Characterization of Nanoparticles in Antimicrobial Coatings for Medical Applications—A Review
Abstract
:1. Antimicrobial Coatings with Metal and Metal Oxide Nanoparticles
1.1. Silver Nanoparticles in Antimicrobial Coatings
1.2. Titanium Oxide Nanoparticles in Antimicrobial Coatings
1.3. Zinc Oxide and Magnesium Oxide in Antimicrobial Coatings
1.4. Chromium Oxide NPs in Antimicrobial Coatings
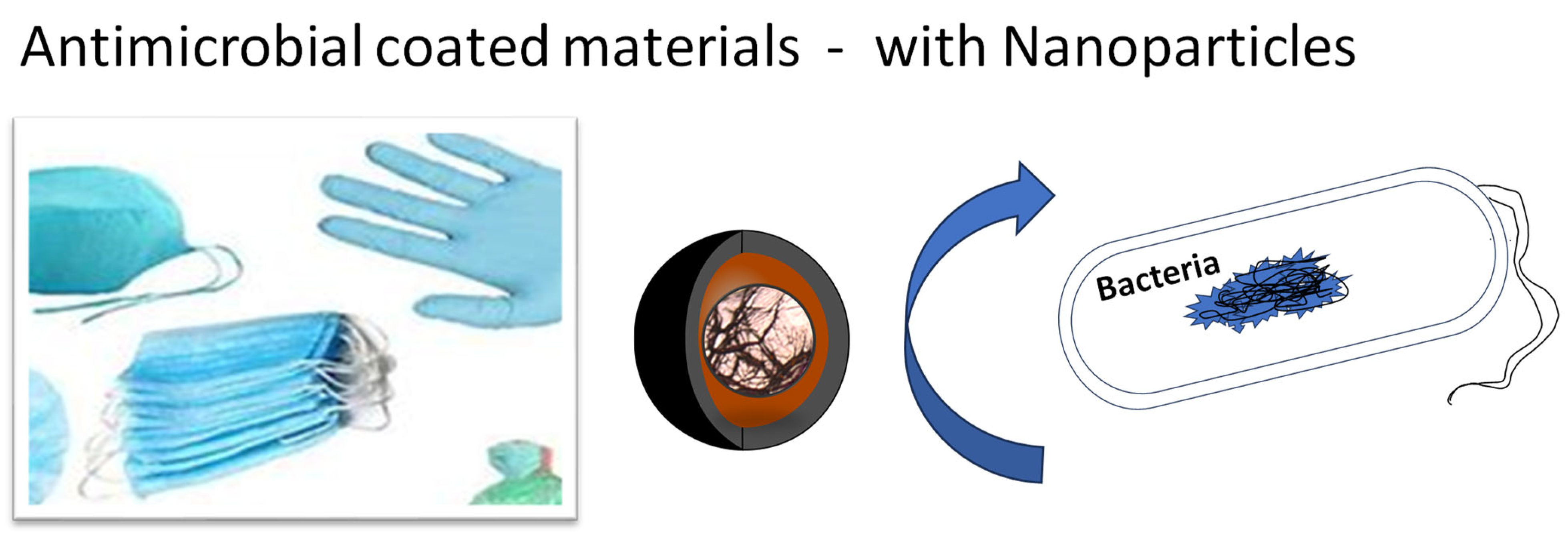
2. Antimicrobial Coatings on Polymers
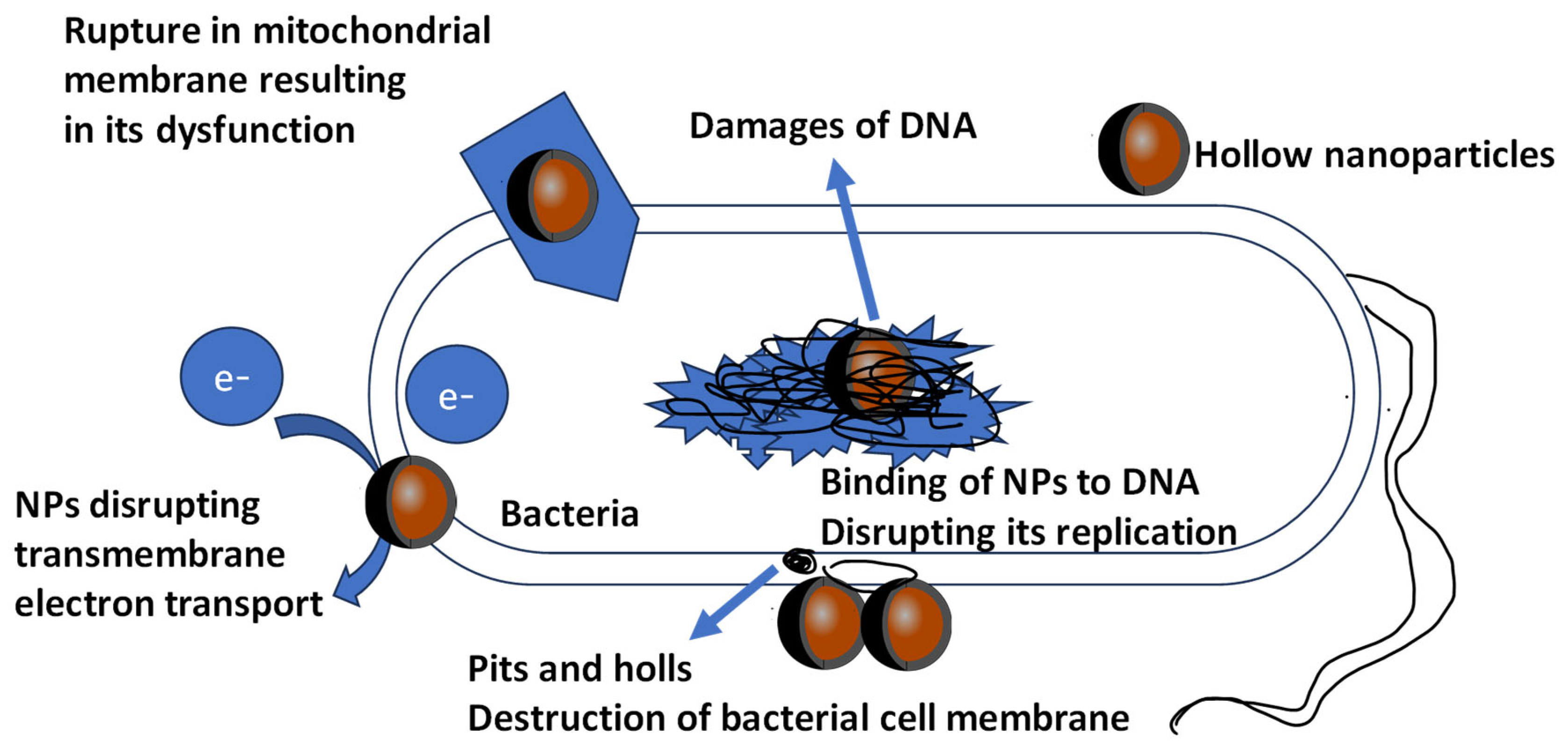
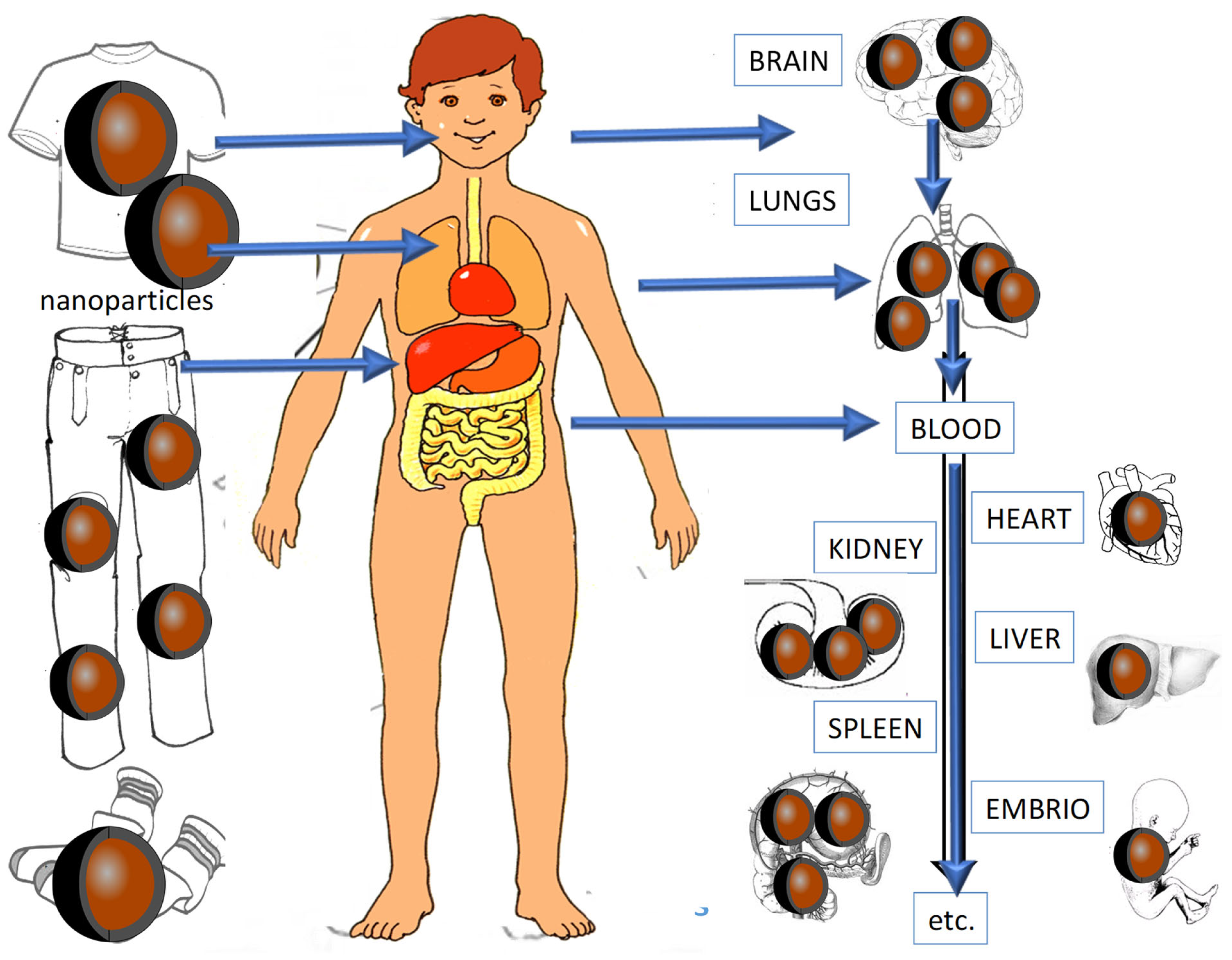
3. Toxic Effects of Antimicrobial NPs from Coatings
4. Separation Methods of Antimicrobial NPs in Coatings
5. Instrumental Analysis of Antimicrobial NPs in Coatings
6. Microscopical Investigation of Antimicrobial NPs in Coatings
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Acoustic spectroscopy | (AS) |
| Analytical electron microscopy | (AEM) |
| Asymmetric flow field-flow fractionation | (AF4) |
| Atmospheric pressure photo-ionization mass spectrometry | (APPI-MS) |
| Atomic force microscopy | (AFM) |
| Capillary electrophoresis | (CE) |
| Confocal laser scanning microscopy | (CLSM) |
| Cross-flow filtration | (CFF) |
| Dynamic light scattering | (DLS) |
| Electron microscopy | (EM) |
| Energy dispersive X-ray spectroscopy | (EDS) |
| Antimicrobial nanoparticles | (NPs) |
| Environmental scanning electron microscopy | (ESEM) |
| Field-flow fractionation | (FFF) |
| Flame atomic absorption spectrometry | (F-AAS) |
| Graphite furnace atomic absorption spectrometry | (GF-AAS) |
| High-performance liquid chromatography | (HPLC) |
| Hydrodynamic chromatography | (HDC) |
| Infrared spectroscopy | (IR) |
| Inductively coupled plasma mass spectrometry | (ICP-MS) |
| Inductively coupled plasma–optical emission spectrometry | (ICP-OES) |
| Ion-exchange chromatography | (IEC) |
| Laser ablation ICP-MS | (LA-ICP-MS) |
| Laser desorption/ionization time-of-flight-mass spectrometry | (LDI-TOF-MS) |
| Laser Doppler velocimetry | (LDV) |
| Liquid chromatography | (LC) |
| Matrix-assisted laser desorption/ionization-time-of-flight-mass spectrometry | (MALDI-TOF-MS) |
| Nanoparticles | (NP) |
| Nanoparticle-tracking analysis | (NTA) |
| Nuclear magnetic resonance spectroscopy | (NMR) |
| Photon correlation spectroscopy | (PCS) |
| Raman spectroscopy | (RS) |
| Scanning electron microscopy | (SEM) |
| Secondary ion mass spectroscopy | (SIMS) |
| Silver nanoparticles | (AgNPs) |
| Size exclusion chromatography | (SEC) |
| Solid-phase extraction | (SPE) |
| Static light scattering | (SLS) |
| Transmission electron microscopy | (TEM) |
| Ultraviolet–visible spectroscopy | (UV-VIS) |
| X-ray photoelectron spectrometry | (XPS) |
| X-ray powder diffraction | (XRPD) |
References
- He, P.; Tang, H.; Zheng, Y.; Xiong, Y.; Cheng, H.; Li, J.; Zhang, Y.; Liu, G. Advances in nanomedicines for lymphatic imaging and therapy. J. Nanobiotechnol. 2023, 24, 292. [Google Scholar] [CrossRef]
- Berhe, M.G.; Gebreslassie, Y.T. Biomedical Applications of Biosynthesized Nickel Oxide Nanoparticles. Int. J. Nanomed. 2023, 27, 4229–4251. [Google Scholar] [CrossRef]
- Rezić, I. Nanoparticles for Biomedical Application and Their Synthesis. Polymers 2022, 14, 4961. [Google Scholar] [CrossRef]
- Han, J.; Ma, Q.; An, Y.; Wu, F.; Zhao, Y.; Wu, G.; Wang, J. The current status of stimuli-responsive nanotechnologies on orthopedic titanium implant surfaces. J. Nanobiotechnol. 2023, 21, 277. [Google Scholar] [CrossRef]
- Rezić, I.; Somogyi Škoc, M.; Majdak, M.; Jurić, S.; Sopko Stracenski, K.; Vinceković, M. Functionalization of Polymer Surface with Antimicrobial Microcapsules. Polymers 2022, 14, 1961. [Google Scholar] [CrossRef]
- Younis, A.B.; Haddad, Y.; Kosaristanova, L.; Smerkova, K. Titanium dioxide nanoparticles: Recent progress in antimicrobial applications, Wiley. Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1860. [Google Scholar] [CrossRef]
- Venkatesan, R.; Rajeswari, N. TiO2 nanoparticles/poly(butylene adipate-co-terephthalate) bionanocomposite films for packaging applications. Polym. Adv. Technol. 2017, 223, 4042. [Google Scholar] [CrossRef]
- Markowicz, A.; Borymski, S.; Adamek, A.; Sułowicz, S. The influence of ZnO nanoparticles on horizontal transfer of resistance genes in lab and soil conditions. Environ. Res. 2023, 223, 115420. [Google Scholar] [CrossRef]
- Kumari, A.; Mandzhieva, S.S.; Minkina, T.M.; Rajput, V.D.; Shuvaeva, V.A.; Nevidomskaya, D.G.; Kirichkov, M.V.; Veligzhanin, A.A.; Svetogorov, R.D.; Khramov, E.V.; et al. Speciation of macro- and nanoparticles of Cr2O3 in Hordeum vulgare L. and subsequent toxicity: A comparative study. Environ. Res. 2023, 223, 115485. [Google Scholar] [CrossRef]
- Stolar, T.; Lukin, S.; Etter, M.; Rajić Linarić, M.L.; Užarević, K.; Meštrović, E.; Halasz, I. DNA-specific selectivity in pairing of model nucleobases in the solid state. Chem. Commun. 2020, 56, 13524. [Google Scholar] [CrossRef]
- Martinaga Pintarić, L.; Somogi Škoc, M.; Ljoljić Bilić, V.; Pokrovac, I.; Kosalec, I.; Rezić, I. Synthesis, Modification and Characterization of Antimicrobial Textile Surface Containing ZnO Nanoparticles. Polymers 2020, 12, 1210. [Google Scholar] [CrossRef]
- Vigneshwaran, N.; Kathe, A.A.; Varadarajan, P.V.; Nachane, R.P.; Balasubramanya, R.H.J. Functional finishing of cotton fabrics using silver nanoparticles. Nanosci. Nanotechnol. 2007, 7, 1893. [Google Scholar] [CrossRef]
- Ahamed, M.; Posgai, R.; Gorey, T.J.; Nielsen, M.; Hussain, S.M.; Rowe, J.J. Silver nanoparticles induced heat shock protein. Toxicol. Appl. Pharmacol. 2010, 242, 263. [Google Scholar] [CrossRef]
- Kasemets, K.; Ivask, A.; Dubourguier, H.C.; Kahru, A. Toxicity of nanoparticles of ZnO, CuO and TiO2 to yeast Saccharomyces cerevisiae. Toxicol. Vitr. 2009, 23, 1116. [Google Scholar] [CrossRef]
- Rezić, I.; Majdak, M.; Ljoljić Bilić, V.; Pokrovac, I.; Martinaga, L.; Somogyi Škoc, M.; Kosalec, I. Development of Antibacterial Protective Coatings Active Against MSSA and MRSA on Biodegradable Polymers. Polymers 2021, 13, 659. [Google Scholar] [CrossRef]
- Rahman, M.F.; Wang, J.; Patterson, T.A.; Saini, U.T.; Robinson, B.L.; Newport, G.D.; Murdock, R.C.; Schlager, J.J.; Hussain, S.M.; Ali, S.F. Expression of genes related to oxidative stress in the mouse brain after exposure to silver-25 nanoparticles. Toxicol. Lett. 2009, 187, 15. [Google Scholar] [CrossRef]
- Rezić, I.; Somogyi Škoc, M.; Majdak, M.; Jurić, S.; Sopko Stracenski, K.; Vlahoviček-Kahlina, K.; Vinceković, M. ICP-MS Determination of Antimicrobial Metals in Microcapsules. Molecules 2022, 27, 3219. [Google Scholar] [CrossRef]
- Vukoja, D.; Vlainić, J.; Ljoljić Bilić, V.; Martinaga, L.; Rezić, I.; Brlek Gorski, D.; Kosalec, I. Innovative Insights into In Vitro Activity of Colloidal Platinum Nanoparticles against ESBL-Producing Strains of Escherichia coli and Klebsiella pneumoniae. Pharmaceutics 2022, 14, 1714. [Google Scholar] [CrossRef]
- Casals, E.; Vazquez-Campos, S.; Bastus, N.G.; Puntes, V. Distribution and potential toxicity of engineered inorganic nanoparticles. Trends Anal. Chem. 2008, 27, 672. [Google Scholar] [CrossRef]
- Kirchner, C.; Liedl, T.; Kudera, S.; Pellegrino, T.; Javier, A.M.; Gaub, H.E.; Stolzle, S.; Fertig, N.; Parak, W.J. Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles. Nano Lett. 2005, 5, 331. [Google Scholar] [CrossRef]
- Hoshino, A.; Fujioka, K.; Oku, T.; Suga, M.; Sasaki, Y.F.; Ohta, T.; Yasuhara, M.; Suzuki, K.; Yamamoto, K. Bright and Stable Core−Shell Fluorescent Silica Nanoparticles. Nano Lett. 2004, 4, 2163. [Google Scholar] [CrossRef]
- Service, R.F. Is Nanotechnology Dangerous? Science 2000, 290, 1526. [Google Scholar] [CrossRef]
- Gatti, A.M. Biocompatibility of micro- and nano-particles in the colon. Biomaterials 2004, 25, 385. [Google Scholar] [CrossRef]
- Green, M.; Howman, E. Semiconductor quantum dots and free radical induced DNA nicking. Chem. Commun. 2005, 121, 121. [Google Scholar]
- Maynard, A.D.; Aitken, R.J.; Butz, T.; Colvin, V.; Donaldson, K.; Oberdörster, G.; Philbert, M.A.; Ryan, J.; Seaton, A.; Stone, V.; et al. Safe handling of nanotechnology. Nature 2006, 444, 267. [Google Scholar] [CrossRef]
- Eom, H.J.; Choi, J. Oxidative stress of silica nanoparticles in human bronchial epithelial cell. Toxicol. Vitr. 2009, 23, 1326. [Google Scholar] [CrossRef]
- Tinkle, S.S.; Antonini, J.M.; Rich, B.A.; Roberts, J.R.; Salmen, R.; DePree, K.; Adkins, E.J. Skin as a route of exposure and sensitization in chronic beryllium disease. Environ. Health Perspect. 2003, 111, 1202. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- EU Communication. Communication from the Commission—Towards a European Strategy for Nanotechnology; European Commision: Brussels, Belgium, 2004; Volume 338, p. 1. [Google Scholar]
- Lo, K.C.; Paau, M.C.; Xiao, D.; Choi, M.M.F. Applications of CE SDS gel in development of biopharmaceutical antibody-based products. Electrophoresis 2008, 29, 2330. [Google Scholar] [CrossRef]
- Liu, F. Analysis and applications of nanoparticles in the separation sciences: A case of gold nanoparticles. J. Chromatogr. A 2009, 1216, 9034. [Google Scholar] [CrossRef]
- Habinovec, I.; Car, Ž.; Ribić, R.; Galić, N.; Novak, P.; Meštrović, E.; Tomić-Pisarović, S. HPLC Monitoring of Acid Catalyzed Conversion of 7-Ethyltryptophol to Methyl Ester of Etodolac. Croat. Chem. Acta 2016, 894, 549–553. [Google Scholar] [CrossRef]
- Šabić Runjavec, M.; Vuković Domanovac, M.; Meštrović, E. Removal of organic pollutants from real pharmaceutical industrial wastewater with environmentally friendly processes. Chem. Pap. 2022, 76, 1423–1431. [Google Scholar] [CrossRef]
- Runje, M.; Babic, S.; Mestrovic, E.; Nekola, I.; Dujmic-Vucinic, Z.; Vojcic, N. Forced degradation of nepafenac: Development and validation of stability indicating UHPLC method. J. Pharm. Biomed. Anal. 2016, 123, 42–52. [Google Scholar] [CrossRef]
- Kammer, F.; Legros, S.; Larsen, E.H.; Löschner, K.; Hofmann, T. Separation and characterization of nanoparticles in complex food and environmental samples by field-flow fractionation. Trends Anal. Chem 2011, 3, 425–436. [Google Scholar] [CrossRef]
- Harča, M.; Habinovec, I.; Meštrović, E.; Biljan, I.; Novak, P. Rapid Identification of Unknown Impurities in 3- Bromo-5-trifluoromethylaniline by LC-SPE/NMR. Croat. Chem. Acta 2016, 89, 543–547. [Google Scholar] [CrossRef]
- Jasprica, I.; Horvat, P.; Zrnc, K.; Bonney, K.J.; Bjornstad, V.; Hok, L.; Vianello, R.; Bregović, N.; Požar, J.; Leko, K.; et al. Utilization of a kinetic isotope effect to decrease decomposition of ceftriaxone in a mixture of D2O/H2O. Eur. J. Pharm. Sci. 2023, 187, 106461. [Google Scholar] [CrossRef]
- Rezić, I. Optimization of ultrasonic extraction of 23 elements from cotton. Ultrason. Sonochem. 2009, 16, 63. [Google Scholar] [CrossRef]
- Jović, F.; Sučec, A.; Nekola, I.; Čavužić, D.; Marcelić, E.; Meštrović, E. Application of Safety by Design Methodology in Evaluating Process Safety for a Duff Reaction Using Predictive Process Simulators. Org. Process Res. Dev. 2015, 19, 1268–1273. [Google Scholar] [CrossRef]
- Biljan, T.; Gajović, A.; Meić, Z.; Meštrović, E. Preparation, characterization and luminescence of nanocrystalline Y2O3:Ho. J. Alloys Compd. 2007, 431, 217–220. [Google Scholar] [CrossRef]
- Doucet, F.J.; Maguire, L.; Lead, J.R. Size fractionation of aquatic colloids and particles by cross-flow filtration: Analysis by scanning electron and atomic force microscopy. Anal. Chim. Acta 2004, 52, 59. [Google Scholar] [CrossRef]
- Meier, F. Online parallel accumulation–serial fragmentation (PASEF) with a novel trapped ion mobility mass spectrometer. Mol. Cell. Proteom. 2018, 17, 2534–2545. [Google Scholar] [CrossRef]
- Rezić, I.; Ćurković, L.; Ujević, M. Simple methods for characterization of metals in historical items. Talanta 2010, 82, 237. [Google Scholar] [CrossRef]
- Guan, B.; Lu, W.; Fang, J.; Cole, R.B. Characterization of synthesized titanium oxide nanoclusters by MALDI-TOF mass spectrometry. J. Am. Soc. Mass Spectrom. 2007, 18, 517. [Google Scholar] [CrossRef]
- Weinberg, H.; Galyean, A.; Leopold, M. Evaluating engineered nanoparticles in natural waters. Trends Anal. Chem. 2011, 30, 72. [Google Scholar] [CrossRef]
- Rezić, I.; Steffan, I. ICP-OES determination of metals present in textile materials. Microchem. J. 2007, 85, 46. [Google Scholar] [CrossRef]
- Rezić, I.; Zeiner, M.; Steffan, I. Determination of 28 selected elements in textiles by axially viewed inductively coupled plasma optical emission spectrometry. Talanta 2011, 83, 865. [Google Scholar] [CrossRef]
- Brar, S.K.; Verma, M. Measurement of nanoparticles by light-scattering techniques. Trends Anal. Chem. 2011, 304, 4–17. [Google Scholar] [CrossRef]
- Gallego-Urrea, J.A.; Tuoriniemi, J.; Hassellöv, M. Size discrimination and detection capabilities of single-particle ICPMS for environmental analysis of silver nanoparticles. Trends Anal. Chem. 2012, 84, 3965–3972. [Google Scholar]
- Rezić, I. Determination of engineered nanoparticles on textiles and in textile wastewaters. Trends Anal. Chem. 2011, 30, 1159–1167. [Google Scholar]
- Bonacucina, G.; Perinelli, D.R.; Cespi, M.; Casettari, L.; Cossi, R.; Blasi, P.; Palmieri, G.F. Acoustic spectroscopy: A powerful analytical method for the pharmaceutical field. Int. J. Pharm. 2016, 503, 174–195. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef]
- Dukhin, A.S. Acoustic Spectroscopy for Particle Size Measurement of Concentrated Nanodispersions. In Micro and Nano Technologies, Characterization of Nanoparticles; Vasile-Dan, H., Wolfgang, E.S., Unger, A., Shard, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 197–211. [Google Scholar]
- Tanaka, N. Elemental Analysis by Electron Microscopes. In Electron Nano-Imaging; Springer: Tokyo, Japan, 2017. [Google Scholar] [CrossRef]
- Vladitsi, M.; Nikolaou, C.; Kalogiouri, N.P.; Samanidou, V.F. Analytical Methods for Nanomaterial Determination in Biological Matrices. Methods Protoc. 2022, 15, 61. [Google Scholar] [CrossRef]
- Alasonati, E.; Caebergs, T.; Pétry, J.; Sebaïhi, N.; Fisicaro, P.; Feltin, N. Size measurement of silica nanoparticles by Asymmetric Flow Field-Flow Fractionation coupled to Multi-Angle Light Scattering: A comparison exercise between two metrological institutes. J. Chrom. A 2021, 1638, 461859. [Google Scholar] [CrossRef]
- Caputo, F.; Mehn, D.; Clogston, J.D.; Rösslein, M.; Prina-Mello, A.; Borgos, S.E.; Gioria, S.; Calzolai, L. Asymmetric-flow field-flow fractionation for measuring particle size, drug loading and (in)stability of nanopharmaceuticals. The joint view of European Union Nanomedicine Characterization Laboratory and National Cancer Institute—Nanotechnology Characterization Laboratory. J. Chrom. A 2021, 1635, 461–767. [Google Scholar]
- Ayala-Cabrera, J.F.; Montero, L.; Meckelmann, S.W.; Uteschil, F.; Schmitz, O.J. Review on atmospheric pressure ionization sources for gas chromatography-mass spectrometry. Part I: Current ion source developments and improvements in ionization strategies. Anal. Chim. Acta 2023, 1238, 340–353. [Google Scholar] [CrossRef]
- Fang, J.; Zhao, H.; Zhang, Y.; Lu, M.; Cai, Z. Atmospheric pressure chemical ionization in gas chromatography-mass spectrometry for the analysis of persistent organic pollutants. Trends Environ. Anal. Chem. 2020, 25, e00076. [Google Scholar] [CrossRef]
- Grobelny, J.; DelRio, F.W.; Pradeep, N.; Kim, D.I.; Hackley, V.A.; Cook, R.F. Size Measurement of Nanoparticles Using Atomic Force Microscopy. In: McNeil, S. (eds) Characterization of Nanoparticles Intended for Drug Delivery. Methods Mol. Biol. 2011, 697, 71–82. [Google Scholar] [PubMed]
- Bellotti, R.; Picotto, G.B.; Ribotta, L. AFM Measurements and Tip Characterization of Nanoparticles with Different Shapes. Nanomanuf. Metrol. 2022, 5, 127–138. [Google Scholar] [CrossRef]
- Gao, Z.; Zhong, W. Recent (2018–2020) development in capillary electrophoresis. Anal. Bioanal. Chem. 2022, 414, 115–130. [Google Scholar] [CrossRef]
- Raffaele, J.; Loughney, J.W.; Rustandi, R.R. Development of a microchip capillary electrophoresis method for determination of the purity and integrity of mRNA in lipid nanoparticle vaccines. Electrophoresis 2022, 43, 9–10. [Google Scholar] [CrossRef]
- Amaldoss, M.J.N.; Pandzic, E.; Koshy, P.; Kumar, N.; Sorrell, C.C.; Unnikrishnan, A. Detection and quantification of nanoparticle-induced intracellular ROS in live cells by laser scanning confocal microscopy. Methods 2022, 207, 11–19. [Google Scholar] [CrossRef]
- Zou, Y.; Celli, A.; Zhu, H.; Elmahdy, A.; Cao, Y.; Hui, X.; Maibach, H. Confocal laser scanning microscopy to estimate nanoparticles’ human skin penetration in vitro. Int. J. Nanomed. 2017, 12, 8035–8041. [Google Scholar] [CrossRef]
- Robertson, J.D.; Rizzello, L.; Avila-Olias, M.; Gaitzsch, J.; Contini, C.; Magoń, M.S.; Renshaw, S.A.; Battaglia, G. Purification of Nanoparticles by Size and Shape. Sci. Rep. 2016, 6, 27494. [Google Scholar] [CrossRef]
- Shah, N.K.; Ivone, R.; Shen, J.; Meenach, S.A. comparison of centrifugation and tangential flow filtration for nanoparticle purification: A case study on acetalated dextran nanoparticles. Particuology 2020, 50, 189–196. [Google Scholar] [CrossRef]
- Farkas, N.; Kramar, J.A. Dynamic light scattering distributions by any means. J. Nanopart Res. 2021, 23, 120. [Google Scholar] [CrossRef]
- Malm, A.V.; Corbett, J.C.W. Improved Dynamic Light Scattering using an adaptive and statistically driven time resolved treatment of correlation data. Sci. Rep. 2019, 9, 13519. [Google Scholar] [CrossRef]
- Li, L.W.; Yuki, K.; Yu, H.; Yanbin, L. Recent advances and challenges in electron microscopy characterizations of radiation-sensitive nanoparticles. Front. Chem. 2023, 11, 1171240. [Google Scholar]
- Faraz, K.; Grenier, T.; Ducottet, C.; Epicier, T. Deep learning detection of nanoparticles and multiple object tracking of their dynamic evolution during in situ ETEM studies. Sci. Rep. 2022, 12, 2484. [Google Scholar] [CrossRef]
- Wang, J.; Hsu, C.S.; Wu, T.S.; Chan, T.-S.; Suen, N.-T.; Lee, J.-F.; Chen, H.M. In situ X-ray spectroscopies beyond conventional X-ray absorption spectroscopy on deciphering dynamic configuration of electrocatalysts. Nat. Commun. 2023, 14, 6576. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Bernareggi, M.; Selli, E. Redox dynamics of Pt and Cu nanoparticles on TiO2 during the photocatalytic oxidation of methanol under aerobic and anaerobic conditions studied by in situ modulated excitation X-ray absorption spectroscopy. ACS Catal. 2022, 12, 12879–12889. [Google Scholar] [CrossRef]
- Du, C.; Mills, J.P.; Yohannes, A.G.; Wei, W.; Wang, L.; Lu, S.; Lian, J.-X.; Wang, M.; Guo, T.; Wang, X.; et al. Cascade electrocatalysis via AgCu single-atom alloy and Ag nanoparticles in CO2 electroreduction toward multicarbon products. Nat. Commun. 2023, 14, 6142. [Google Scholar] [CrossRef]
- Vladár, A.E.; Hodoroaba, V.D. Characterization of Nanoparticles by Scanning Electron Microscopy. In Micro and Nano Technologies, Characterization of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 7–27. [Google Scholar]
- Graewert, M.A.; Wilhelmy, C.; Bacic, T. Quantitative size-resolved characterization of mRNA nanoparticles by in-line coupling of asymmetrical-flow field-flow fractionation with small angle X-ray scattering. Sci. Rep. 2023, 13, 15764. [Google Scholar] [CrossRef]
- Huber, M.J. Physicochemical characterization and quantification of nanoplastics: Applicability, limitations and complementarity of batch and fractionation methods. Anal. Bioanal. Chem. 2023, 415, 3007–3031. [Google Scholar] [CrossRef]
- Fu, Y.; Wan, Q.; Qin, Z. Concentration determination of gold nanoparticles by flame atomic absorption spectrophotometry. Acta Geochim. 2021, 40, 498–506. [Google Scholar] [CrossRef]
- Godoy, N.V.; Galazzi, R.M.; Chacon-Madrid, K.; Arruda, M.A.Z.; Mazali, I.O. Evaluating the total gold concentration in metallic nanoparticles with a high content of organic matter through microwave-assisted decomposition platform and plasma-based spectrometric techniques ICP-MS and ICP OES. Talanta 2021, 224, 7. [Google Scholar] [CrossRef]
- García-Mesa, J.C.; Montoro-Leal, P.; Rodríguez-Moreno, A.; López Guerrero, M.M.; Vereda Alonso, E.I. Direct solid sampling for speciation of Zn2+ and ZnO nanoparticles in cosmetics by graphite furnace atomic absorption spectrometry. Talanta 2021, 223 Pt 1, 121795. [Google Scholar] [CrossRef]
- Galbács, G.; Kéri, A.; Kohut, A.; Veres, M.; Geretovszky, Z. Nanoparticles in analytical laser and plasma spectroscopy—A review of recent developments in methodology and applications. J. Anal. At. Spectrom. 2021, 36, 1826. [Google Scholar] [CrossRef]
- De Souza, L.P.; Alseekh, S.; Scossa, F.; Fernie, A.R. Ultra-high-performance liquid chromatography high-resolution mass spectrometry variants for metabolomics research. Nat. Methods 2021, 18, 733–746. [Google Scholar] [CrossRef]
- Roman, M. Hydrodynamic Chromatography for the Characterization of Inorganic Nanoparticles. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2021; Volume 93, pp. 121–171. [Google Scholar]
- Williams, A.; Varela, E.; Meehan, E.; Tribe, K. Characterisation of nanoparticulate systems by hydrodynamic chromatography. Int. J. Pharm. 2002, 242, 295–299. [Google Scholar] [CrossRef]
- López-Lorente, Á.I.; Mizaikoff, B. Recent advances on the characterization of nanoparticles using infrared spectroscopy. TrAC Trends Anal. Chem. 2016, 84 Pt A, 97–106. [Google Scholar] [CrossRef]
- Fahelelbom, K.M.; Saleh, A.; Al-Tabakha, M.M.A.; Ashames, A.A. Recent applications of quantitative analytical FTIR spectroscopy in pharmaceutical, biomedical, and clinical fields: A brief review. Rev. Anal. Chem. 2022, 41, 21–33. [Google Scholar] [CrossRef]
- Biswas, A.; Lemcoff, N.; Shelonchik, O.; Yesodi, D.; Yehezkel, E.; Finestone, E.Y.; Upcher, A.; Weizmann, Y. Photothermally heated colloidal synthesis of nanoparticles driven by silica-encapsulated plasmonic heat sources. Nat. Commun. 2023, 14, 6355. [Google Scholar] [CrossRef]
- Loeschner, K.; Johnson, M.E.; Montoro Bustos, A.R. Application of Single Particle ICP-MS for the Determination of Inorganic Nanoparticles in Food Additives and Food: A Short Review. Nanomaterials 2023, 13, 2547. [Google Scholar] [CrossRef]

| Metal | Diameter Minimal | Diameter Maximal |
|---|---|---|
| Ag | 1.5 nm | 35 nm |
| Al | 18 nm | 80 nm |
| Au | 50 nm | 150 nm |
| Co | 28 nm | 50 nm |
| Cr | 50 nm | 100 nm |
| Cu | 25 nm | 500 nm |
| MgO | 20 nm | 100 nm |
| Mn | 30 nm | 60 nm |
| Mo | 70 nm | 100 nm |
| Ni | 20 nm | 50 nm |
| TiO2 | 30 nm | 150 nm |
| ZnO | 80 nm | 130 nm |
| Method | Name Abbreviation | References |
|---|---|---|
| Acoustic spectroscopy | AS | [51,52,53] |
| Analytical electron microscopy | AEM | [54,55] |
| Asymmetric flow field-flow fractionation | AF4 | [56,57] |
| Atmospheric pressure photo-ionization mass spectrometry | APPI-MS | [58,59] |
| Atomic force microscopy | AFM | [60,61] |
| Capillary electrophoresis | CE | [62,63] |
| Confocal laser scanning microscopy | CLSM | [64,65] |
| Cross-flow filtration | CFF | [66,67] |
| Dynamic light scattering | DLS | [68,69] |
| Electron microscopy | EM | [70,71] |
| Energy dispersive X-ray spectroscopy | EDS | [72,73] |
| Environmental scanning electron microscopy | ESEM | [74,75] |
| Field-flow fractionation | FFF | [76,77] |
| Flame atomic absorption spectrometry | F-AAS | [78,79] |
| Graphite furnace atomic absorption spectrometry | GF-AAS | [80,81] |
| High-performance liquid chromatography | HPLC | [42,82] |
| Hydrodynamic chromatography | HDC | [83,84] |
| Infrared spectroscopy | IR | [85,86] |
| Inductively coupled plasma mass spectrometry | ICP-MS | [87,88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezić, I.; Meštrović, E. Characterization of Nanoparticles in Antimicrobial Coatings for Medical Applications—A Review. Coatings 2023, 13, 1830. https://doi.org/10.3390/coatings13111830
Rezić I, Meštrović E. Characterization of Nanoparticles in Antimicrobial Coatings for Medical Applications—A Review. Coatings. 2023; 13(11):1830. https://doi.org/10.3390/coatings13111830
Chicago/Turabian StyleRezić, Iva, and Ernest Meštrović. 2023. "Characterization of Nanoparticles in Antimicrobial Coatings for Medical Applications—A Review" Coatings 13, no. 11: 1830. https://doi.org/10.3390/coatings13111830
APA StyleRezić, I., & Meštrović, E. (2023). Characterization of Nanoparticles in Antimicrobial Coatings for Medical Applications—A Review. Coatings, 13(11), 1830. https://doi.org/10.3390/coatings13111830







