Flexible Finely and Directly Patternable Liquid Metal Electrodes via Selective Surface Wetting Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Patterned EGaIn Electrodes
2.3. Characterization
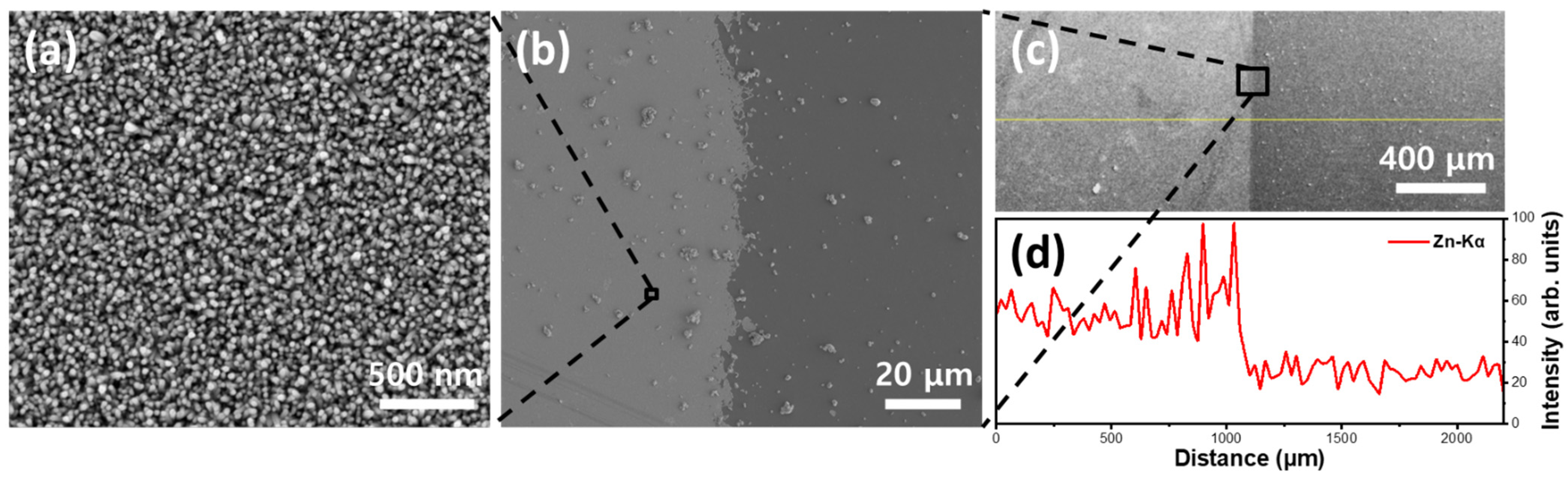
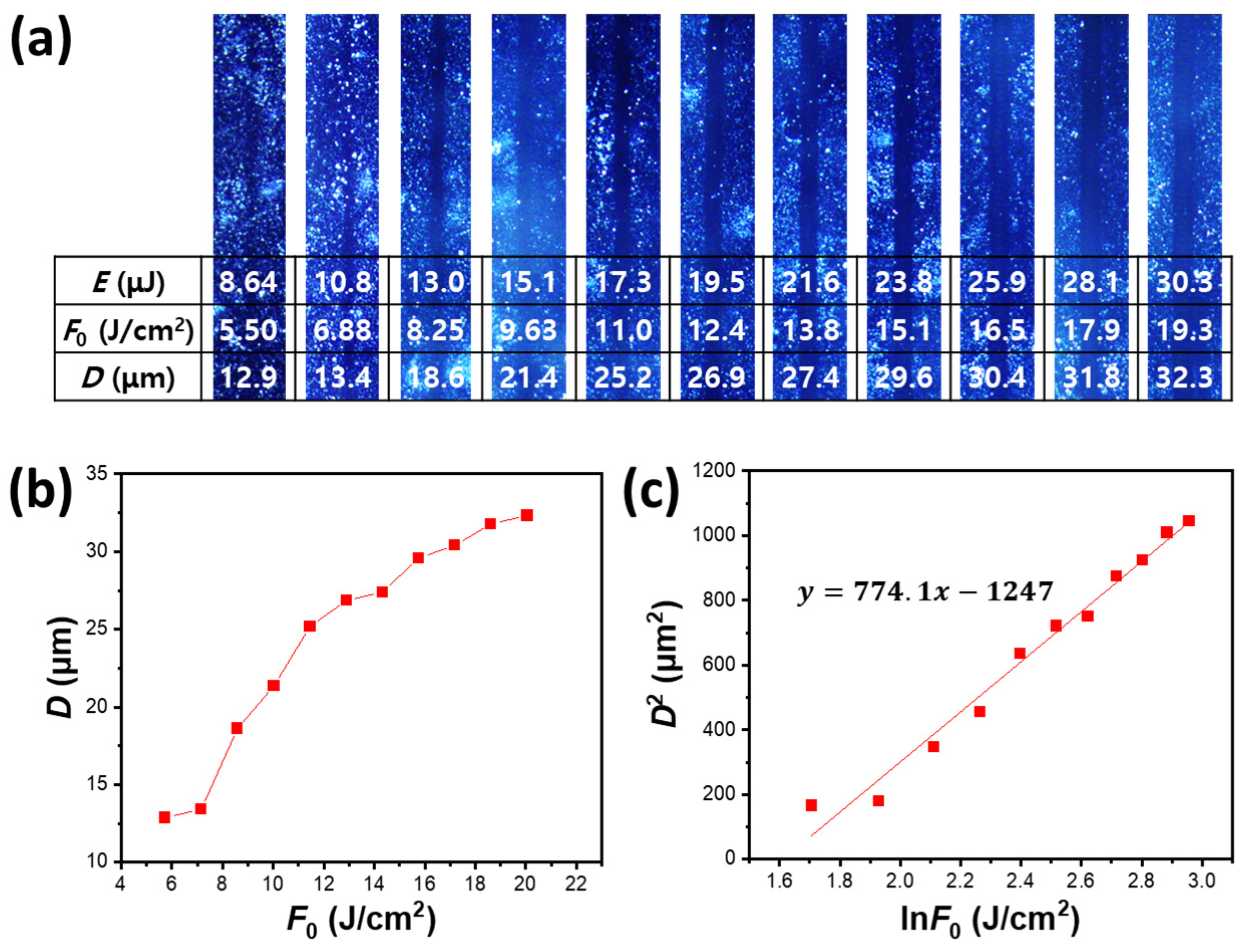
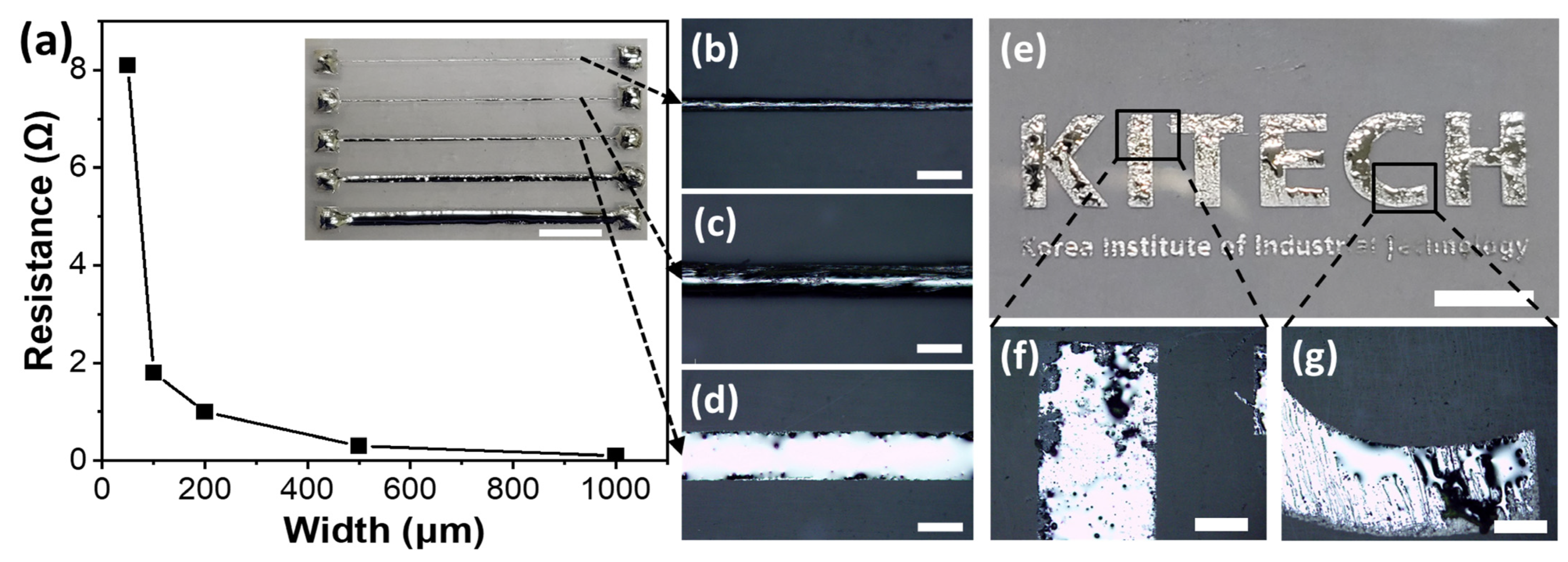
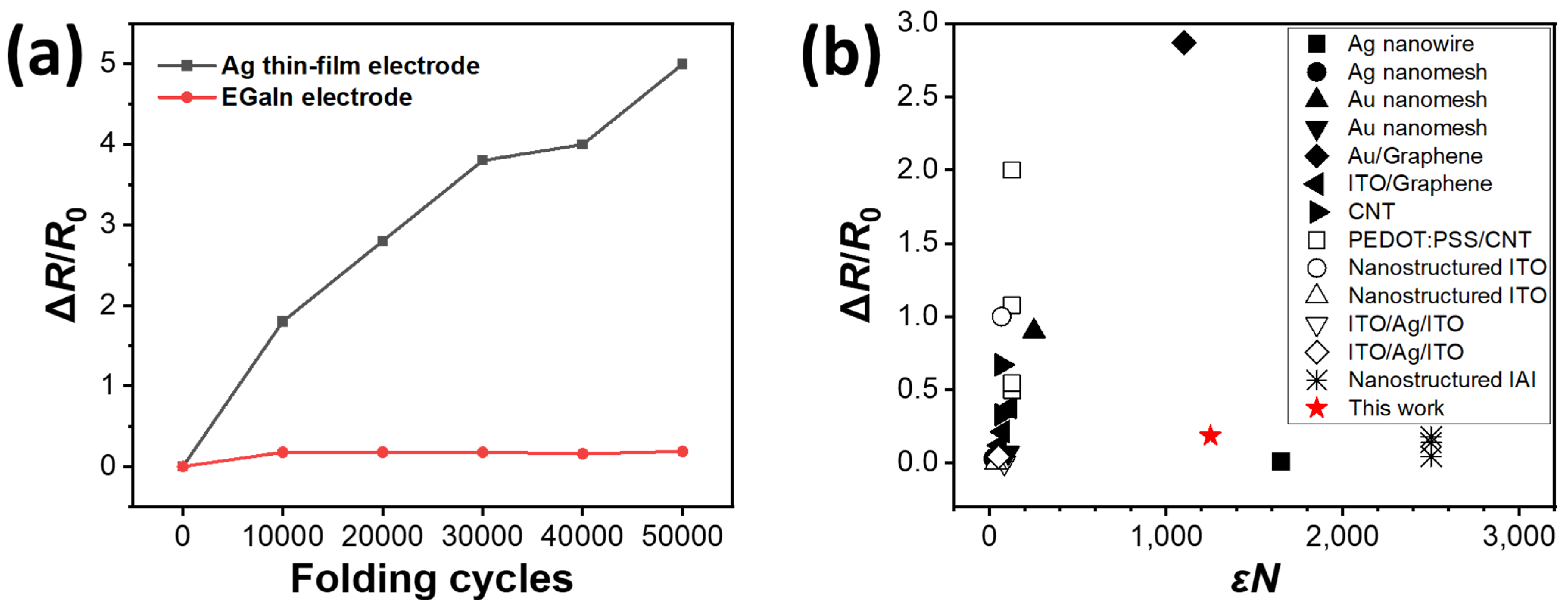
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, G.; Wang, H.; Guo, R.; Duan, M.; Zhang, Y.; Liu, J. Superelastic EGaIn composite fibers sustaining 500% tensile strain with superior electrical conductivity for wearable electronics. ACS Appl. Mater. Interfaces 2020, 12, 6112–6118. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, X.; Li, X.; Tian, H.; Wang, C.; Nie, B.; He, J.; Shao, J. Bioinspired robot skin with mechanically gated electron channels for sliding tactile perception. Sci. Adv. 2022, 8, eade0720. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-J.; Shin, H.-A.S.; Jung, S.-Y.; Cho, Y.; Kraft, O.; Choi, I.-S.; Joo, Y.-C. Crack nucleation during mechanical fatigue in thin metal films on flexible substrates. Acta Mater. 2013, 61, 3473–3481. [Google Scholar] [CrossRef]
- Bo, G.; Ren, L.; Xu, X.; Du, Y.; Dou, S. Recent progress on liquid metals and their applications. Adv. Phys. X 2018, 3, 1446359. [Google Scholar] [CrossRef]
- Clarkson, T.W.; Magos, L. The toxicology of mercury and its chemical compounds. Crit. Rev. Toxicol. 2006, 36, 609–662. [Google Scholar] [CrossRef] [PubMed]
- French, S.J.; Saunders, D.J.; Ingle, G.W. The system gallium-indium. J. Phys. Chem. 2002, 42, 265–274. [Google Scholar] [CrossRef]
- Chen, X.; Sun, P.; Tian, H.; Li, X.; Wang, C.; Duan, J.; Luo, Y.; Li, S.; Chen, X.; Shao, J. Self-healing and stretchable conductor based on embedded liquid metal patterns within imprintable dynamic covalent elastomer. J. Mater. Chem. C 2022, 10, 1039–1047. [Google Scholar] [CrossRef]
- Hao, X.P.; Li, C.Y.; Zhang, C.W.; Du, M.; Ying, Z.; Zheng, Q.; Wu, Z.L. Self-shaping soft electronics based on patterned hydrogel with stencil-printed liquid metal. Adv. Funct. Mater. 2021, 31, 2105481. [Google Scholar] [CrossRef]
- Yan, J.; Lu, Y.; Chen, G.; Yang, M.; Gu, Z. Advances in liquid metals for biomedical applications. Chem. Soc. Rev. 2018, 47, 2518–2533. [Google Scholar] [CrossRef]
- Boley, J.W.; White, E.L.; Chiu, G.T.C.; Kramer, R.K. Direct writing of gallium-indium alloy for stretchable electronics. Adv. Funct. Mater. 2014, 24, 3501–3507. [Google Scholar] [CrossRef]
- Park, C.W.; Moon, Y.G.; Seong, H.; Jung, S.W.; Oh, J.-Y.; Na, B.S.; Park, N.-M.; Lee, S.S.; Im, S.G.; Koo, J.B. Photolithography-based patterning of liquid metal interconnects for monolithically integrated stretchable circuits. ACS Appl. Mater. Interfaces 2016, 8, 15459–15465. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhou, T.; Zhang, L.; Zhu, D.; Handschuh-Wang, S.; Liu, Z.; Kong, T.; Liu, Y.; Zhang, J.; Zhou, X. Defect-free, high resolution patterning of liquid metals using reversibly sealed, reusable polydimethylsiloxane microchannels for flexible electronic applications. J. Mater. Chem. C 2017, 5, 6790–6797. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, B.; Jiang, J.; Wu, K.; Guo, C.F.; Wu, Z. High-fidelity conformal printing of 3D liquid alloy circuits for soft electronics. ACS Appl. Mater. Interfaces 2019, 11, 7148–7156. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Su, S.; Peng, H.; Kwok, H.S.; Zhou, X.; Chen, S. Selective wetting/dewetting for controllable patterning of liquid metal electrodes for all-printed device application. J. Mater. Chem. C 2017, 5, 12378–12383. [Google Scholar] [CrossRef]
- Yang, C.; Hong, K.; Jang, J.; Chung, D.S.; An, T.K.; Choi, W.S.; Park, C.E. Solution-processed flexible ZnO transparent thin-film transistors with a polymer gate dielectric fabricated by microwave heating. Nanotechnology 2009, 20, 465201. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Kwack, Y.; Kim, S.H.; An, T.K.; Hong, K.; Nam, S.; Park, M.; Choi, W.-S.; Park, C.E. Ambipolar thin-film transistors and an inverter based on pentacene/self-assembled monolayer modified ZnO hybrid structures for balanced hole and electron mobilities. Org. Electron. 2011, 12, 411–418. [Google Scholar] [CrossRef]
- Çolak, H.; Karaköse, E.; Kartopu, G. Effect of consumption of the sol–gel deposited ZnO seed layer on the growth and properties of high quality ZnO nanorods. J. Mater. Sci. Mater. Electron. 2018, 29, 11964–11971. [Google Scholar] [CrossRef]
- Usha, A.; Christy, J. Characterization, thermal effect on optical band gap energy and photoluminescence in wurtzite ZnO: Er nanocrystallites. Mater. Today Proc. 2016, 3, 145–151. [Google Scholar] [CrossRef]
- Ravi-Kumar, S.; Lies, B.; Zhang, X.; Lyu, H.; Qin, H. Laser ablation of polymers: A review. Polym. Int. 2019, 68, 1391–1401. [Google Scholar] [CrossRef]
- Lee, H.-L.; Hussain, A.; Moon, Y.-J.; Hwang, J.Y.; Moon, S.J. Influence of pre-sintering on the nanosecond pulsed laser ablation patterns of spin-coated silver nanoparticles. Appl. Phys. A 2023, 129, 705. [Google Scholar] [CrossRef]
- Lee, J.; Yong, K. Combining the lotus leaf effect with artificial photosynthesis: Regeneration of underwater superhydrophobicity of hierarchical ZnO/Si surfaces by solar water splitting. NPG Asia Mater. 2015, 7, e201. [Google Scholar] [CrossRef]
- Kinloch, A.J. Adhesion and Adhesives: Science and Technology; Springer: Berlin, Germany, 1987; ISBN 978-0-412-27440-4. [Google Scholar]
- Sim, I.; Park, S.; Shin, K.Y.; Yang, C.; Kang, H.; Hwang, J.Y.; Moon, S.J. Inkjet printing of high aspect ratio silver lines via laser-induced selective surface wetting technique. Coatings 2023, 13, 683. [Google Scholar] [CrossRef]
- Mao, L.; Meng, Q.; Ahmad, A.; Wei, Z. Mechanical analyses and structural design requirements for flexible energy storage devices. Adv. Energy Mater. 2017, 7, 1700535. [Google Scholar] [CrossRef]
- Han, S.; Ju, B.K.; Yang, C. Ultra-flexible and transparent electrodes with controllable crack length via metal–polymer hybrid nanostructure. Thin Solid Film. 2022, 757, 139388. [Google Scholar] [CrossRef]
- Zhao, Z.; Soni, S.; Lee, T.; Nijhuis, C.A.; Xiang, D. Smart eutectic gallium–indium: From properties to applications. Adv. Mater. 2023, 35, 2203391. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-G.; Lee, G.-Y.; Jang, J.; Yun, S.M.; Kim, E.; Park, J.-U. Liquid metal-based soft electronics for wearable healthcare. Adv. Healthc. Mater. 2021, 10, 2002280. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, H.-Z.; Zhao, R.-Q.; Rao, W.; Liu, J. Liquid metal composites. Matter 2020, 2, 1446. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.; Kim, D.; Ha, H.; Qaiser, N.; Yi, H.; Hwang, B. Ethylcellulose/Ag nanowire composites as multifunctional patchable transparent electrodes. Surf. Coat. Technol. 2020, 394, 125898. [Google Scholar] [CrossRef]
- Song, M.; Kim, H.-J.; Kim, C.S.; Jeong, J.-H.; Cho, C.; Lee, J.-Y.; Jin, S.-H.; Choi, D.-G.; Kim, D.-H. ITO-free highly bendable and efficient organic solar cells with Ag nanomesh/ZnO hybrid electrodes. J. Mater. Chem. A 2015, 3, 65–70. [Google Scholar] [CrossRef]
- Guo, C.F.; Sun, T.; Liu, Q.; Suo, Z.; Ren, Z. Highly stretchable and transparent nanomesh electrodes made by grain boundary lithography. Nat. Commun. 2014, 5, 3121. [Google Scholar] [CrossRef]
- Chauvin, A.; Txia Cha Heu, W.; Buh, J.; Tessier, P.-Y.; El Mel, A.-A. Vapor dealloying of ultra-thin films: A promising concept for the fabrication of highly flexible transparent conductive metal nanomesh electrodes. NPJ Flex. Electron. 2019, 3, 5. [Google Scholar] [CrossRef]
- Cho, C.; Kang, P.; Taqieddin, A.; Jing, Y.; Yong, K.; Kim, J.M.; Haque, M.F.; Aluru, N.R.; Nam, S.W. Strain-resilient electrical functionality in thin-film metal electrodes using two-dimensional interlayers. Nat. Electron. 2021, 4, 126–133. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, Y.; Hwang, J.-Y.; Lee, J.-H.; Jung, S.; Park, H.; Cho, S.; Nahm, S.; Yang, W.S.; Kim, H.; et al. Flexible indium-tin oxide crystal on plastic substrates supported by graphene monolayer. Sci. Rep. 2017, 7, 3131. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Han, S.-H.; Kim, B.-J.; Park, J.-S. Surface modification of plastic substrates via corona-pretreatment and its effects on the properties of carbon nanotubes for use of flexible transparent electrodes. Surf. Coat. Technol. 2015, 271, 100–105. [Google Scholar] [CrossRef]
- Kim, B.-J.; Han, S.-H.; Park, J.-S. Properties of CNTs coated by PEDOT:PSS films via spin-coating and electrophoretic deposition methods for flexible transparent electrodes. Surf. Coat. Technol. 2015, 271, 22–26. [Google Scholar] [CrossRef]
- Königer, T.; Münstedt, H. Coatings of indium tin oxide nanoparticles on various flexible polymer substrates: Influence of surface topography and oscillatory bending on electrical properties. J. Soc. Inf. Disp. 2008, 16, 559–568. [Google Scholar] [CrossRef]
- Yun, J.; Park, Y.H.; Bae, T.-S.; Lee, S.; Lee, G.-H. Fabrication of a completely transparent and highly flexible ITO nanoparticle electrode at room temperature. ACS Appl. Mater. Interfaces 2013, 5, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Park, S.-H.; Kim, D.-H.; Nah, Y.-C.; Kim, H.-K. Roll-to-roll sputtered ITO/Ag/ITO multilayers for highly transparent and flexible electrochromic applications. Sol. Energy Mater. Sol. Cells 2017, 160, 203–210. [Google Scholar] [CrossRef]
- Wu, C.-C. Highly flexible touch screen panel fabricated with silver-inserted transparent ITO triple-layer structures. RSC Adv. 2018, 8, 11862–11870. [Google Scholar] [CrossRef]


| Sample | Contact Angle (°) | (mJ m−2) | (mJ m−2) | (mJ m−2) | ||
|---|---|---|---|---|---|---|
| DI Water | DIM | |||||
| Pristine ZnO-PFOTES |  141.0 |  70.63 | 5.906 | 29.01 | 34.92 | 0.1691 |
| Laser-ablated ZnO-PFOTES |  42.87 |  26.50 | 24.71 | 33.97 | 58.68 | 0.4210 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.J.; Yang, C. Flexible Finely and Directly Patternable Liquid Metal Electrodes via Selective Surface Wetting Technique. Coatings 2023, 13, 1922. https://doi.org/10.3390/coatings13111922
Park SJ, Yang C. Flexible Finely and Directly Patternable Liquid Metal Electrodes via Selective Surface Wetting Technique. Coatings. 2023; 13(11):1922. https://doi.org/10.3390/coatings13111922
Chicago/Turabian StylePark, Seong Ju, and Chanwoo Yang. 2023. "Flexible Finely and Directly Patternable Liquid Metal Electrodes via Selective Surface Wetting Technique" Coatings 13, no. 11: 1922. https://doi.org/10.3390/coatings13111922
APA StylePark, S. J., & Yang, C. (2023). Flexible Finely and Directly Patternable Liquid Metal Electrodes via Selective Surface Wetting Technique. Coatings, 13(11), 1922. https://doi.org/10.3390/coatings13111922









