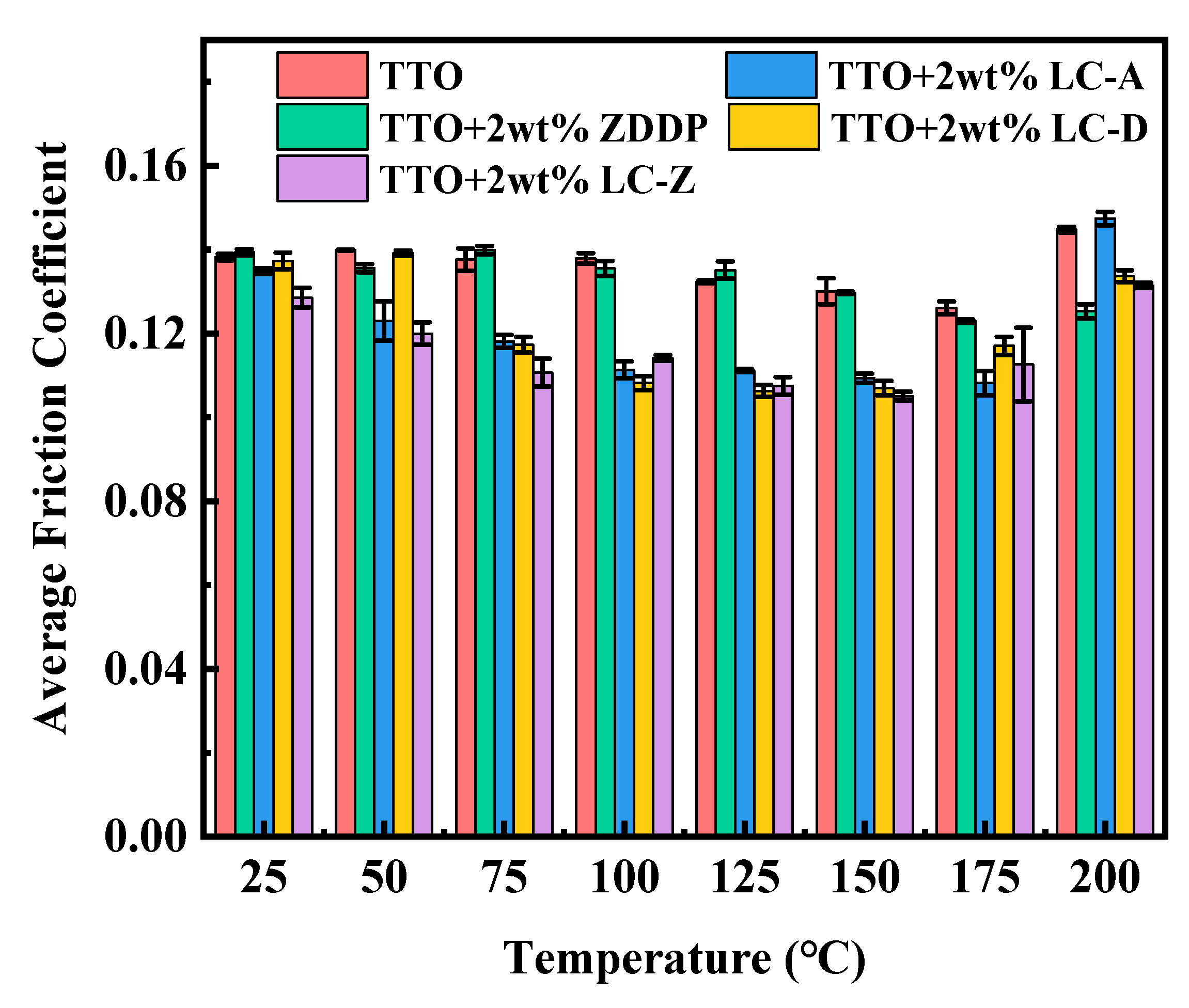
3.3. Anti-friction Analysis
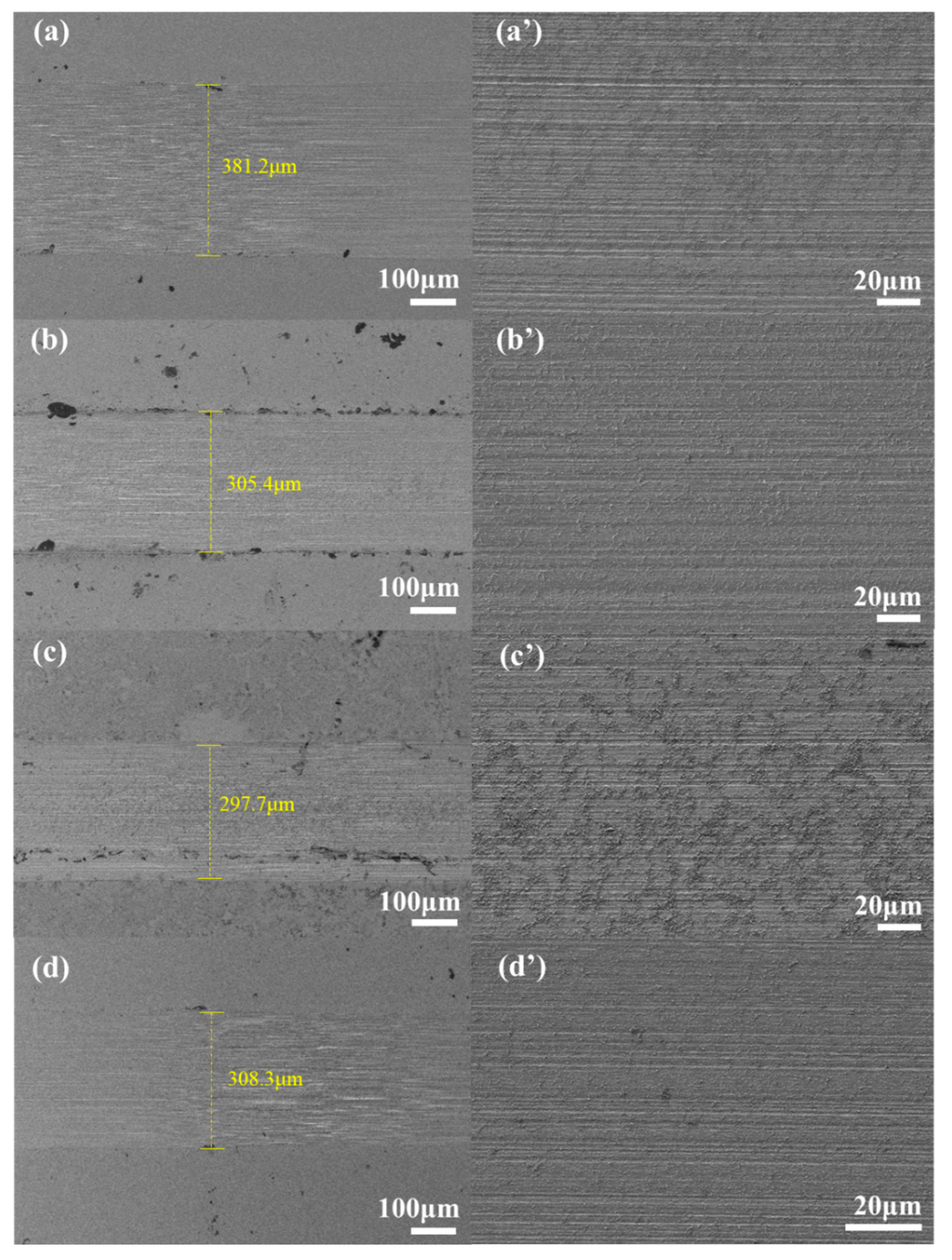
3.4. Anti-Wear Analysis
3.5. Surface Analysis
3.5.1. SEM Analysis
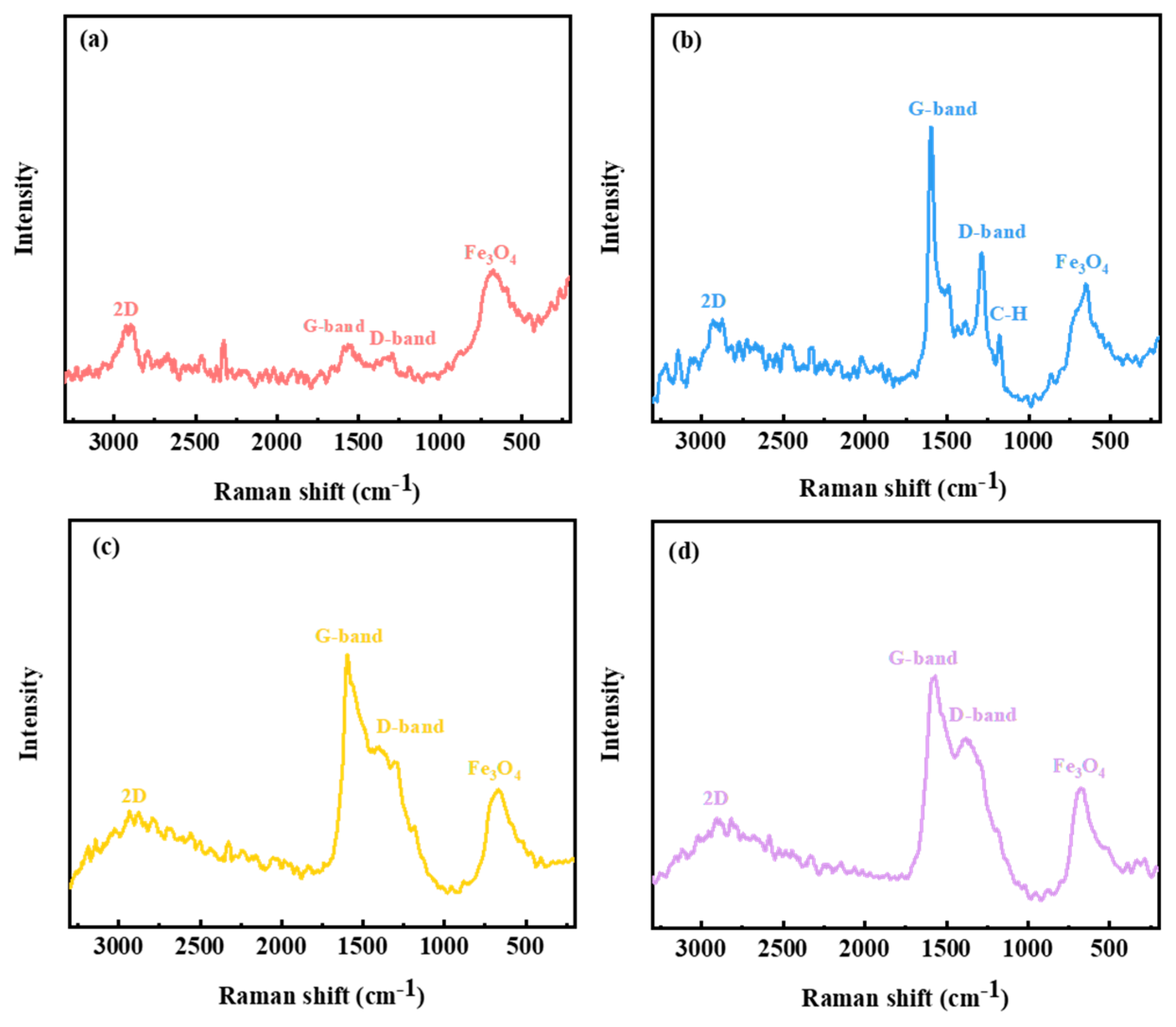
3.5.2. Raman Spectroscopy Analysis
4. Conclusions
- Three LCs were synthesized and characterized for their structures and thermal stability. The results indicate that they have excellent thermal stability. The LCs’ properties were examined, and it was found that three LCs exhibited good mesogenic-phase behavior and that the chain length was an essential factor affecting the width of the mesogenic-phase temperature ranges. Among the LCs, LC-A showed the longest chain length and the widest mesogenic-phase temperature range.
- The synthesized LCs were used as lubricant additives to investigate tribological behavior. The three LCs showed excellent friction-reduction and anti-wear properties when compared to the base oil. Furthermore, the tribological properties of the LCs were very closely connected with their mesogenic-phase temperature ranges, and the best tribological properties of LCs are found in or close to the temperature range.
- To analyze the lubrication mechanism of LCs, it is found that the addition of LCs additive can reduce the width of wear marks and the surface furrow, which can form a lubricant film on the wear surface and prevent the direct contact of frictional pairs on the sliding surface, leading to the improvement of tribological properties of the base oil.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, H.; Adukure, A.R.; Iglesias, P. Effect of Ionicity of Three Protic Ionic Liquids as Neat Lubricants and Lubricant Additives to a Biolubricant. Coatings 2019, 9, 713. [Google Scholar] [CrossRef] [Green Version]
- Liao, M.; Nicolini, P.; Du, L.; Yuan, J.; Wang, S.; Yu, H.; Tang, J.; Cheng, P.; Watanabe, K.; Taniguchi, T.; et al. UItra-low friction and edge-pinning effect in large-lattice-mismatch van der Waals heterostructures. Nat. Mater. 2022, 21, 47–53. Available online: https://www.nature.com/articles/s41563-021-01058-4 (accessed on 6 January 2023). [CrossRef]
- Wang, J.; Li, Z.; Xu, Y.; Hu, W.; Zheng, G.; Zheng, L.; Ren, T. Synthesis and Tribological Behavior of Bridged Bicyclic Polymers as Lubricants. Ind. Eng. Chem. Res. 2022, 169, 107458. [Google Scholar] [CrossRef]
- Uzoma, P.C.; Hu, H.; Khadem, M.; Penkov, O.V. Tribology of 2D Nanomaterials: A Review. Coatings 2020, 10, 897. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Qiu, Y.; Guo, R.; Xu, F.; Liu, S.; Ye, Q.; Zhou, F. Nitrogen-doped porous carbon nanospheres derived from hyper-crosslinked polystyrene as lubricant additives for friction and wear reduction. Tribol. Int. 2022, 169, 107458. [Google Scholar] [CrossRef]
- Yaqoob, H.; Teoh, Y.H.; Sher, F.; Jamil, M.A.; Nuhanovi, M.; Razmkhah, O.; Erten, B. Tribological behaviour and lubricating mechanism of tire pyrolysis oil. Coatings 2021, 11, 386. [Google Scholar] [CrossRef]
- Pandey, P.; Somers, A.; Hait, S.; Ramakumar, S. Synthesis of oil miscible novel silane functionalised imidazoline-based ILs as lubricant additives: Characterization and tribological evaluations. Tribol. Int. 2022, 70, 25. Available online: https://link.springer.com/article/10.1007/s11249-022-01567-6 (accessed on 6 January 2023). [CrossRef]
- Zhang, X.J.; Liu, X.X.; Zhang, X.H.; Tian, Y.; Meng, Y.G. Ordering of the 7CB liquid crystal induced by nanoscale confinement and boundary lubrication. Liq. Cryst. 2012, 39, 1305–1313. [Google Scholar] [CrossRef]
- Peng, J.F.; Shen, M.X.; Cai, Z.B. Nano Diesel Soot Particles Reduce Wear and Friction Performance Using an Oil Additive on a Laser Textured Surface. Coatings 2018, 8, 89. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Demir, K.G.; Gu, G.X. Developments in 4D-printing: A review on current smart materials, technologies, and applications. Int. J. Smart. Nano. Mat. 2019, 10, 205–224. [Google Scholar] [CrossRef]
- Jeong, U.; Yin, Y. Smart and responsive micro and nanostructured materials. Adv. Funct. Mater. 2020, 30, 1907059. [Google Scholar] [CrossRef]
- Huang, C.Y. Effect of Thicknesses of Liquid Crystal Layers on Shift of Resonance Frequencies of Metamaterials. Coatings 2021, 11, 578. [Google Scholar] [CrossRef]
- Guo, Y.M.; Li, J.S.; Zhou, X.J.; Tang, Y.Z.; Zeng, X.Q. Formulation of lyotropic liquid crystal emulsion based on natural sucrose ester and its tribological behavior as novel lubricant. Friction 2022, 10, 1879–1892. Available online: https://link.springer.com/article/10.1007/s40544-021-0565-6 (accessed on 6 January 2023). [CrossRef]
- Chiang, W.F.; Lu, Y.Y.; Chen, Y.P.; Lin, X.Y.; Huang, C.Y. Passively Tunable Terahertz Filters Using Liquid Crystal Cells Coated with Metamaterials. Coatings 2021, 11, 381. [Google Scholar] [CrossRef]
- Yang, J.; Yuan, Y.; Li, K.; Amann, C.W.; Yuan, C.Q.; Neville, A. Ultralow friction of 5CB liquid crystal on steel surfaces using a 1,3-diketone additive. Wear 2021, 480–481, 203934. [Google Scholar] [CrossRef]
- Mokshin, V. Tribological capabilities of chiral nematic liquid crystal additives in mineral motor oil. Adv. Mech. Eng. 2020, 12, 1–9. [Google Scholar] [CrossRef]
- Ghosh, P.; Upadhyay, M.; Das, M.K. Studies on the additive performance of liquid crystal blended polyacrylate in lubricating oil. Liq. Cryst. 2013, 41, 30–35. [Google Scholar] [CrossRef]
- Nakano, K. Scaling Law on Molecular Orientation and Effective Viscosity of Liquid-Crystalline Boundary Films. Tribol. Lett. 2003, 14, 17–24. [Google Scholar] [CrossRef]
- Novotná, V.; Hamplová, V.; Kapar, M.; Glogarová, M. New chlorine-substituted ferroelectric liquid crystals with four aromatic rings in the mesogenic core. Liq. Cryst. 2002, 29, 1435–1439. [Google Scholar] [CrossRef]
- Gao, M.; Ma, L.; Luo, J.B. Effect of alkyl chain length on the orientational behavior of liquid crystals nano-film. Tribol. Lett. 2016, 62, 24. [Google Scholar] [CrossRef]
- Qiao, X.X.; Zhang, X.J.; Guo, Y.B.; Yang, S.K.; Tian, Y.; Meng, Y.G. Boundary layer viscosity of CNT-doped liquid crystals: Effects of phase behavior. Rheol. Acta. 2013, 52, 939–947. [Google Scholar] [CrossRef]
- Hird, M. Fluorinated liquid crystals—Properties and applications. Chem. Soc. Rev. 2007, 36, 2070. [Google Scholar] [CrossRef] [PubMed]
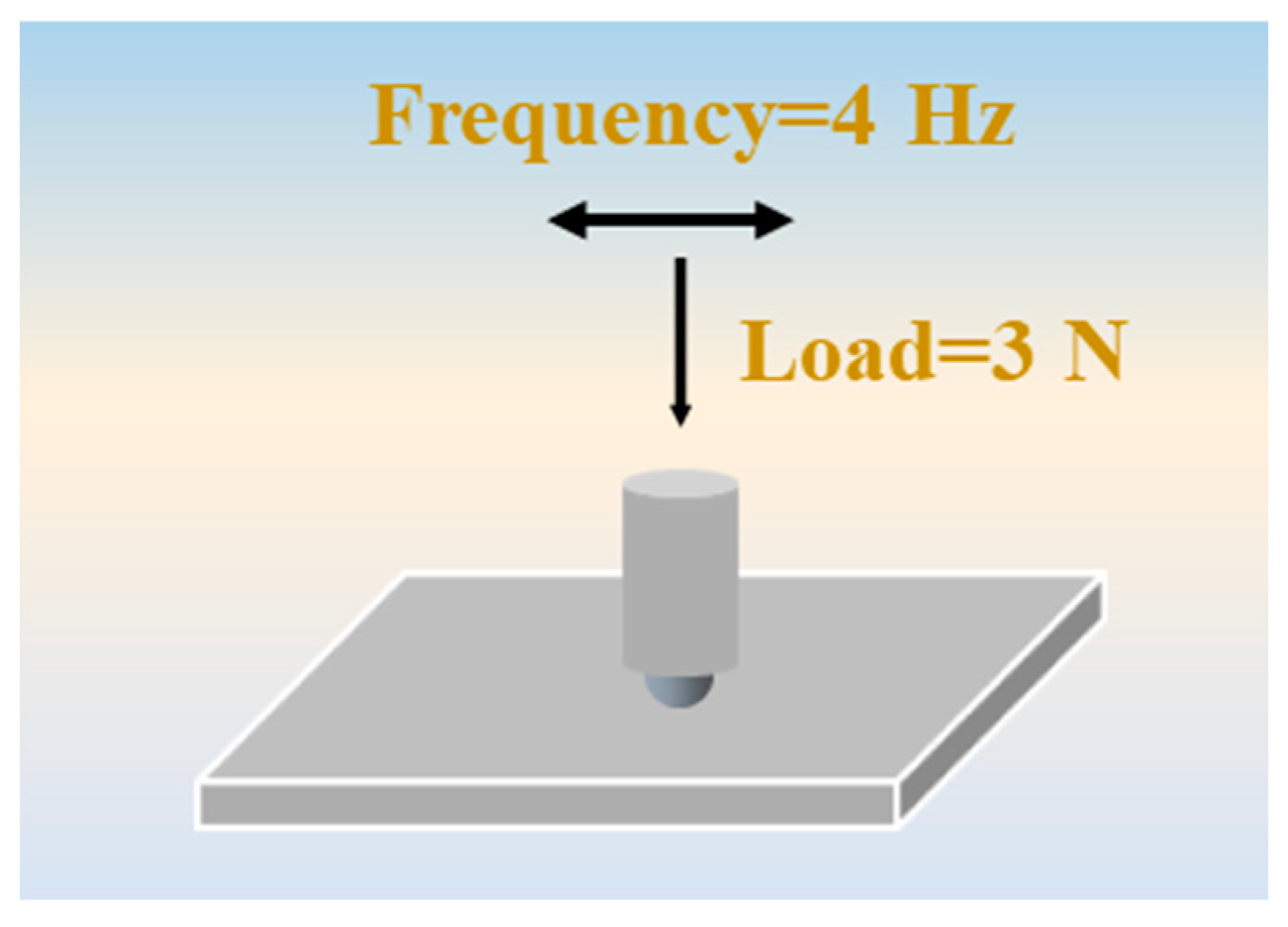


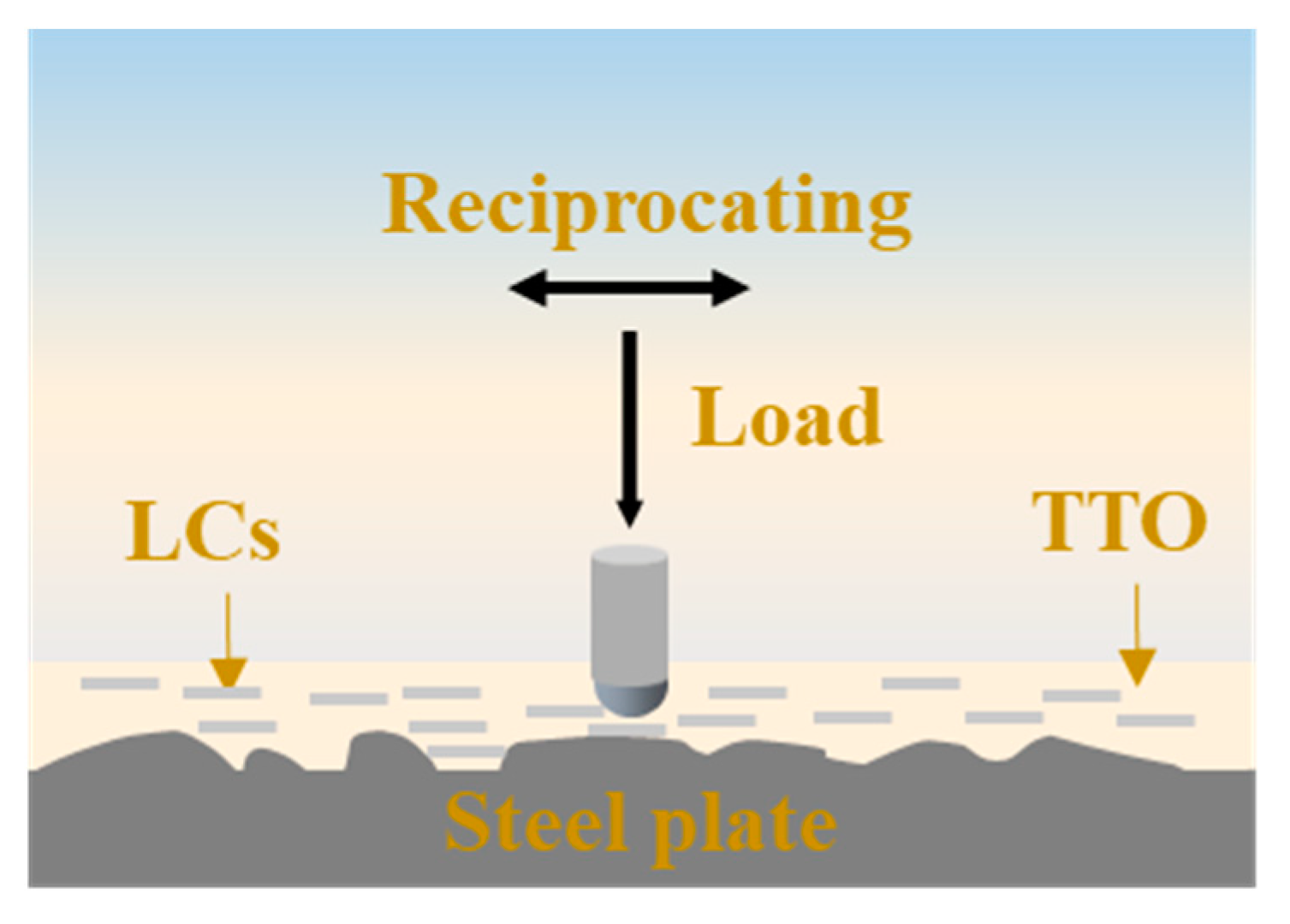
| Load (N) | Frequency (Hz) | Time (min) | Stroke Length (mm) |
|---|---|---|---|
| 3 | 4 | 10 | 10 |
| Samples | Tm (°C) | Td (°C) | ΔT 1 (°C) |
|---|---|---|---|
| LC-A | 33.7 | 120.8 | 87.1 |
| LC-D | 100.5 | 100.9 | 0.4 |
| LC-Z | 37.8 | 86.6 | 48.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Jiang, Y.; Hu, W.; Feng, S.; Li, J. Effect of Mesogenic Phase and Structure of Liquid Crystals on Tribological Properties as Lubricant Additives. Coatings 2023, 13, 168. https://doi.org/10.3390/coatings13010168
Wu H, Jiang Y, Hu W, Feng S, Li J. Effect of Mesogenic Phase and Structure of Liquid Crystals on Tribological Properties as Lubricant Additives. Coatings. 2023; 13(1):168. https://doi.org/10.3390/coatings13010168
Chicago/Turabian StyleWu, Han, Ying Jiang, Wenjing Hu, Sijing Feng, and Jiusheng Li. 2023. "Effect of Mesogenic Phase and Structure of Liquid Crystals on Tribological Properties as Lubricant Additives" Coatings 13, no. 1: 168. https://doi.org/10.3390/coatings13010168







