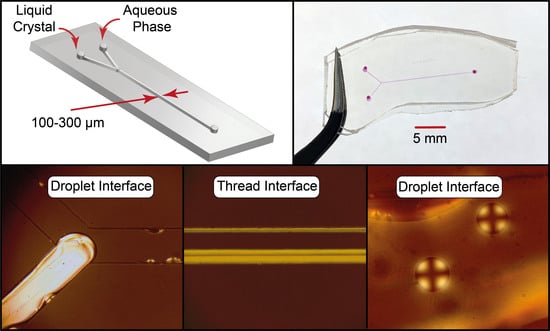
Orientation Behavior of Nematic Liquid Crystals at Flow-Wall Interfaces in Microfluidic Channels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
3.1. Selection of Flow Modes for Generating Microfluidic Liquid Crystal Systems
3.2. Orientation Behavior of Microfluidic Liquid Crystal Systems at Liquid-Wall Interfaces
3.2.1. Single-Phase Flows
3.2.2. Two-Phase Flows
3.3. Orientation Behavior of Microfluidic Liquid Crystal Systems at Liquid-Liquid Interfaces
3.3.1. Comparative Analysis of Mesophase Orientation in Droplets and Threads
3.3.2. Orientation Patterns of Immobilized LC Droplets in Aqueous Flows
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Votava, M.; Ravoo, B.J. Principles and applications of cyclodextrin liquid crystals. Chem. Soc. Rev. 2021, 50, 10009–10024. [Google Scholar] [CrossRef]
- Shang, Y.; Wang, J.; Ikeda, T.; Jiang, L. Bio-inspired liquid crystal actuator materials. J. Mater. Chem. C 2019, 7, 3413–3428. [Google Scholar] [CrossRef]
- Wen, Z.; Yang, K.; Raquez, J.M. A review on liquid crystal polymers in free-standing reversible shape memory materials. Molecules 2020, 25, 1241. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Lub, J.; Schenning, A.P.H.J.; Zhou, G.; de Haan, L.T. Polymer Stabilized Cholesteric Liquid Crystal Siloxane for Temperature-Responsive Photonic Coatings. Int. J. Mol. Sci. 2020, 21, 1803. [Google Scholar] [CrossRef] [Green Version]
- Belmonte, A.; Pilz da Cunha, M.; Nickmans, K.; Schenning, A.P.H.J. Brush-Paintable, Temperature and Light Responsive Triple Shape-Memory Photonic Coatings Based on Micrometer-Sized Cholesteric Liquid Crystal Polymer Particles. Adv. Opt. Mater. 2020, 8, 2000054. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.Q.; Wang, X.Y.; Bisoyi, H.K.; Chen, L.J.; Li, Q. Liquid Crystals in Curved Confined Geometries: Microfluidics Bring New Capabilities for Photonic Applications and beyond. Langmuir 2021, 37, 3789–3807. [Google Scholar] [CrossRef]
- Deng, J.; Han, D.; Yang, J. Applications of microfluidics in liquid crystal-based biosensors. Biosensors 2021, 11, 385. [Google Scholar] [CrossRef]
- Khan, M.; Liu, S.; Qi, L.; Ma, C.; Munir, S.; Yu, L.; Hu, Q. Liquid crystal-based sensors for the detection of biomarkers at the aqueous/LC interface. TrAC Trends Anal. Chem. 2021, 144, 116434. [Google Scholar] [CrossRef]
- Gollapelli, B.; Rama Raju Ganji, S.; Kumar Tatipamula, A.; Vallamkondu, J. Bio-derived chlorophyll dye doped cholesteric liquid crystal films and microdroplets for advanced anti-counterfeiting security labels. J. Mol. Liq. 2022, 363, 119952. [Google Scholar] [CrossRef]
- Gollapelli, B.; Suguru Pathinti, R.; Vallamkondu, J. Carbon Quantum Dots doped Cholesteric Liquid Crystal Films and Microdroplets for Anti-Counterfeiting. ACS Appl. Nano Mater. 2022, 5, 11912–11922. [Google Scholar] [CrossRef]
- Yang, T.; Yuan, D.; Liu, W.; Zhang, Z.; Wang, K.; You, Y.; Ye, H.; de Haan, L.T.; Zhang, Z.; Zhou, G. Thermochromic Cholesteric Liquid Crystal Microcapsules with Cellulose Nanocrystals and a Melamine Resin Hybrid Shell. ACS Appl. Mater. Interfaces 2022, 14, 4588–4597. [Google Scholar] [CrossRef] [PubMed]
- Mochalov, K.; Bobrovsky, A.; Solovyeva, D.; Samokhvalov, P.; Nabiev, I.; Oleinikov, V. Microstructure and optical properties of composites consisting of nanoporous stretched polypropylene doped with liquid crystals and quantum dots at a high concentration. Orient. J. Chem. 2016, 32, 2863–2872. [Google Scholar] [CrossRef] [Green Version]
- Pandey, S.; Vimal, T.; Singh, D.P.; Gupta, S.K.; Mahamuni, S.; Srivastava, A.; Manohar, R. Analysis of physical parameters and collective dielectric relaxations in core/shell quantum dot ferroelectric liquid crystal composite. J. Mol. Liq. 2015, 211, 157–163. [Google Scholar] [CrossRef]
- Osipova, V.V.; Kurilov, A.D.; Galyametdinov, Y.G.; Muravsky, A.A.; Kumar, S.; Chausov, D.N. Optical Properties of Nematic Liquid Crystal Composites with Semiconducting Quantum Dots. Zhidkie Krist. I Ikh Prakt. Ispol’zovanie 2020, 20, 84–92. [Google Scholar] [CrossRef]
- Liu, D.; Broer, D.J. Surface dynamics and mechanics in liquid crystal polymer coatings. In Proceedings of the SPIE—The International Society for Optical Engineering, San Francisco, CA, USA, 7–12 February 2015. [Google Scholar]
- Fuh, A.Y.G.; Chen, K.N.; Wu, S.T. Smart electro-optical iris diaphragm based on liquid crystal film coating with photoconductive polymer of poly(N-vinylcarbazole). Appl. Opt. 2016, 55, 6034–6039. [Google Scholar] [CrossRef]
- Yoon, H.J.; Lee, D.; Yang, J.; Song, J.K. Self-aligned liquid crystal smart windows with dual modes. J. Mol. Liq. 2022, 348, 118014. [Google Scholar] [CrossRef]
- Kurylo, I.; van der Tol, J.; Colonnese, N.; Broer, D.J.; Liu, D. Photo-responsive liquid crystal network-based material with adaptive modulus for haptic application. Sci. Rep. 2022, 12, 19512. [Google Scholar] [CrossRef]
- Kurilov, A.D.; Chausov, D.N.; Osipova, V.V.; Kucherov, R.N.; Belyaev, V.V.; Galyametdinov, Y.G. Highly luminescent nanocomposites of nematic liquid crystal and hybrid quantum dots CdSe/CdS with ZnS shell. J. Mol. Liq. 2021, 339, 116747. [Google Scholar] [CrossRef]
- Sengupta, A. Topological microfluidics: Present and prospects. Liq. Cryst. Today 2015, 24, 70–80. [Google Scholar] [CrossRef]
- Kim, J.-W.; Oh, Y.; Lee, S.; Kim, S.-H. Thermochromic Microcapsules Containing Chiral Mesogens Enclosed by Hydrogel Shell for Colorimetric Temperature Reporters. Adv. Funct. Mater. 2022, 32, 2107275. [Google Scholar] [CrossRef]
- Sengupta, A.; Tkalec, U.; Ravnik, M.; Yeomans, J.M.; Bahr, C.; Herminghaus, S. Liquid crystal microfluidics for tunable flow shaping. Phys. Rev. Lett. 2013, 110, 048303. [Google Scholar] [CrossRef] [PubMed]
- Sevim, S.; Sorrenti, A.; Franco, C.; Furukawa, S.; Pané, S.; Demello, A.J.; Puigmartí-Luis, J. Self-assembled materials and supramolecular chemistry within microfluidic environments: From common thermodynamic states to non-equilibrium structures. Chem. Soc. Rev. 2018, 47, 3788–3803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Chen, Q.; Ma, Y.; Sun, J. Microfluidic Methods for Fabrication and Engineering of Nanoparticle Drug Delivery Systems. ACS Appl. Bio Mater. 2020, 3, 107–120. [Google Scholar] [CrossRef]
- Seiffert, S. Microfluidics and Macromolecules: Top-Down Analytics and Bottom-Up Engineering of Soft Matter at Small Scales. Macromol. Chem. Phys. 2017, 218, 1600280. [Google Scholar] [CrossRef]
- Cohen, C.; Giles, R.; Sergeyeva, V.; Mittal, N.; Tabeling, P.; Zerrouki, D.; Baudry, J.; Bibette, J.; Bremond, N. Parallelised production of fine and calibrated emulsions by coupling flow-focusing technique and partial wetting phenomenon. Microfluid. Nanofluid. 2014, 17, 959–966. [Google Scholar] [CrossRef]
- Gwon, S.; Park, S. Preparation of uniformly sized interpenetrating polymer network polyelectrolyte hydrogel droplets from a solid-state liquid crystal shell. J. Ind. Eng. Chem. 2021, 99, 235–245. [Google Scholar] [CrossRef]
- Dreyfus, R.; Tabeling, P.; Willaime, H. Ordered and Disordered Patterns in Two-Phase Flows in Microchannels. Phys. Rev. Lett. 2003, 90, 4. [Google Scholar] [CrossRef] [Green Version]
- Zilio, C.; Sola, L.; Damin, F.; Faggioni, L.; Chiari, M. Universal hydrophilic coating of thermoplastic polymers currently used in microfluidics. Biomed. Microdevices 2014, 16, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Babaei, M.; Bonakdar, S.; Nasernejad, B. Selective biofunctionalization of 3D cell-imprinted PDMS with collagen immobilization for targeted cell attachment. Sci. Rep. 2022, 12, 1–9. [Google Scholar]
- Wu, T.; Suzuki, H.; Su, Y.; Tang, Z.; Zhang, L.; Yomo, T. Bio-inspired three-dimensional self-patterning of functional coatings for PDMS microfluidics. Soft Matter 2013, 9, 3473–3477. [Google Scholar] [CrossRef]
- Shakeri, A.; Imani, S.M.; Chen, E.; Yousefi, H.; Shabbir, R.; Didar, T.F. Plasma-induced covalent immobilization and patterning of bioactive species in microfluidic devices. Lab Chip 2019, 19, 3104–3115. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, T.; Mawatari, K.; Kitamori, T. On-chip antibody immobilization for on-demand and rapid immunoassay on a microfluidic chip. Biomicrofluidics 2010, 4, 032207. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Yin, Y.; Yang, Y.; Russell, T.P.; Shi, S. Layer-by-Layer Engineered All-Liquid Microfluidic Chips for Enzyme Immobilization. Adv. Mater. 2022, 34, 2105386. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.; Hoffmann, L.; Müller, J.; Alhorn, P.; Fleger, M.; Neyer, A.; Niemeyer, C.M. Addressable microfluidic polymer chip for DNA-directed immobilization of oligonucleotide-tagged compounds. Small 2009, 5, 1547–1552. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A.; Tkalec, U.; Bahr, C. Nematic textures in microfluidic environment. Soft Matter 2011, 7, 6542–6549. [Google Scholar] [CrossRef]
- Čopar, S.; Kos, Ž.; Emeršič, T.; Tkalec, U. Microfluidic control over topological states in channel-confined nematic flows. Nat. Commun. 2020, 11, 59. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, A.; Herminghaus, S.; Bahr, C. Liquid crystal microfluidics: Surface, elastic and viscous interactions at microscales. Liq. Cryst. Rev. 2014, 2, 73–110. [Google Scholar] [CrossRef]
- Wiese, O.; Marenduzzo, D.; Henrich, O. Microfluidic flow of cholesteric liquid crystals. Soft Matter 2016, 12, 9223–9237. [Google Scholar] [CrossRef] [Green Version]
- Anderson, T.G.; Mema, E.; Kondic, L.; Cummings, L.J. Transitions in Poiseuille flow of nematic liquid crystal. Int. J. Non-Linear Mech. 2015, 75, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Takenaka, Y.; Škarabot, M.; Muševič, I. Nematic Liquid-Crystal Necklace Structure Made by Microfluidics System. Langmuir 2020, 36, 3234–3241. [Google Scholar] [CrossRef]
- Copar, S.; Ravnik, M.; Žumer, S. Introduction to colloidal and microfluidic nematic microstructures. Crystals 2021, 11, 956. [Google Scholar] [CrossRef]
- Śliwa, I.; Maslennikov, P.V.; Zakharov, A.V. Two shear driven flow regimes in microfluidic nematic devices: Tumbling and laminar. J. Mol. Liq. 2021, 340, 117205. [Google Scholar] [CrossRef]
- Shojaei-Zadeh, S.; Anna, S.L. Role of surface anchoring and geometric confinement on focal conic textures in smectic-a liquid crystals. Langmuir 2006, 22, 9986–9993. [Google Scholar] [CrossRef]
- Sargazi, M.; Linford, M.R.; Kaykhaii, M. Liquid Crystals in Analytical Chemistry: A Review. Crit. Rev. Anal. Chem. 2019, 49, 243–255. [Google Scholar] [CrossRef]
- Wang, H.; Xu, T.; Fu, Y.; Wang, Z.; Leeson, M.S.; Jiang, J.; Liu, T. Liquid Crystal Biosensors: Principles, Structure and Applications. Biosensors 2022, 12, 639. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Rather, A.M.; Yao, Y.; Fang, J.-C.; Mamtani, R.S.; Bennett, R.K.A.; Atta, R.G.; Adera, S.; Tkalec, U.; Wang, X. Liquid crystal—Based open surface microfluidics manipulate liquid mobility and chemical composition on demand. Sci. Adv. 2021, 7, eabi7607. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Shao, F.; Xianyu, Y. Microfluidics-Implemented Biochemical Assays: From the Perspective of Readout. Small 2020, 16, 1903388. [Google Scholar] [CrossRef]
- Fan, Y.-J.; Chen, F.-L.; Liou, J.-C.; Huang, Y.-W.; Chen, C.-H.; Hong, Z.-Y.; Lin, J.-D.; Hsiao, Y.-C. Label-Free Multi-Microfluidic Immunoassays with Liquid Crystals on Polydimethylsiloxane Biosensing Chips. Polymers 2020, 12, 395. [Google Scholar] [CrossRef] [Green Version]
- Wang, I.-T.; Lee, Y.-H.; Chuang, E.-Y.; Hsiao, Y.-C. Sensitive, Color-Indicating and Labeling-Free Multi-Detection Cholesteric Liquid Crystal Biosensing Chips for Detecting Albumin. Polymers 2021, 13, 1463. [Google Scholar] [CrossRef]
- Gollapelli, B.; Tatipamula, A.K.; Dewanjee, S.; Pathinti, R.S.; Vallamkondu, J. Detection of bile acids using optical biosensors based on cholesteric liquid crystal droplets. J. Mater. Chem. C 2021, 9, 13991–14002. [Google Scholar] [CrossRef]
- Bao, P.; Paterson, D.A.; Harrison, P.L.; Miller, K.; Peyman, S.; Jones, J.C.; Sandoe, J.; Evans, S.D.; Bushby, R.J.; Gleeson, H.F. Lipid coated liquid crystal droplets for the on-chip detection of antimicrobial peptides. Lab Chip 2019, 19, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-H.; Park, S.-Y. pH-responsive cholesteric liquid crystal double emulsion droplets prepared by microfluidics. Sens. Actuators B Chem. 2017, 241, 636–643. [Google Scholar] [CrossRef]
- Hamlington, B.D.; Steinhaus, B.; Feng, J.J.; Link, D.; Shelley, M.J.; Shen, A.Q. Liquid crystal droplet production in a microfluidic device. Liq. Cryst. 2007, 34, 861–870. [Google Scholar] [CrossRef]
- Priest, C.; Quinn, A.; Postma, A.; Zelikin, A.N.; Ralston, J.; Caruso, F. Microfluidic polymer multilayer adsorption on liquid crystal droplets for microcapsule synthesis. Lab Chip 2008, 8, 2182–2187. [Google Scholar] [CrossRef]
- Xu, S.; Nisisako, T. Polymer Capsules with Tunable Shell Thickness Synthesized via Janus-to-core shell Transition of Biphasic Droplets Produced in a Microfluidic Flow-Focusing Device. Sci. Rep. 2020, 10, 4549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zana, R. Dynamics of Surfactant Self-Assemblies; CRC Press: Boca Raton, FL, USA, 2019; p. 537. [Google Scholar]
- McDonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T.; Wu, H.; Schueller, O.J.A.; Whitesides, G.M. Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Anna, S.L.; Bontoux, N.; Stone, H.A. Formation of dispersions using “flow focusing” in microchannels. Appl. Phys. Lett. 2003, 82, 364–366. [Google Scholar] [CrossRef]
- Link, D.R.; Anna, S.I.; Weitz, D.A.; Stone, H.A. Geometrically Mediated Breakup of Drops in Microfluidic Devices. Phys. Rev. Lett. 2004, 92, 545031–545034. [Google Scholar] [CrossRef] [Green Version]
- Christopher, G.F.; Anna, S.L. Microfluidic methods for generating continuous droplet streams. J. Phys. D Appl. Phys. 2007, 40, R319–R336. [Google Scholar] [CrossRef]
- Anna, S.L. Droplets and Bubbles in Microfluidic Devices. Annu. Rev. Fluid Mech. 2016, 48, 285–309. [Google Scholar] [CrossRef]
- Sarman, S.; Wang, Y.-L.; Laaksonen, A. Non-Newtonian rheological properties of shearing nematic liquid crystal model systems based on the Gay–Berne potential. Phys. Chem. Chem. Phys. 2015, 17, 16615–16623. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.; Majumdar, A.; Ramos, A.M.; Griffiths, I.M. Solution landscapes in nematic microfluidics. Phys. D Nonlinear Phenom. 2017, 351–352, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Bezrukov, A.N.; Osipova, V.V.; Galyametdinov, Y.G. Orientational behavior of a nematic liquid crystal and its composite with quantum dots in a microfluidic channel. Russ. Chem. Bull. 2022, 71, 2092–2097. [Google Scholar] [CrossRef]
- Song, H.; Chen, D.L.; Ismagilov, R.F. Reactions in droplets in microfluidic channels. Angew. Chem. Int. Ed. Engl. 2006, 45, 7336–7356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, K.C.; Hwang, Y.H.; Sheu, T.W.H.; Yusoff, M.Z. A Numerically Consistent Multiphase Poiseuille Flow Computation by a New Particle Method. J. Teknol. 2015, 76, 83–87. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Huang, Y. A GPU-Based δ-Plus-SPH Model for Non-Newtonian Multiphase Flows. Water 2022, 14, 1734. [Google Scholar] [CrossRef]
- Zainali, A.; Tofighi, N.; Shadloo, M.S.; Yildiz, M. Numerical investigation of Newtonian and non-Newtonian multiphase flows using ISPH method. Comput. Methods Appl. Mech. Eng. 2013, 254, 99–113. [Google Scholar] [CrossRef]
- Sinha, N.; Yang, H.; Janse, D.; Hendriks, L.; Rand, U.; Hauser, H.; Köster, M.; van de Vosse, F.N.; de Greef, T.F.A.; Tel, J. Microfluidic chip for precise trapping of single cells and temporal analysis of signaling dynamics. Commun. Eng. 2022, 1, 18. [Google Scholar] [CrossRef]
- Narayanamurthy, V.; Nagarajan, S.; Firus Khan, A.A.Y.; Samsuri, F.; Sridhar, T.M. Microfluidic hydrodynamic trapping for single cell analysis: Mechanisms, methods and applications. Anal. Methods 2017, 9, 3751–3772. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bezrukov, A.; Galyametdinov, Y. Orientation Behavior of Nematic Liquid Crystals at Flow-Wall Interfaces in Microfluidic Channels. Coatings 2023, 13, 169. https://doi.org/10.3390/coatings13010169
Bezrukov A, Galyametdinov Y. Orientation Behavior of Nematic Liquid Crystals at Flow-Wall Interfaces in Microfluidic Channels. Coatings. 2023; 13(1):169. https://doi.org/10.3390/coatings13010169
Chicago/Turabian StyleBezrukov, Artem, and Yury Galyametdinov. 2023. "Orientation Behavior of Nematic Liquid Crystals at Flow-Wall Interfaces in Microfluidic Channels" Coatings 13, no. 1: 169. https://doi.org/10.3390/coatings13010169
APA StyleBezrukov, A., & Galyametdinov, Y. (2023). Orientation Behavior of Nematic Liquid Crystals at Flow-Wall Interfaces in Microfluidic Channels. Coatings, 13(1), 169. https://doi.org/10.3390/coatings13010169