Corrosion Behavior in Saline Solution of Electrodeposited Nanocomposite Zn-CeO2 Coatings Deposited onto Low Alloyed Steel
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Coating Elaboration
2.2. Coating Characterization
2.3. Corrosion Behavior in Saline Solution
2.4. Neutral Salt Spray
3. Results and Discussion
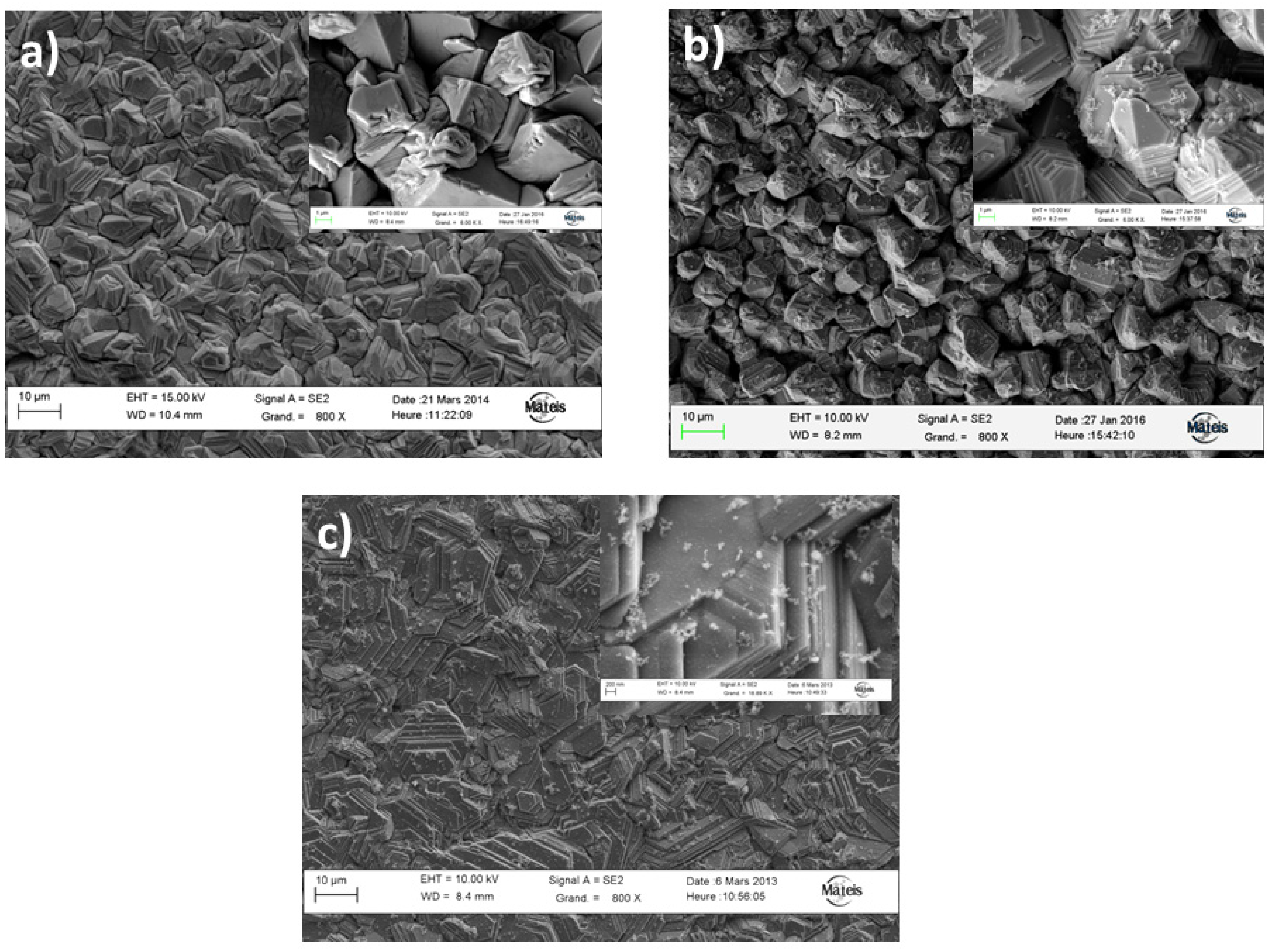
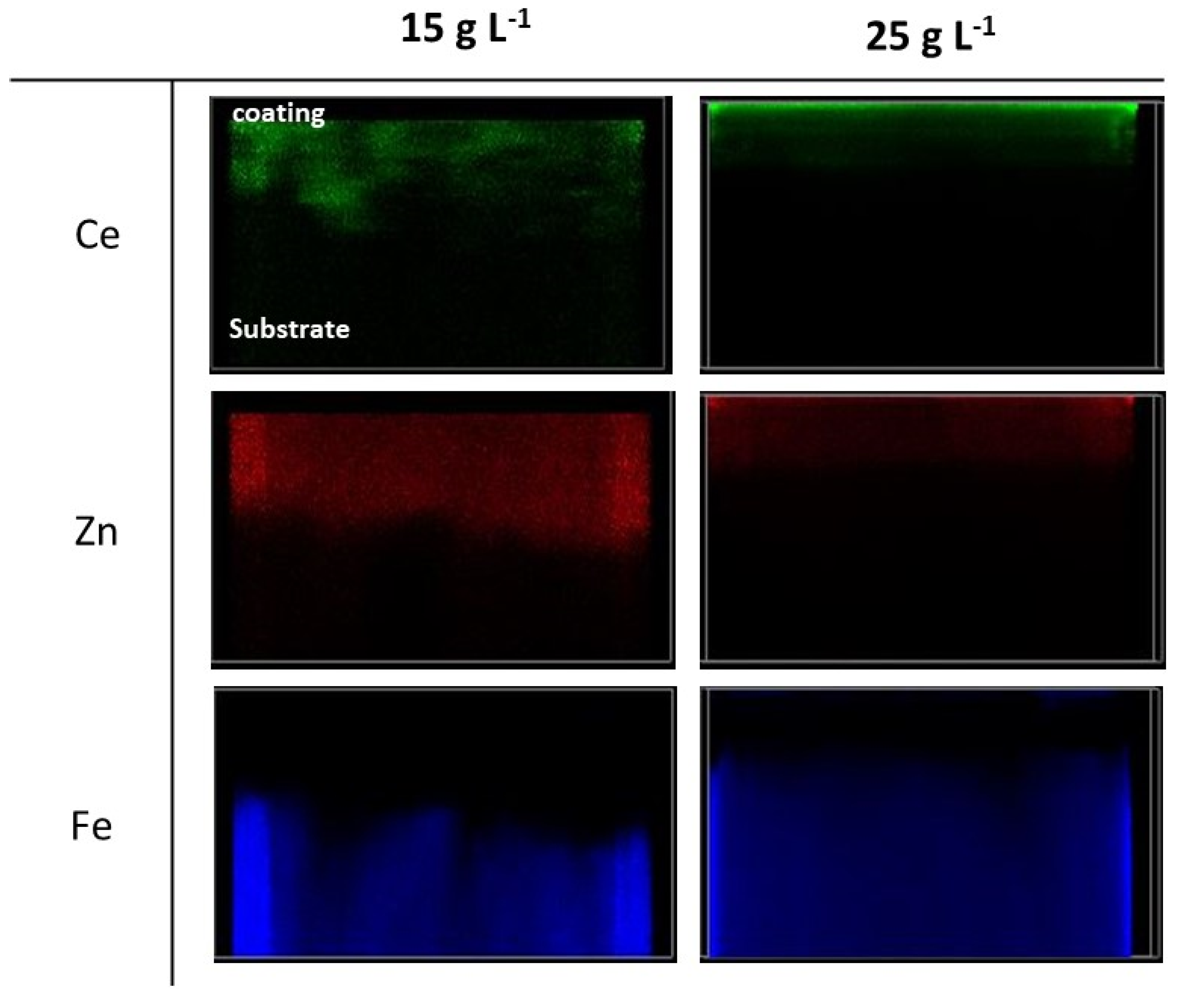
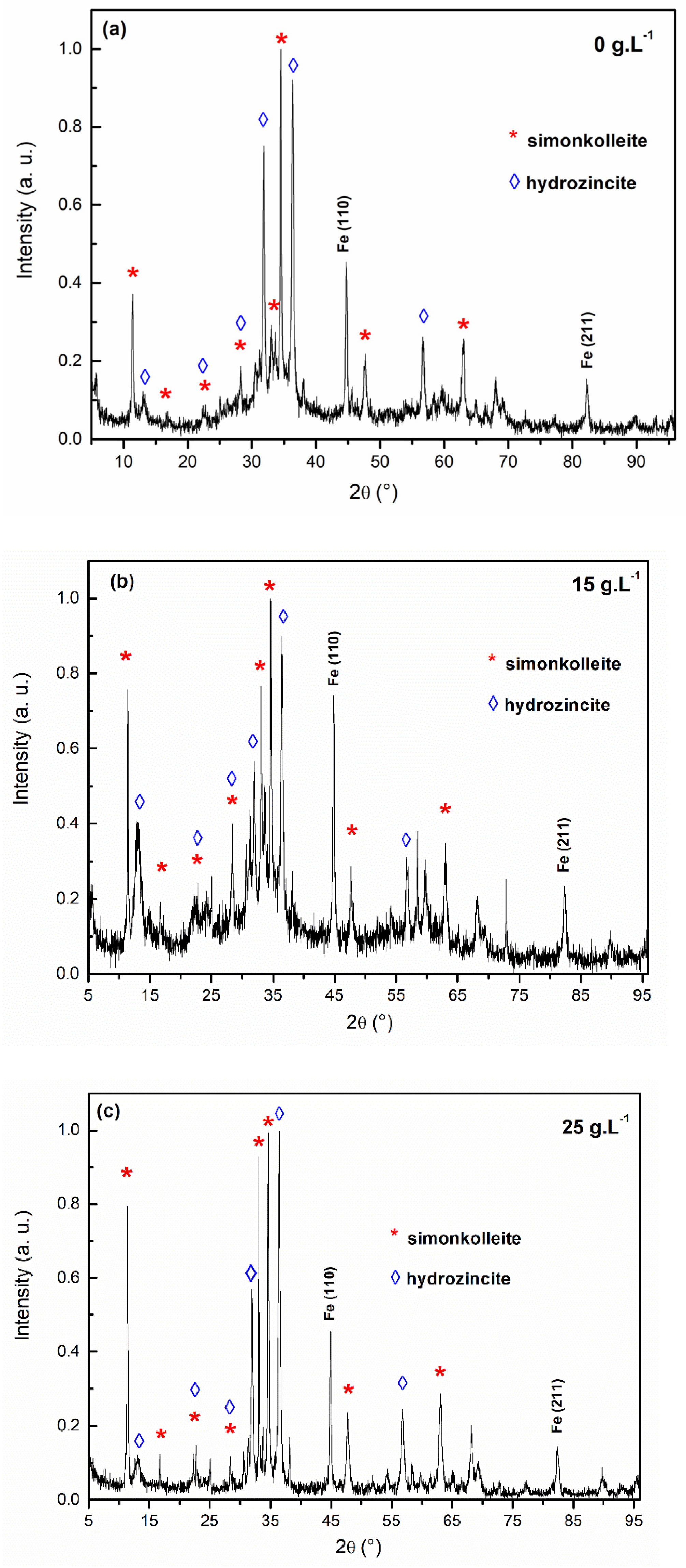
3.1. Metallurgical Study
3.2. Corrosion Properties of Nanocomposites Zn-Ceria Coatings
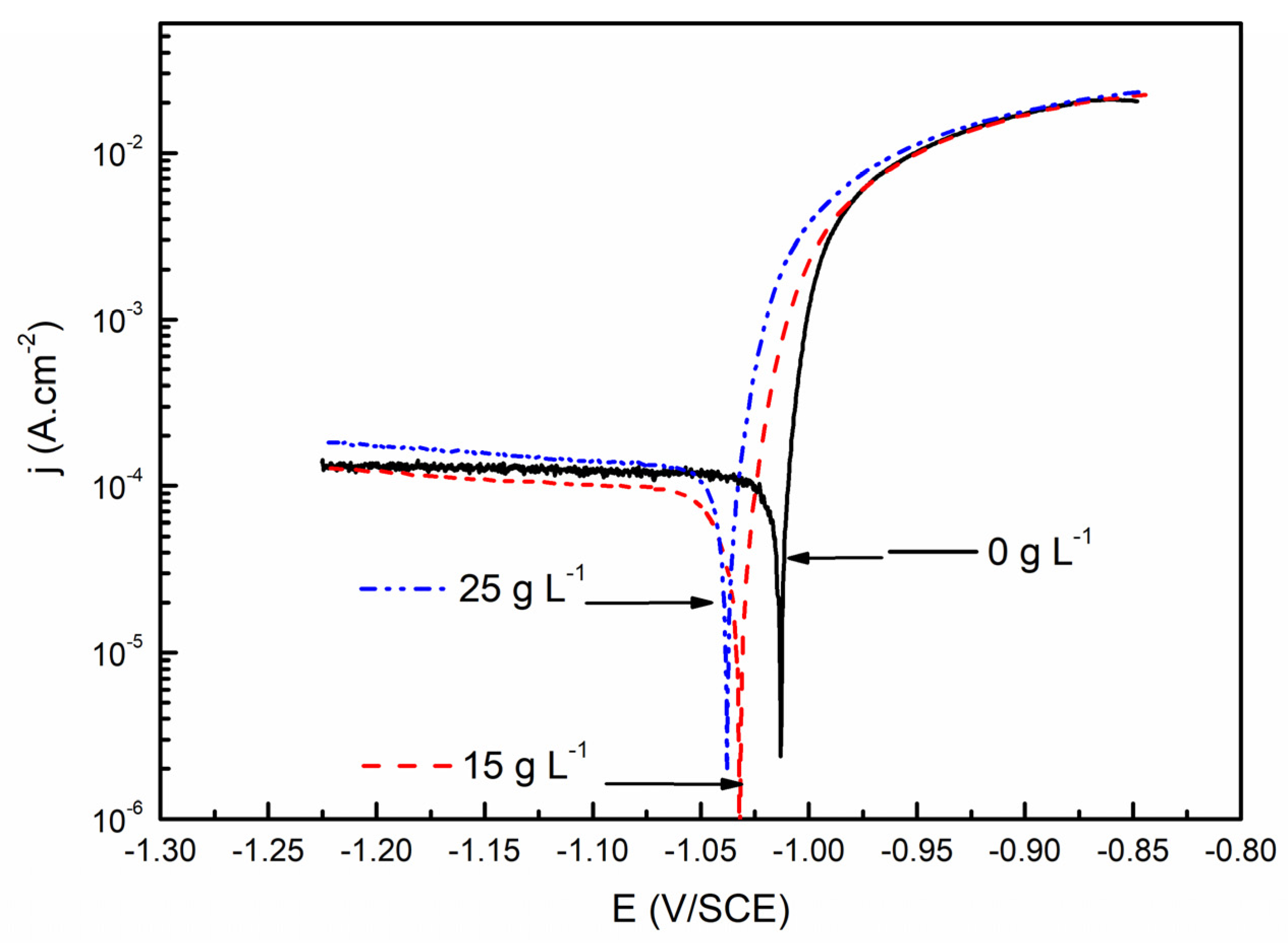
3.2.1. Polarization Curves after One Hour of Immersion in Saline Solution
3.2.2. OCP and Rp Evolutions during Extended Immersion Tests
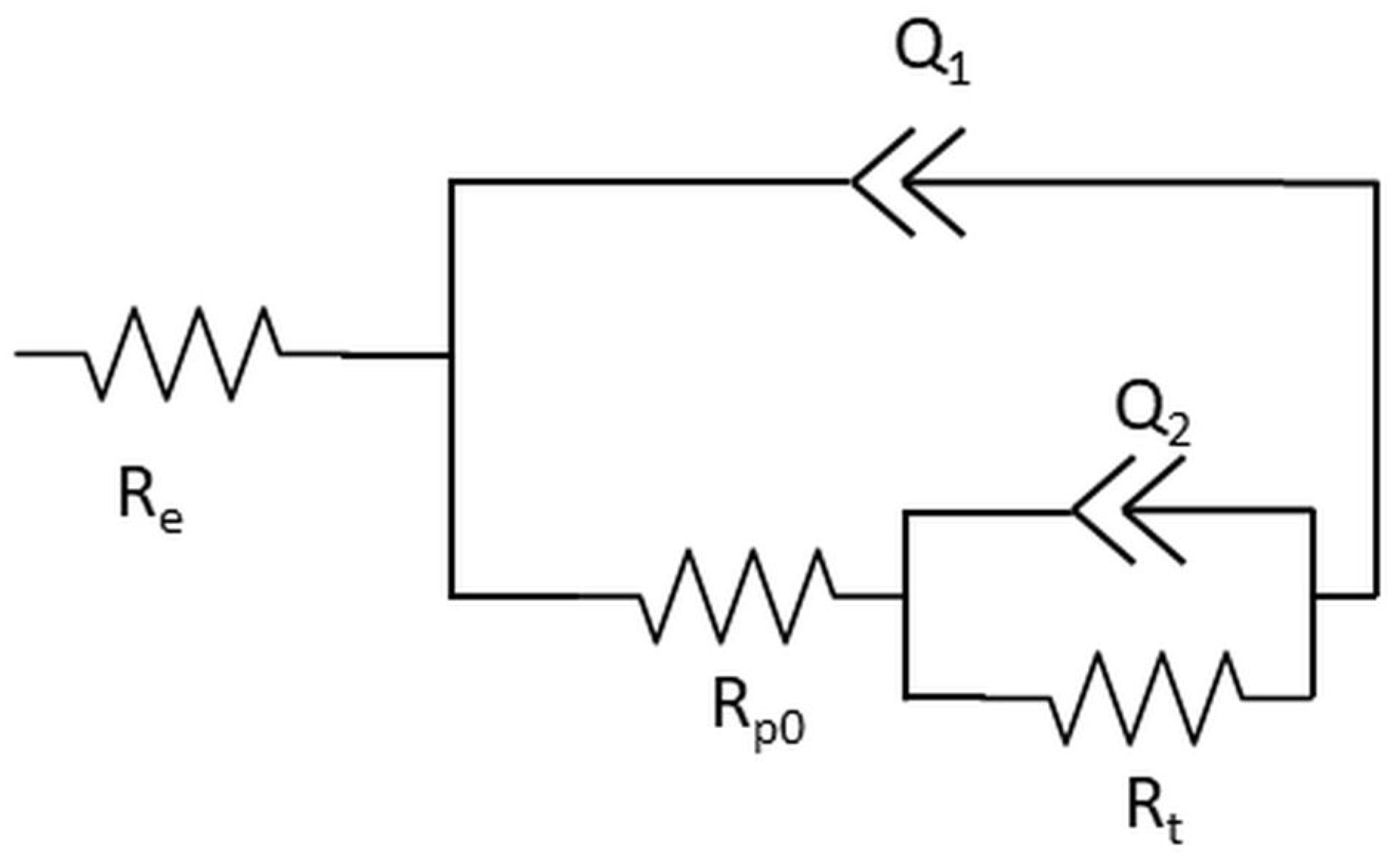
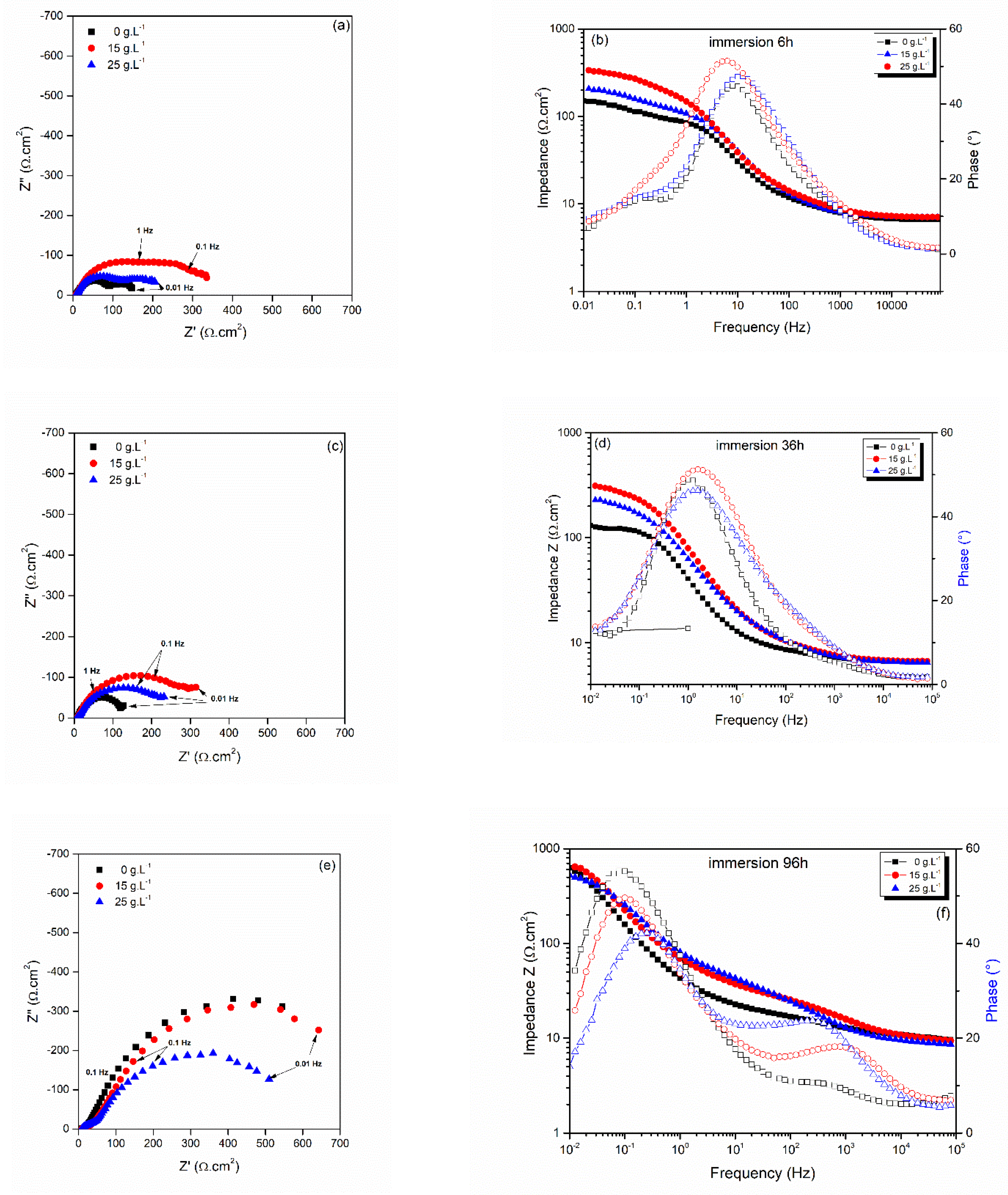
3.2.3. Electrochemical Impedance Spectroscopy during Extended Immersion Tests
3.3. Salt Spray Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, H.; Szpunar, J.A. The role of texture and morphology in optimizing the corrosion resistance of zinc-based electrogalvanized coatings. Corros. Sci. 1998, 40, 525–545. [Google Scholar] [CrossRef]
- Landner, L.; Reuther, R. Metals in Society and in the Environment: A Critical Review of Current Knowledge on Fluxes, Speciation, Bioavailability and Risk for Adverse Effects of Copper, Chromium, Nickel and Zinc; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Bodar, C.W.M.; Pronk, M.E.J.; Sijm, D.T.H.M. The european union risk assessment on zinc and zinc compounds: The process and the facts. Integr. Environ. Assess. 2005, 1, 301–319. [Google Scholar] [CrossRef]
- Gavrila, M.; Millet, J.P.; Mazille, H.; Marchandise, D.; Cuntz, J.M. Corrosion behavior of zinc-nickel coatings, electrodeposited on steel. Surf. Coat. Technol. 2000, 123, 164–172. [Google Scholar] [CrossRef]
- Bielawski, M. Alternative technologies and coatings for electroplated cadmium and hard chromium. Can. Aeronaut. Space J. 2010, 56, 67–80. [Google Scholar] [CrossRef]
- Barcelo, G.; Garcia, J.; Sarret, M.; Muller, C.; Pregonas, J. Properties of Zn-Ni alloy deposits from ammonium baths. J. Appl. Electrochem. 1994, 24, 1249–1255. [Google Scholar] [CrossRef]
- Muller, C.; Sarret, M.; Andreu, T. Electrodeposition of Zn-Mn alloys at low current densities. J. Electrochem. Soc. 2002, 149, C600–C606. [Google Scholar] [CrossRef]
- Sylla, D.; Savall, C.; Gadouleau, M.; Rebere, C.; Creus, J.; Refait, P. Electrodeposition of Zn-Mn alloys on steel using an alkaline pyrophosphate-based electrolytic bath. Surf. Coat. Technol. 2005, 200, 2137–2145. [Google Scholar] [CrossRef]
- Amirat, S.; Rehamnia, R.; Bordes, M.; Creus, J. Zn-Fe electrodeposition from chloride bath: Influence of deposition parameters on coatings morphology and structure. Mater. Corros. 2013, 64, 328–334. [Google Scholar] [CrossRef]
- Arrighi, C.; Savall, C.; Cohendoz, S.; Grosseau-Poussard, J.L.; Baissac, L.; Olivier, M.-G.; Creus, J. Optimization of the morphology, structure and properties of high iron content Zn-Fe coatings by pulse electrodeposition. Mater. Chem. Phys. 2021, 263, 124366. [Google Scholar] [CrossRef]
- Low, C.T.J.; Wills, R.G.A.; Walsh, F.C. Electrodeposition of composite coatings containing nanoparticles in a metal deposit. Surf. Coat.Technol. 2006, 201, 371–383. [Google Scholar] [CrossRef]
- Ahmadkhaniha, D.; Zanella, C. The effects of additives, particles load and current density on codeposition of SiC particles in NiP nanocomposite coatings. Coatings 2019, 9, 554. [Google Scholar] [CrossRef]
- Frade, T.; Gomes, A.; Da Silva Pereira, M.I.; Alberts, D.; Pereiro, R.; Fernández, B. Studies on the Stability of Zn and Zn – TiO2 Nanocomposite Coatings Prepared by Pulse Reverse Current. J. Electrochem. Soc. 2011, 158, C63–C70. [Google Scholar] [CrossRef]
- Anwar, S.; Khan, F.; Zhang, Y.; Caines, S. Zn composite corrosion resistance coatings: What works and what does not work? J. Loss Prev. Process Ind. 2021, 69, 104376. [Google Scholar] [CrossRef]
- Xia, X.; Zhitomirsky, I.; McDermid, J.R. Electrodeposition of zinc and composite zinc–yttria stabilized zirconia coatings. J. Mater. Process Technol. 2009, 209, 2632–2640. [Google Scholar] [CrossRef]
- Popoola, A.P.I.; Aigbodion, V.S.; Fayomi, O.S.I. Surface characterization, mechanical properties and corrosion behaviour of ternary based Zn-ZnO-SiO2 composite coating of mild steel. J. Alloys Compd. 2016, 654, 561–566. [Google Scholar] [CrossRef]
- Ghorbani, M.; Salehi, F.; Razavizadeh, O. Enhanced Hardness and Corrosion Resistance of Zn/SiO2 Films by Electrodeposition. J. Electrochem. Soc. 2015, 162, D480–D485. [Google Scholar] [CrossRef]
- Ohgai, T.; Ogushi, K.; Takao, K. Morphology Control of Zn-SiO2 Composite Films Electrodeposited from Aqueous Solution Containing Quaternary Ammonium Cations. J. Phys. Conf. Ser. 2013, 417, 012006. [Google Scholar] [CrossRef]
- Vathsala, K.; Venkatesha, T.V. Zn–ZrO2 nanocomposite coatings: Electrodeposition and evaluation of corrosion resistance. Appl. Surf. Sci. 2011, 257, 8929–8936. [Google Scholar] [CrossRef]
- Al-Dhire, T.M.; Zuhailawati, H.; Anasyida, A.S. Effect of current density on corrosion and mechanical properties of Zn-SiC composite coating. Mater. Today Proc. 2019, 17, 664–671. [Google Scholar] [CrossRef]
- Kanagalasara, V.; Venkatesha, T. Studies on electrodeposition of Zn–MoS2 nanocomposite coatings on mild steel and its properties. J. Solid. State Electrochem. 2012, 16, 993–1001. [Google Scholar] [CrossRef]
- Rekha, M.Y.; Srivastava, C. Electrogalvanization using Zn-graphene oxide composite coatings with enhance corrosion resistance performance. J. Coat. Technol. Res. 2021, 18, 753–760. [Google Scholar] [CrossRef]
- Da, S.; Lopes, C.; Rigoli, I.C.; Rovere, C.A.D.; Da Rocha, C.L.F.; De Souza, C.A.C. Electrodeposition and the properties of a Zn-cotton nanocrystal composite coating. J. Mater. Res. Technol. 2022, 17, 852–864. [Google Scholar]
- Baissac, L.; Buron, C.C.; Hihn, J.-Y.; Chantegrel, L. Electrolytic zinc composite coatings: Ultrasound assisted deposition for morphological and composition modification. ECS Trans. 2019, 92, 25–46. [Google Scholar] [CrossRef]
- Praveen Kumar, C.M.; Gowdru Chandrashekarppa, M.P.; Mahabaleshwar Kulkarni, R.; Yurievich Pimenov, D.; Giasin, K. The effect of Zn and Zn-WO3 composites nanocoatings deposition on hardness and corrosion resistance n steel substrate. Materials 2021, 14, 2253. [Google Scholar] [CrossRef]
- Anwar, S.; Khan, F.; Zhang, Y. Corrosion behaviour of Zn-Ni alloy and Zn-Ni nano-TiO2 composite coatings electrodeposited from ammonium citrate baths. Process Saf. Environ. Prot. 2020, 141, 366–379. [Google Scholar] [CrossRef]
- Safavi, M.S.; Tanhaei, M.; Farshbaf Ahmadipour, M.; Ghaffari Adli, R.; Mahdavi, S.; Walsh, F.C. Electrodeposited Ni-Co alloy-particle composite coatings: A comprehensive review. Surf. Coat. Technol. 2020, 382, 125153. [Google Scholar] [CrossRef]
- Ridosic, M.; Salicio-Paz, A.; Garcia-Lecina, E.; Zabinski, P.; Zivkovic, L.S.; Bajat, J.B. The effect of the ultrasound agitation and source of ceria particles on the morphology and structure of the Zn-Co-CeO2 composite coatings. J. Mater. Res. Technol. 2021, 13, 1336–1349. [Google Scholar] [CrossRef]
- Ridosic, M.; Bucko, M.; Salicio-Paz, A.; Garcia-Lecina, E.; Zivkovic, L.S.; Bajat, J.B. Ceria particles as efficient dopant in the electrodeposition of Zn-Co-CeO2 composite coatings with enhanced corrosion resistance. The effect of current density and particle concentration. Molecules 2021, 26, 4578. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, W. Synthesis of Ni-Co-ZrO2 nanocomposites doped with ceria particles via electrodeposition as highly protective coating. J. Alloy Compds. 2020, 820, 153158. [Google Scholar] [CrossRef]
- Azizi, M.; Schneider, W.; Plieth, W. Electrolytic co-deposition of silicate and mica particles with zinc. J. Solid. State Electrochem. 2005, 9, 429–437. [Google Scholar] [CrossRef]
- Nemeş, P.; Zaharescu, M.; Muresan, L. Initial corrosion behavior of composite coatings obtained by co-electrodeposition of zinc with nanoparticles of Ti and Ce oxides. J. Solid. State Electrochem. 2013, 17, 511–518. [Google Scholar] [CrossRef]
- ASTM B117; Standard Practice for Operating Salt Spray (Fog) Apparatus. ASTM: West Conshohocken, PA, USA, 2019; 11p.
- Ranganatha, S.; Venkatesha, T.V.; Vathsala, K.; Kumar, M.K.P. Electrochemical studies on Zn/nano-CeO2 electrodeposited composite coatings. Surf. Coat. Technol. 2012, 208, 64–72. [Google Scholar] [CrossRef]
- Exbrayat, L.; Steyer, P.; Rébéré, C.; Berziou, C.; Savall, C.; Ayrault, P.; Tertre, E.; Joly-Pottuz, G.L.; Creus, J. Electrodeposition of zinc–ceria nanocomposite coatings in alkaline bath. J. Solid. State Electrochem. 2014, 18, 223–233. [Google Scholar] [CrossRef]
- Vaezi, M.R.; Sachnezhaad, S.K.; Nikzad, L. Electrodeposition on Ni-SiC nanocomposite coatings and evaluation of the wear and corrosion resistance and electroplating characteristics. Colloids Surf. A 2008, 315, 176–182. [Google Scholar] [CrossRef]
- Abdeen, D.H.; El Hachach, M.; Koc, M.; Atieh, M.A. A review on the corrosion behaviour of nanocoatings on metallic substrates. Materials 2019, 12, 210. [Google Scholar] [CrossRef] [PubMed]
- Exbrayat, L.; Calvié, E.; Douillard, T.; Marcos, G.; Savall, C.; Berziou, C.; Creus, J.; Steyer, P. Role of Ceria Nanoparticles on the Electrodeposited Zinc Coating’s Growth: Interest of a TEM-Scale Investigation. ECS Electrochem. Lett. 2014, 3, D33–D35. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Brug, G.J.; Van den Eeden, A.L.G.; SluytersRehbach, M.; Sluyters, J.H. The Analysis of electrodes Impedances Complicated by the Presence of a Constant Phase element. J. Electroanal. Chem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Hsu, C.H.; Mansfeld, F. Concerning the conversion of the constant phase element parameter Y0 into a capacitance. Corrosion 2001, 57, 747–748. [Google Scholar] [CrossRef]
- Ramanauskas, R. Structural factor of Zn alloy electrodeposit corrosion. Appl. Surf. Sci. 1999, 153, 53–64. [Google Scholar] [CrossRef]
- Khorsand, S.; Raeissi, K.; Golozar, M.A. An investigation on the role of texture and surface morphology in the corrosion resistance of zinc electrodeposits. Corros. Sci. 2011, 53, 2676–2678. [Google Scholar] [CrossRef]
- Nayana, K.O.; Venkatesha, T.V. Synergistic effects of additives on morphology, texture and discharge mechanism of zinc during electrodeposition. J. Electroanal. Chem. 2011, 663, 98–107. [Google Scholar] [CrossRef]
- Loto, C.A. Electrodeposition of Zinc from acid based solutions: A review and experimental study. Asian J. Appl. Sci. 2012, 5, 314–326. [Google Scholar] [CrossRef]
- Kondo, K.; Ohgishi, A.; Tanaka, Z. Electrodeposition of Zinc-SiO2 Composite. J. Electrochem. Soc. 2000, 147, 2611–2613. [Google Scholar] [CrossRef]
- Stern, M.; Geary, A.L. Electrochemical polarization I. A theoretical analysis of the shape of polarization curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Meng, Y.; Lui, L.; Zhang, D.; Dong, C.; Yan, Y.; Volinsky, A.A.; Wang, L.-N. Initial formation of corrosion products on pure zinc in saline solution. Bioact. Mater. 2019, 4, 87–96. [Google Scholar] [CrossRef]
- Migani, A.; Vayssilov, G.N.; Bromley, S.T.; Illas, F.; Neyman, K.M. Dramatic reduction of the oxygen vacancy formation energy in ceria particles: A possible key to their remarkable reactivity at the nanoscale. J. Mater. Chem. 2010, 20, 10535–10546. [Google Scholar] [CrossRef]
- Sun, C.; Xue, D. Size-dependent oxygen storage ability of nano-sized ceria. Phys. Chem. Chem. Phys. 2013, 15, 14414–14419. [Google Scholar] [CrossRef]
- Xiang, T.; Zhang, M.; Li, C.; Dong, C.; Yang, L.; Chan, W. CeO2 modified SiO2 acted as additive in electrodeposition of Zn-Ni alloy coating with enhanced corrosion resistance. J. Alloys Compd. 2018, 736, 62–70. [Google Scholar] [CrossRef]
- Lakshmanan, P.; Averseng, F.; Bion, N.; Delannoy, L.; Tatibouët, J.-M.; Louis, C. Understanding of the oxygen activation on ceria-and ceria/alumina-supported gold catalysts: A study combining 18O/16O isotopic exchange and EPR spectroscopy. Gold. Bull. 2013, 46, 233–242. [Google Scholar] [CrossRef]
- Sovizi, M.R.; Afshari, M. Effect of nano-zirconia on electrochemical performance, corrosion behavior and microstructure of Al-Mg-Sn-Ga anode for aluminium batteries. J. Alloys Compd. 2019, 792, 1088–1094. [Google Scholar] [CrossRef]
- Vlasa, A.; Varvara, S.; Pop, A.; Bulea, C.; Muresan, L. Electrodeposited Zn–TiO2 nanocomposite coatings and their corrosion behavior. J. Appl. Electrochem. 2010, 40, 1519–1527. [Google Scholar] [CrossRef]
- Sayle, T.X.T.; Caddeo, F.; Zhang, X.; Sakthivel, T.; Das, S.; Seal, S.; Ptasinska, S.; Sayle, D.C. Structure—Activity Map of Ceria Nanoparticles, Nanocubes, and Mesoporous Architectures. Chem. Mater. 2016, 28, 7287–7295. [Google Scholar] [CrossRef]
- Bruix, A.; Neyman, K.M. Modeling ceria-based nanomaterials for catalysis and related applications. Catal. Lett. 2016, 146, 2053–2080. [Google Scholar] [CrossRef]
- Barranco, V.; Feliu Jr, S.; Feliu, S. EIS study of the corrosion behavior of zinc-based coatings on steel in quiescent 3% NaCl solution. Part 1: Directly exposed coatings. Corros. Sci. 2004, 46, 2203–2220. [Google Scholar] [CrossRef]
- El-Mahdy, G.A.; Nishikata, A.; Tsuru, T. Electrochemical corrosion monitoring of galvanized steel under cyclic wet-dry conditions. Corros. Sci. 2000, 42, 183–194. [Google Scholar] [CrossRef]
- Sriraman, K.; Brahimi, S.; Szpunar, J.; Osborne, J.; Yue, S. Characterization of corrosion resistance of electrodeposited Zn–Ni Zn and Cd coatings. Electrochim. Acta 2013, 105, 314–323. [Google Scholar] [CrossRef]
- Sriraman, K.; Manimunda, P.; Chromik, R.; Yue, S. Effect of crystallographic orientation on the tribological behavior of electrodeposited Zn coatings. RSC Adv. 2016, 6, 17360–17372. [Google Scholar] [CrossRef]
- Sajjadnejad, M.; Mozafari, A.; Omidvar, H.; Javanbakht, M. Preparation and corrosion resistance of pulse electrodeposited Zn and Zn-SiC nanocomposite coatings. Appl. Surf. Coat. 2014, 300, 1–7. [Google Scholar] [CrossRef]
- Poupard, S.; Pedraza, F.; Creus, J. Diffusion of a Corroding Electrolyte through Defective Electroplated Ceria Based Coatings. Defect and Diffusion Forum; Trans Tech Publications: Zurich, Switzerland, 2009; Volume 289–292, pp. 235–242. [Google Scholar]
- Martínez, L.; Román, E.; De Segovia, J.; Poupard, S.; Creus, J.; Pedraza, F. Surface study of cerium oxide based coatings obtained by cathodic electrodeposition on zinc. Appl. Surf. Sci. 2011, 257, 6202–6207. [Google Scholar] [CrossRef]
- Hashimoto, S.; Abe, M. The characterization of electrodeposited Zn-SiO2 composites before and after corrosion test. Corros. Sci. 1994, 36, 2125–2137. [Google Scholar] [CrossRef]
- Tang, N.; Van Ooij, W.J.; Górecki, G. Comparative EIS study of pretreatment performance in coated metals. Prog. Org. Coat. 1997, 30, 255–263. [Google Scholar] [CrossRef]
- Jüttner, K. Electrochemical impedance spectroscopy (EIS) of corrosion processes on inhomogeneous surfaces. Electrochim. Acta 1990, 35, 1501–1508. [Google Scholar] [CrossRef]
- Akbarinezhad, E.; Ebrahimi, M.; Faridi, H.R. Corrosion inhibition of steel in sodium chloride solution by undoped polyaniline epoxy blend coating. Prog. Org. Coat. 2009, 64, 361–364. [Google Scholar] [CrossRef]
- Jüttner, K.; Lorenz, W.J.; Kendig, M.W.; Mansfeld, F. Electrochemical Impedance Spectroscopy on 3-D Inhomogeneous Surfaces: Corrosion in Neutral Aerated Solutions. J. Electrochem. Soc. 1988, 135, 332–339. [Google Scholar] [CrossRef]
- Liu, C.; Bi, Q.; Leyland, A.; Matthews, A. An electrochemical impedance spectroscopy study of the corrosion behavior of PVD coated steels in 0.5 M NaCl aqueous solution: Part I. Establishment of equivalent circuits for EIS data modelling. Corros. Sci. 2003, 45, 1243–1256. [Google Scholar] [CrossRef]
- Exbrayat, L.; Rébéré, C.; Ndong Eyame, R.; Steyer, P.; Creus, J. Corrosion behavior in saline solution of pulsed electrodeposited zinc-nickel-ceria nanocomposite coatings. Mater. Corros. 2017, 68, 1129–1142. [Google Scholar] [CrossRef]
- Ramanauskas, R.; Quintana, P.; Maldonado, L.; Pomés, R.; Pech-Canul, M.A. Corrosion resistance and microstructure of electrodeposited Zn and Zn alloy coatings. Surf. Coat. Technol. 1997, 92, 16–21. [Google Scholar] [CrossRef]
- Short, N.R.; Abibsi, A.; Dennis, J.K. Corrosion resistance of electroplated zinc alloy coatings. Trans. Inst. Met. Finish. 1989, 67, 73–77. [Google Scholar] [CrossRef]
- Ligier, V.; Wery, M.; Hihn, J.-Y.; Faucheu, J.; Tachez, M. Formation of the main atmospheric zinc end products: NaZn4Cl(OH)6SO4. 6H2O, Zn4SO4(OH)6. nH2O and Zn4Cl2 (OH) 4SO4. 5H2O in [Cl−] [SO2−4] [HCO−3] [H2O2] electrolytes. Corros. Sci. 1999, 41, 1139–1164. [Google Scholar] [CrossRef]
- Yoo, J.D.; Volovitch, P.; Abdel Aal, A.; Allely, C.; Ogle, K. The effect of an artificially synthesized simonkolleite layer on the corrosion of electrogalvanized steel. Corros. Sci. 2013, 70, 1–10. [Google Scholar] [CrossRef]
- Vasilakopoulos, D.; Bouroushian, M. Electrochemical codeposition of PMMA particles with zinc. Surf. Coat. Technol. 2010, 205, 110–117. [Google Scholar] [CrossRef]




| Compound | Massic Concentration (g L−1) | Molar Concentration (mol L−1) |
|---|---|---|
| ZnCl2 | 63 | 0.45 |
| NH4Cl | 40 | 1.5 |
| SDS (anionic surfactant) | 0.5 | 1.7 × 10−3 |
| CeO2 nanoparticles | 0–25 | 0–0.15 |
| Nanoparticle Concentration in the Electrolytic Bath | Ecorr (V/SCE) | jcorr (μA/cm2) | bA (mV/dec) | bC (V/dec) | Rp (Ω.cm2) |
|---|---|---|---|---|---|
| 0 g L−1 | −1.013 | 100 | 15 | −3.2 | 65 |
| 15 g L−1 | −1.032 | 90 | 25 | −2.4 | 122 |
| 25 g L−1 | −1.038 | 120 | 21 | −1.6 | 76 |
| Time (h) | Re (Ω·cm2) | Rpo (Ω·cm2) | Q1 (Ω−1·cm−2·s−n) | n1 | Rt (Ω·cm2) | Q2 (Ω−1·cm−2·s−n) | n2 | C2 (mF·cm2) | |
|---|---|---|---|---|---|---|---|---|---|
| Zn-CeO2 0 g·L−1 | 1 h | 8 | 91 | 4.1 × 10−4 | 0.91 | 128 | 1.38 × 10−2 | 0.5 | 10.1 |
| 6 h | 8 | 91 | 1.12 × 10−3 | 0.81 | 60 | 0.05 | 0.89 | 53.8 | |
| 36 h | 7 | 130 | 5.6 × 10−3 | 0.8 | 2.5 | ||||
| 60 h | 8 | 616 | 7.8 × 10−3 | 0.81 | 4.1 | ||||
| 96 h | 8 | 973 | 8.8 × 10−3 | 0.78 | 4.2 | ||||
| Zn-CeO2 15 g·L−1 | 1 h | 8 | 168 | 2.8 × 10−4 | 0.78 | 133 | 7.7 × 10−3 | 0.5 | 7.1 |
| 6 h | 7 | 73 | 1.2 × 10−3 | 0.99 | 329 | 4. × 10−3 | 0.54 | 1.2 | |
| 36 h | 7 | 157 | 2.8 × 10−3 | 0.88 | 483 | 1.5 × 10−2 | 0.5 | 26.7 | |
| 60 h | 8 | 293 | 5.0 × 10−3 | 0.7 | 1.2 | ||||
| 96 h | 8 | 800 | 6.0 × 10−3 | 0.76 | 2.3 | ||||
| Zn-CeO2 25 g·L−1 | 1 h | 8 | 92 | 4.1 × 10−4 | 0.81 | 112 | 2 × 10−2 | 0.6 | 29.6 |
| 6 h | 8 | 73 | 4.1 × 10−4 | 0.9 | 211 | 0.1 | 0.6 | 542.4 | |
| 36 h | 7 | 96 | 4.1 × 10−4 | 0.89 | 166 | 1.0 × 10−2 | 0.6 | 6.1 | |
| 60 h | 8 | 357 | 7.4 × 10−3 | 0.63 | 1.3 | ||||
| 96 h | 8 | 600 | 3.6 × 10−5 | 0.71 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Exbrayat, L.; Rébéré, C.; Milet, R.; Calvié, E.; Steyer, P.; Creus, J. Corrosion Behavior in Saline Solution of Electrodeposited Nanocomposite Zn-CeO2 Coatings Deposited onto Low Alloyed Steel. Coatings 2023, 13, 1688. https://doi.org/10.3390/coatings13101688
Exbrayat L, Rébéré C, Milet R, Calvié E, Steyer P, Creus J. Corrosion Behavior in Saline Solution of Electrodeposited Nanocomposite Zn-CeO2 Coatings Deposited onto Low Alloyed Steel. Coatings. 2023; 13(10):1688. https://doi.org/10.3390/coatings13101688
Chicago/Turabian StyleExbrayat, Loïc, Christelle Rébéré, Rémy Milet, Emilie Calvié, Philippe Steyer, and Juan Creus. 2023. "Corrosion Behavior in Saline Solution of Electrodeposited Nanocomposite Zn-CeO2 Coatings Deposited onto Low Alloyed Steel" Coatings 13, no. 10: 1688. https://doi.org/10.3390/coatings13101688





