Preparation and Properties of Silane Coupling Agent Modified Basalt Flake Polyurethane Anti-Corrosion Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification of BFs
2.3. Pre-Dispersion of Fillers
2.4. Preparation of Polyurethane Anti-Corrosion Coatings
2.5. Performance Characterization
3. Results
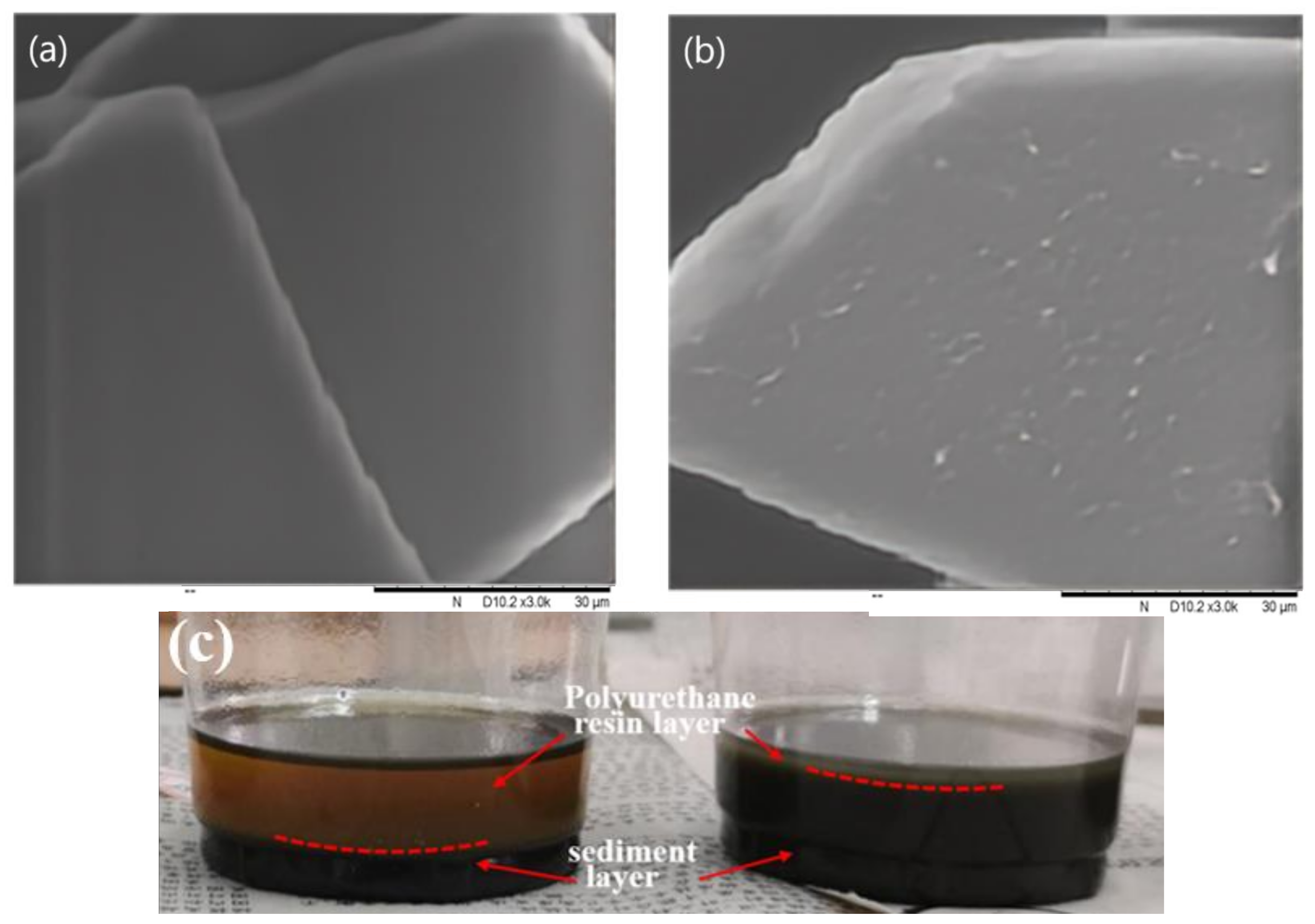
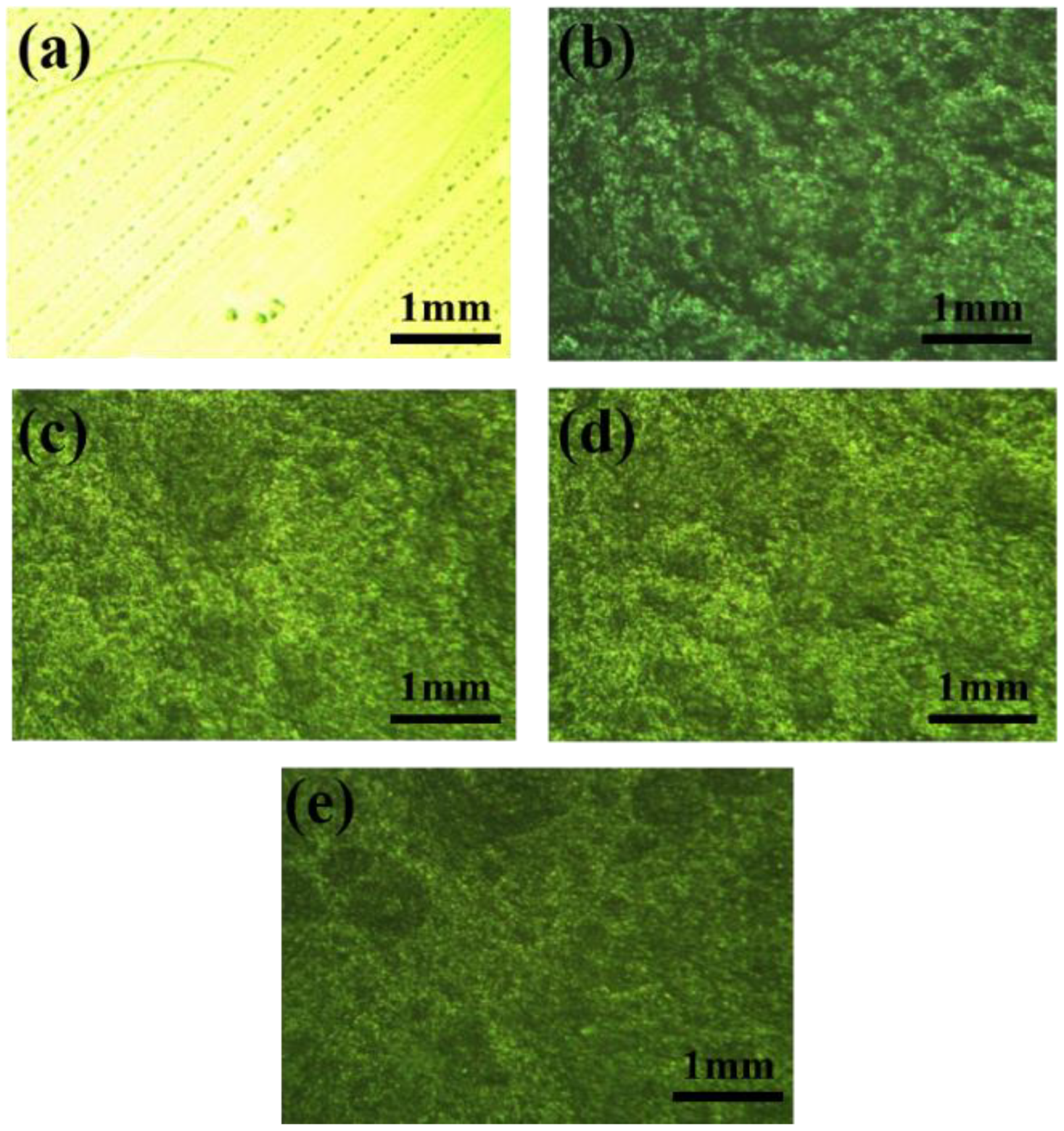
3.1. Structural Characterizations of MBFs and Polyurethane Anti-Corrosion Coatings
3.2. Effect of MBFs Content on Conventional Physicochemical Properties of Coatings
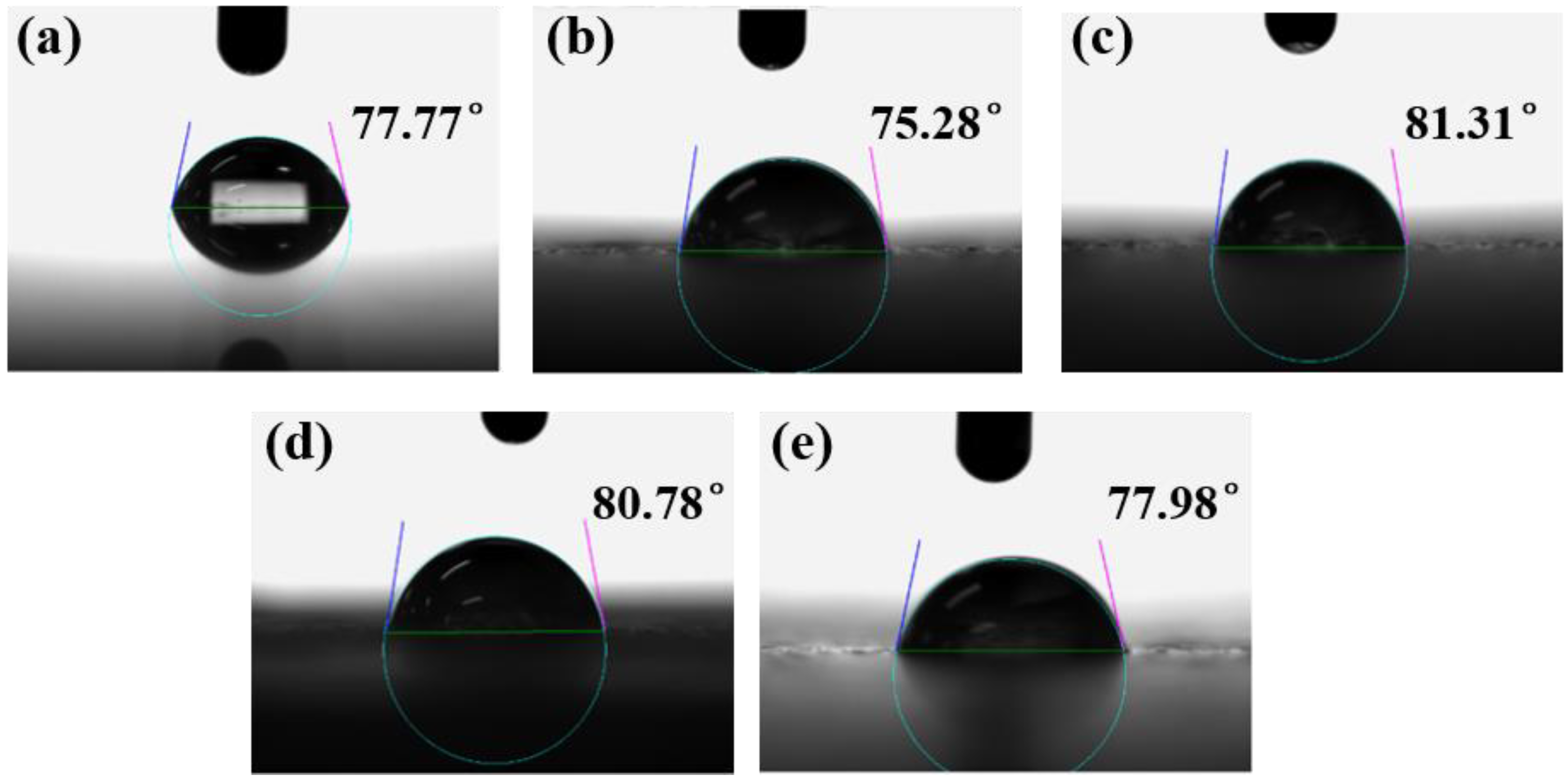
3.3. Effect of MBFs Content on Water Contact Angle of Coatings
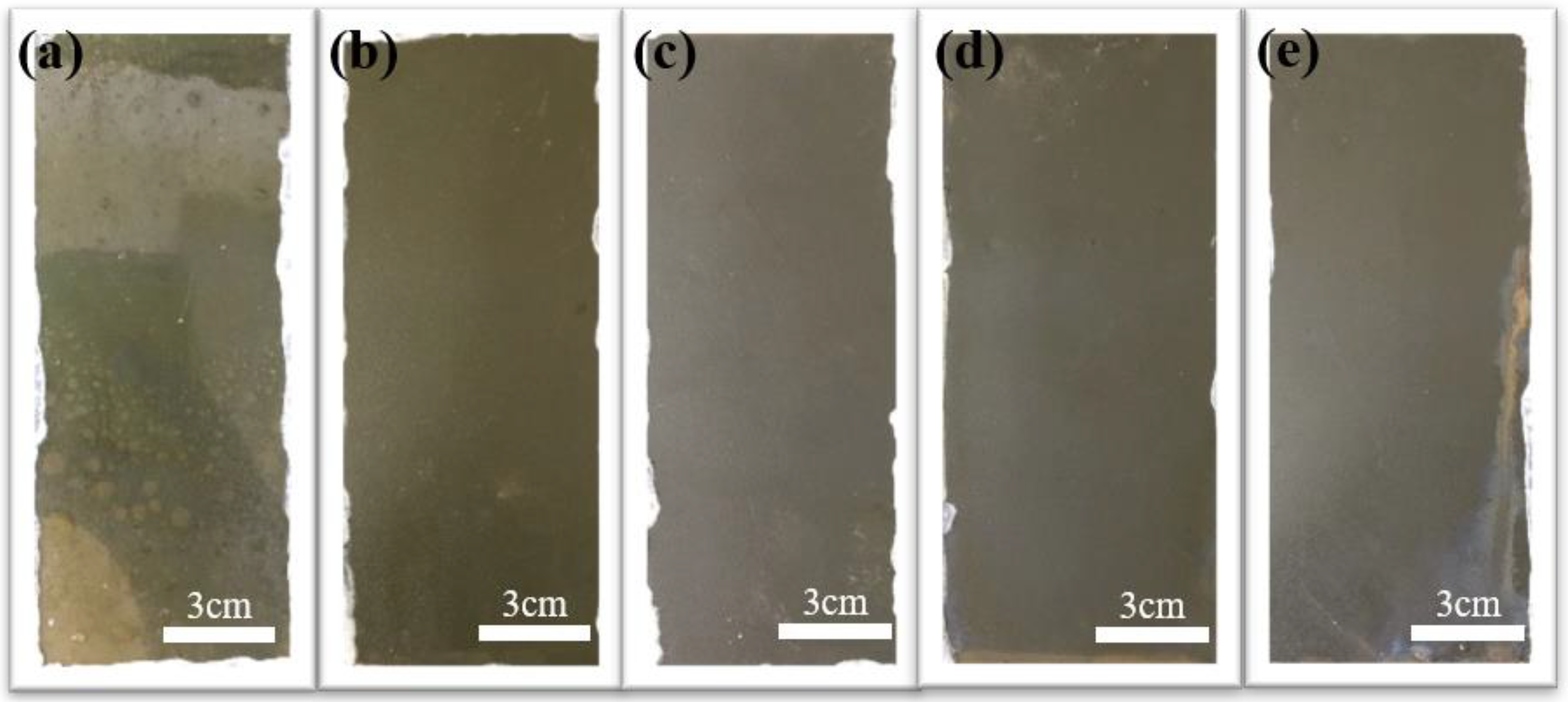
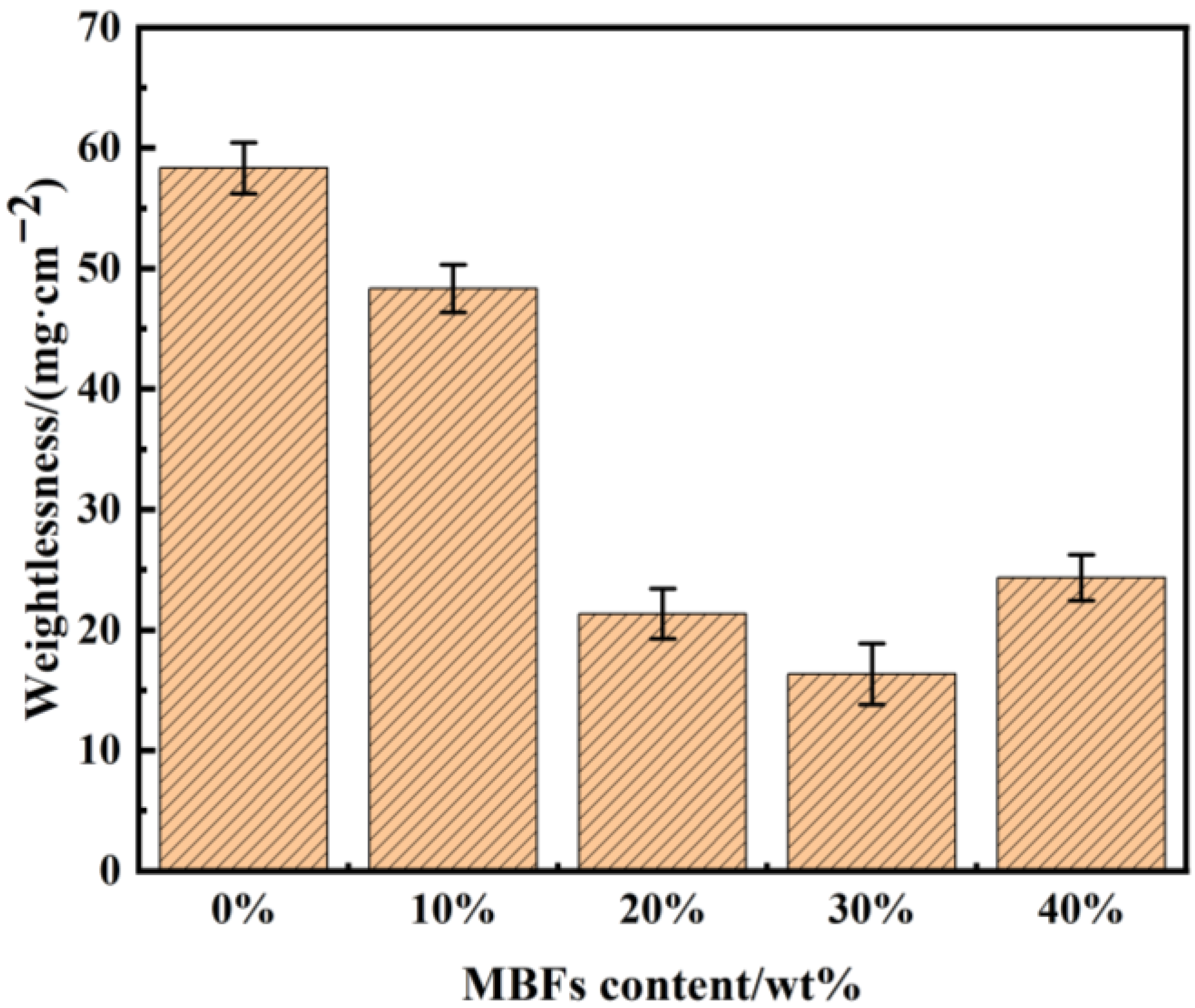
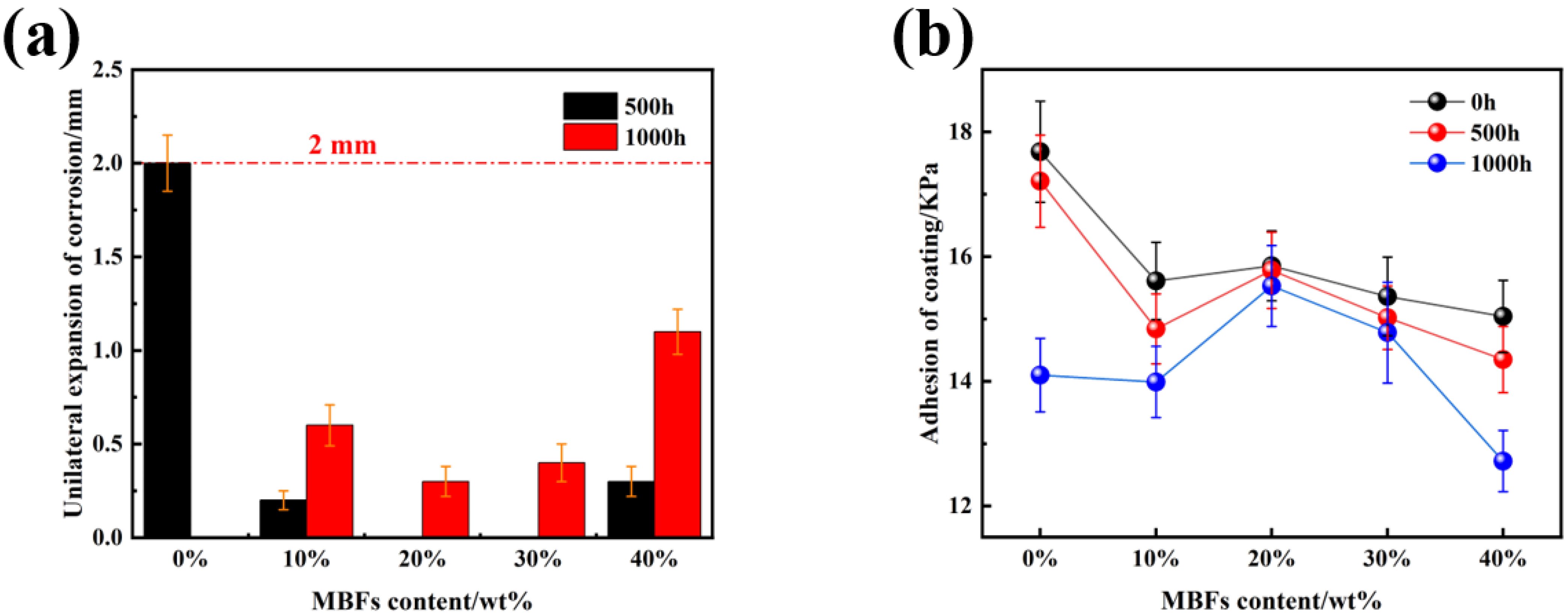
3.4. Effect of MBFs Content on the Weathering Properties of Coatings
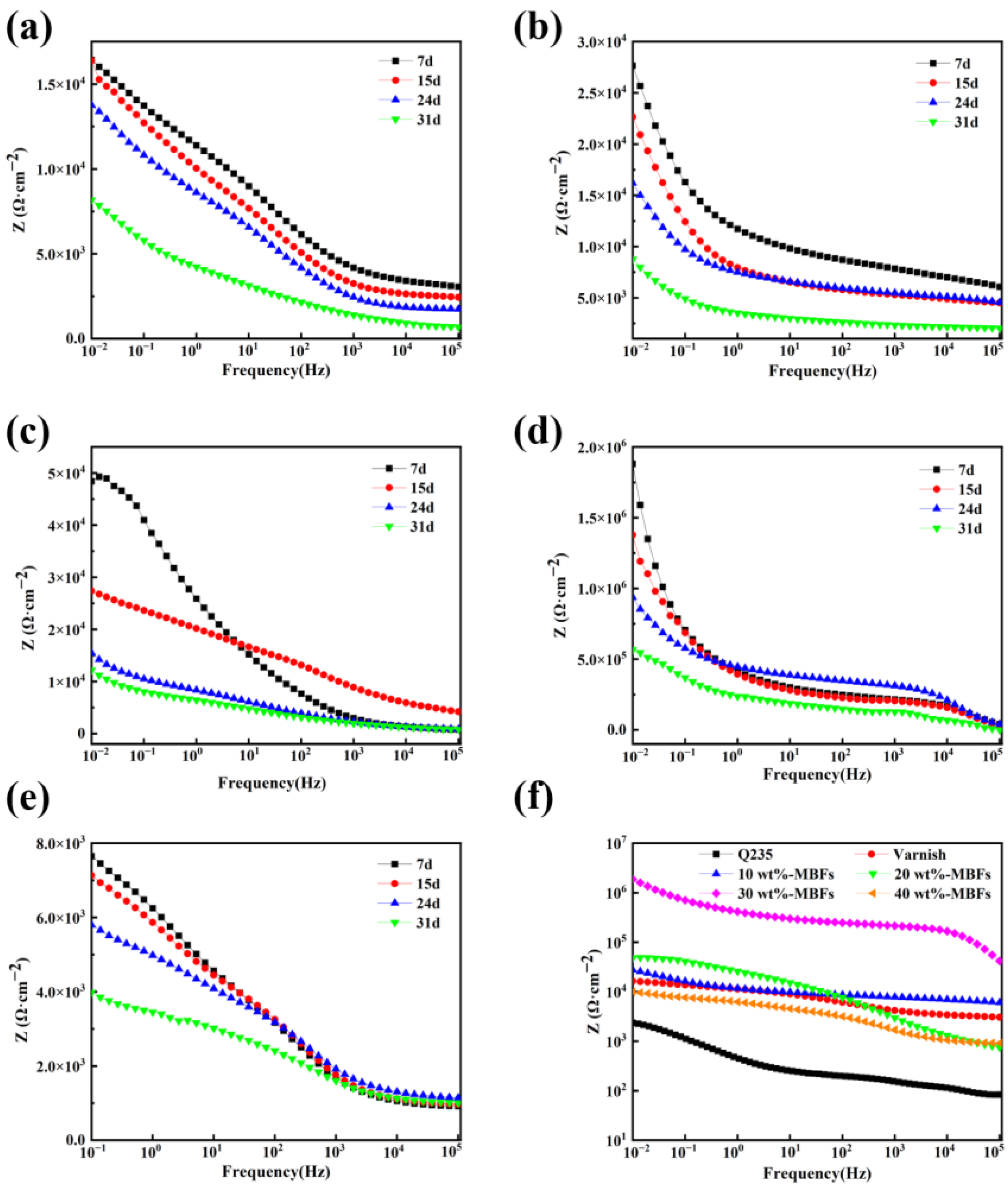
3.5. Effect of MBFs Content on Corrosion Resistance of Coatings
3.6. Analysis of Coating Corrosion Resistance Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Z.; Xia, W.; Yao, C.; Lin, Z.; Zhang, W.; Li, W. Research on the Metal Corrosion Process in the Sea Mud/Seawater/Atmosphere Interface Zone. Coatings 2020, 10, 1219. [Google Scholar] [CrossRef]
- Gruber, M.R.; Hofko, B.; Hoffmann, M.; Stinglmayr, D.; Grothe, H. Analysis of metal corrosion methods and identification of cost-efficient and low corrosion deicing agents. Corros. Eng. Sci. Technol. 2023, 58, 452–463. [Google Scholar] [CrossRef]
- Yu, H.D.; Zhang, Z.; Han, M.Y. Metal Corrosion for Nanofabrication. Small 2012, 8, 2621–2635. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Xing, Q.; Wang, H. Corrosion behaviors of weathering steel 09CuPCrNi welded joints in simulated marine atmospheric environment. Anti-Corros. Methods Mater. 2016, 63, 295–300. [Google Scholar] [CrossRef]
- Sun, B.; Yan, L.; Gao, K. Hydrophobicity and Improved Corrosion Resistance of Weathering Steel via a Facile Sol–Gel Process with a Natural Rust Film. ACS Appl. Mater. Interfaces 2023, 15, 46400–46407. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Qian, B. Effect of graphene on the electrochemical protection of zinc-rich coatings. Mater. Corros. 2018, 69, 1854–1860. [Google Scholar] [CrossRef]
- Wang, Y.; Sahadeo, E.; Lee, S.B. An Electrochemically Polymerized Protective Layer for a Magnesium Metal Anode. ACS Appl. Energy Mater. 2022, 5, 2613–2620. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, R.; Wu, R.; Luo, Y.; Guo, L.; He, Z. Green and high-efficiency corrosion inhibitors for metals: A review. J. Adhes. Sci. Technol. 2022, 37, 1501–1524. [Google Scholar] [CrossRef]
- Sun, W.; Liu, Y.; Li, T.; Cui, S.; Chen, S.; Yu, Q.; Wang, D. Anti-corrosion of amphoteric metal enhanced by MAO/corrosion inhibitor composite in acid, alkaline and salt solutions. J. Colloid Interface Sci. 2019, 554, 488–499. [Google Scholar] [CrossRef]
- Honarvar Nazari, M.; Zhang, Y.; Mahmoodi, A.; Xu, G.; Yu, J.; Wu, J.; Shi, X. Nanocomposite organic coatings for corrosion protection of metals: A review of recent advances. Prog. Org. Coat. 2022, 162, 106573. [Google Scholar] [CrossRef]
- Komalasari, E.; Situmeang, I.D.R.; Heltina, D. Cathodic protection on stuctures of carbon steel using sacrificial anode methode for corrosion control. IOP Conf. Ser. Mater. Sci. Eng. 2020, 845, 012015. [Google Scholar] [CrossRef]
- Sreehari, H.; Sethulekshmi, A.S.; Saritha, A. Bio Epoxy Coatings: An Emergent Green Anticorrosive Coating for the Future. Macromol. Mater. Eng. 2022, 307, 2200004. [Google Scholar] [CrossRef]
- Sørensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anticorrosive coatings: A review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Jin, H.; Wang, J.; Tian, L.; Gao, M.; Zhao, J.; Ren, L. Recent advances in emerging integrated antifouling and anticorrosion coatings. Mater. Des. 2022, 213, 110307. [Google Scholar] [CrossRef]
- Khatoon, H.; Ahmad, S. Vanadium Pentoxide-Enwrapped Polydiphenylamine/Polyurethane Nanocomposite: High-Performance Anticorrosive Coating. ACS Appl. Mater. Interfaces 2018, 11, 2374–2385. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, W.; Li, W.; Zuo, C.; Jiang, Y.; Wang, S. Development of Waterborne Heavy-Duty Anticorrosive Coatings with Modified Nanoscale Titania. Coatings 2022, 12, 1651. [Google Scholar] [CrossRef]
- Zhang, W.; Li, S.; Wei, D.; Zheng, Z.; Han, Z.; Liu, Y. Fabrication of a fluorine-free photocatalytic superhydrophobic coating and its long-lasting anticorrosion and excellent antibacterial abilities. Prog. Org. Coat. 2023, 184, 107806. [Google Scholar] [CrossRef]
- Xiao, Y.-K.; Ji, W.-F.; Chang, K.-S.; Hsu, K.-T.; Yeh, J.-M.; Liu, W.-R. Sandwich-structured rGO/PVDF/PU multilayer coatings for anti-corrosion application. RSC Adv. 2017, 7, 33829–33836. [Google Scholar] [CrossRef]
- Cai, K.; Zuo, S.; Luo, S.; Yao, C.; Liu, W.; Ma, J.; Mao, H.; Li, Z. Preparation of polyaniline/graphene composites with excellent anti-corrosion properties and their application in waterborne polyurethane anticorrosive coatings. RSC Adv. 2016, 6, 95965–95972. [Google Scholar] [CrossRef]
- Qing-Xian, Y.; Jing, L.; Xuan, L.; Yu-Yu, W.; Rui, D.; Yu, L.; Jia-Hao, D.; Peng, H.; Heng, Y.; Tian-Zi, S.; et al. The anti-corrosion performance and catalytic passivation and sorptive corrosion inhibition self-healing of sulfonated aniline trimer modified basalt scale anti-corrosion coatings. J. Taiwan. Inst. Chem. Eng. 2022, 131, 104156. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, L.; Meng, F.; Cui, Y.; Li, Z.; Oguzie, E.E.; Wang, F. Multifunctional superhydrophobic coatings fabricated from basalt scales on a fluorocarbon coating base. J. Mater. Sci. Technol. 2021, 84, 86–96. [Google Scholar] [CrossRef]
- Yue, Q.-X.; Wu, L.-F.; Lv, J.; Wang, A.-M.; Ding, R.; Wang, Y.-Y.; Yue, L.; Gao, W.; Li, X.-L.; Li, X.-Y.; et al. Study on anti-corrosion performance and mechanism of epoxy coatings based on basalt flake loaded aniline trimer. Colloid Interface Sci. Commun. 2021, 45, 100505. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A.; Sharma, S.; Ganjoo, R.; Assad, H. Computational and experimental studies on the efficiency of Sonchus arvensis as green corrosion inhibitor for mild steel in 0.5 M HCl solution. Mater. Today Proc. 2022, 66, 609–621. [Google Scholar] [CrossRef]
- Li, Y.C.; Tang, K.J.; Jin, F.L.; Park, S.J. Enhanced thermal stability and impact strength of phenolic formaldehyde resin using acid-treated basalt scales. J. Appl. Polym. Sci. 2022, 139, e52827. [Google Scholar] [CrossRef]
- Yao, S.-S.; Gao, M.-Z.; Feng, Z.-Y.; Jin, F.-L.; Park, S.-J. Thermal and mechanical properties of poly(latic acid) reinforced with silanized basalt scales. Korean J. Chem. Eng. 2022, 39, 1952–1958. [Google Scholar] [CrossRef]
- Yan, J.; Shi, J.; Zhang, P.; Tian, W.; Zhang, Y.; Sun, Z. Preparation and properties of epoxy/basalt flakes anticorrosive coatings. Mater. Corros. 2018, 69, 1669–1675. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, X.; Jia, H.; Xing, H.; Zhang, H.; Wang, X.; Liu, C. Anticorrosion properties of modified basalt powder/epoxy resin coating. J. Coat. Technol. Res. 2022, 19, 1409–1420. [Google Scholar] [CrossRef]
- Luo, L.; Ma, Q.; Wang, Q.; Ding, L.; Gong, Z.; Jiang, W. Study of a Nano-SiO2 Microsphere-Modified Basalt Flake Epoxy Resin Coating. Coatings 2019, 9, 154. [Google Scholar] [CrossRef]
- GB/T 1728−2020; Paint Film, Putty Film Drying Time Measurement Method. Standardization Administration of the People’s Republic of China: Beijing, China, 2020.
- GB/T 6739−2022; Colour Paints and Varnishes Pencil Method for Determining Film Hardness. Standardization Administration of the People’s Republic of China: Beijing, China, 2022.
- GB/T 5210−2006; Colour Paints and Varnishes Pull Apart Adhesion Test. Standardization Administration of the People’s Republic of China: Beijing, China, 2006.
- GB/T1732−2020; Paint Impact Resistance Measurement Method. Standardization Administration of the People’s Republic of China: Beijing, China, 2020.
- GB/T 11185−2009; Colour Paints and Varnishes Bending Test (Tapered Shaft). Standardization Administration of the People’s Republic of China: Beijing, China, 2009.
- HG/T3343−1985; Measurement of Oil Resistance of Paint Film. Ministry of Chemical Industry of China (MOCI): Beijing, China, 2004.
- GB/T10834−2008; Determination of Salt Water Resistance of Marine Paints Brine and Hot Brine Immersion Method. Standardization Administration of the People’s Republic of China: Beijing, China, 2008.
- GB/T1768−2006; Colours and Varnishes Determination of Abrasion Resistance Rotating Rubber Wheel Method. Standardization Administration of the People’s Republic of China: Beijing, China, 2006.
- Amookht, S.; Gorji Kandi, S.; Mahdavian, M. Quantification of perceptual coarseness of metallic coatings containing aluminum flakes using texture analysis and visual assessment methods. Prog. Org. Coat. 2019, 137, 105375. [Google Scholar] [CrossRef]




| Sample | Polyurethane | Polyurethane Solvents | MBFs | Dispersant | Permutation Agent | Defoamers |
|---|---|---|---|---|---|---|
| 1 | 100 | 0 | 0 | 0 | 0 | 1.5 |
| 2 | 90 | 10 | 10 | 1.5 | 1 | 1.5 |
| 3 | 80 | 20 | 20 | 3 | 2 | 1 |
| 4 | 70 | 30 | 30 | 4.5 | 3 | 1 |
| 5 | 60 | 40 | 40 | 6 | 4 | 0.5 |
| Test Projects | Varnish | 10 wt%-MBFS | 20 wt%-MBFS | 30 wt%-MBFS | 40 wt%-MBFS | Testing Standards |
|---|---|---|---|---|---|---|
| Surface dry h/real dry h | 4/18 | 4/22 | 5/22 | 5/24 | 6/24 | [29] |
| Pencil hardness/H | 2 | 2 | 3 | 3 | 4 | [30] |
| Adhesion/Mpa | 17.68 | 14.61 | 15.15 | 15.36 | 15.04 | [31] |
| Impact resistance/cm | 58 | 59 | 65 | 70 | 70 | [32] |
| Bending resistance/level | 3 | 2 | 1 | 2 | 3 | [33] |
| Oil resistance/level | 0 | 0 | 0 | 0 | 1 | [34] |
| Saltwater resistance/level | 2 | 1 | 1 | 1 | 3 | [35] |
| Resistance to frictional wear/g | 0.22 | 0.18 | 0.19 | 0.23 | 0.27 | [36] |
| Samples | Corrosion Current Density (A/cm2) | Corrosion Potential (V) | Polarization Resistance (kΩ) | Corrosion Rate (mm/Year) |
|---|---|---|---|---|
| Q235 | 2.9474 × 10−4 | −0.766 | 2.36130 | 3.42920 |
| X0 | 5.3961 × 10−5 | −0.612 | 6.87240 | 0.39370 |
| X1 | 7.8156 × 10−5 | −0.609 | 7.23810 | 0.32851 |
| X2 | 8.90670 × 10−6 | −0.549 | 33.0410 | 0.10350 |
| X3 | 6.59110 × 10−6 | −0.323 | 32.7430 | 0.076588 |
| X4 | 3.44351 × 10−5 | −0.603 | 12.81510 | 0.254610 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, K.; Cai, W.; He, X.; Chen, H.; Chen, K.; Jiang, T.; Li, W.; Zhao, Y. Preparation and Properties of Silane Coupling Agent Modified Basalt Flake Polyurethane Anti-Corrosion Coatings. Coatings 2023, 13, 2022. https://doi.org/10.3390/coatings13122022
Sun K, Cai W, He X, Chen H, Chen K, Jiang T, Li W, Zhao Y. Preparation and Properties of Silane Coupling Agent Modified Basalt Flake Polyurethane Anti-Corrosion Coatings. Coatings. 2023; 13(12):2022. https://doi.org/10.3390/coatings13122022
Chicago/Turabian StyleSun, Kuoteng, Weichen Cai, Xuemin He, Hao Chen, Kun Chen, Tao Jiang, Wenge Li, and Yuantao Zhao. 2023. "Preparation and Properties of Silane Coupling Agent Modified Basalt Flake Polyurethane Anti-Corrosion Coatings" Coatings 13, no. 12: 2022. https://doi.org/10.3390/coatings13122022






